Optimizing Hydrazine Activation on Dual-Site Co-Zn Catalysts for Direct Hydrazine-Hydrogen Peroxide Fuel Cells
Qian Liu and Junwei Han contributed equally to this study.
ABSTRACT
Direct hydrazine-hydrogen peroxide fuel cells (DHzHPFCs) offer unique advantages for air-independent applications, but their commercialization is impeded by the lack of high-performance and low-cost catalysts. This study reports a novel dual-site Co-Zn catalyst designed to enhance the hydrazine oxidation reaction (HzOR) activity. Density functional theory calculations suggested that incorporating Zn into Co catalysts can weaken the binding strength of the crucial N2H3* intermediate, which limits the rate-determining N2H3* desorption step. The synthesized p-Co9Zn1 catalyst exhibited a remarkably low reaction potential of −0.15 V versus RHE at 10 mA cm−2, outperforming monometallic Co catalysts. Experimental and computational analyses revealed dual active sites at the Co/ZnO interface, which facilitate N2H3* desorption and subsequent N2H2* formation. A liquid N2H4-H2O2 fuel cell with p-Co9Zn1 catalyst achieved a high open circuit voltage of 1.916 V and a maximum power density of 195 mW cm−2, demonstrating the potential application of the dual-site Co-Zn catalyst. This rational design strategy of tuning the N2H3* binding energy through bimetallic interactions provides a pathway for developing efficient and economical non-precious metal electrocatalysts for DHzHPFCs.
1 Introduction
Direct hydrazine-hydrogen peroxide fuel cells (DHzHPFCs) have long been recognized as unique power sources, particularly suitable for air-independent applications in space and underwater environments [1, 2]. Especially, employing hydrogen peroxide (H2O2) as an oxidant resulted in a simplified internal structure of fuel cells [3-5]. Given the higher theoretical electrode potential and kinetics of the hydrogen peroxide reduction reaction (HRR, 1.8 V vs. RHE) over those of the oxygen reduction reaction (ORR) [6], using H2O2 as an alternative fuel to oxygen elevates the theoretical open circuit voltage (OCV, 2.13 V for DHzHPFC) of a single fuel cell and its power output [2, 3].
While platinum (Pt) has been acknowledged as an active catalyst for both hydrazine oxidation reaction (HzOR) [7, 8] and HRR [9, 10], its scarcity poses challenges for large-scale applications in DHzHPFCs. Cobalt-based catalysts have emerged as a viable alternative due to their low cost and decent catalytic activity in HzOR [11-14]. Previous studies have shown that the desorption of the N2H3* intermediate and formation of N2H2* is the rate-determining step for HzOR on Co catalysts [11, 15]. The relatively strong adsorption of N2H2* on Co represents one of the major energy barriers for the HzOR process. Dual-site catalysts, featuring distinct active sites tailored for different reaction steps, represent an effective strategy to improve electrocatalytic activity [16]. Constructing heterostructures [17, 18], designing diatomic catalysts [19, 20], introducing heteroatoms [21], or forming alloys [22, 23] are promising approaches to create dual active sites that can synergistically improve activity and selectivity.
In the present study, we aim to boost the activity of cobalt-based catalysts by optimizing the adsorption of the key reaction intermediate on dual-site catalysts for HzOR in DHzHPFCs. Density functional theory (DFT) calculations suggest that Zn exhibits a lower adsorption energy of the key intermediate N2H3* than Co, which might help to accelerate the HzOR process on Co [15]. Guided by the theoretical prediction, dual-site Co-Zn catalysts were synthesized through plasma reduction of CoZn hydroxides. The as-synthesized p-Co9Zn1 catalyst demonstrated a remarkable overpotential of −0.15 V versus RHE at 10 mA cm−2, surpassing the monometallic p-Co(OH)2 catalyst. This enhanced intrinsic HzOR activity in p-Co9Zn1, attributed to the incorporation of Zn, is in line with the reduced energy barrier of HzOR manifested by theoretical calculations. Finally, we successfully demonstrate DHzHPFCs with a high OCV of 1.916 V and a maximum power density of 195 mW cm−2 at 80°C, highlighting the potential application of the Co-Zn catalyst.
2 Results and Discussion
2.1 Theoretical Screening of Metallic Catalysts for HzOR
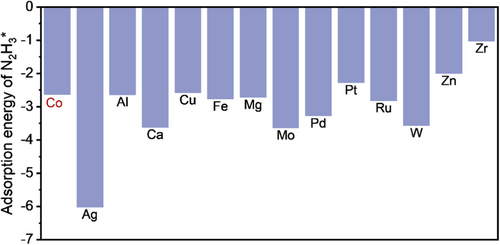
To search for Co-based catalysts with high activity, we conducted DFT calculations to assess the adsorption energies of N2H3* on various metal surfaces (corresponding configurations displayed in Supporting Information S1: Figures S1 and S2). The adsorption energy of N2H3* serves as a crucial parameter, facilitating the comprehension and comparative analysis of whether the incorporation of various elements into cobalt-based catalysts can effectively enhance the catalytic activity in HzOR [11, 15]. Figure 1 illustrates that the adsorption energies of N2H3* on Zn (−2.0 eV) and Zr (−1.03 eV) were significantly lower than that on Co (−2.63 eV) (Supporting Information S1: Table S1). This observation suggests that incorporating Zn or Zr into Co catalysts might mitigate the strong adsorption of N2H3* on the Co surface, thus decreasing the reaction barrier of HzOR. Considering the substantial disparity in atomic radii and coordination structure between Zr and Co, incorporating Zn would be an experimentally feasible approach to modulate the activity of Co for HzOR.
2.2 Synthesis and Structural Characterizations of Co-Zn Catalysts
To validate the theoretical predictions, we systematically synthesized Co(OH)2 and CoZn hydroxides with varying ratios of Co and Zn (denoted as CoxZny, where x:y refers to the feed molar ratio of Co to Zn) as precursors using an electrosynthesis method, followed by H2 plasma reduction (denoted as p-CoxZny). The layered hydroxide [24, 25] with ordered and uniform distribution of Co and Zn cations in the 2D layer ensures the homogeneous incorporation of Zn and Co (refer to Methods in Supplementary Information for details). Subsequently, linear sweep voltammetry (LSV) (Supporting Information S1: Figure S3) was employed to assess the HzOR performance of catalysts with different Zn/Co ratios. Among the catalysts, p-Co9Zn1 exhibited the lowest onset potential and highest current density, affirming that the addition of Zn effectively enhances the HzOR performance of Co-based catalysts. The subsequent HzOR tests and characterizations of Co-Zn catalysts will focus on the sample synthesized with feeding Co:Zn molar ratio of 9:1.
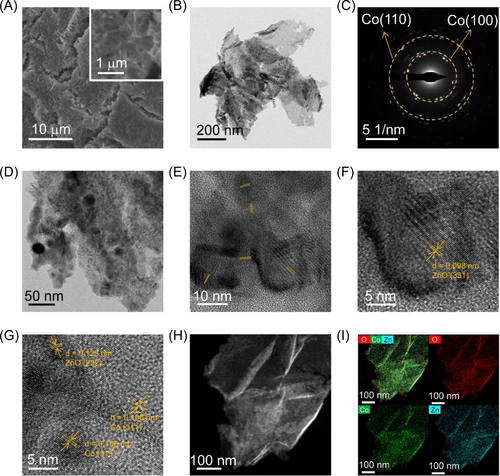
The Co(OH)2 and Co9Zn1 hydroxide precursors exhibit similar X-ray diffraction (XRD) patterns as depicted in Supporting Information S1: Figure S4a,b. Both patterns align closely with the standard pattern of α-Co(OH)2 (PDF# 51-1734). During the electrosynthesis, the Co9Zn1 nanosheet arrays are formed as illustrated in Supporting Information S1: Figure S5a. Remarkably, following H2 plasma reduction, p-Co9Zn1 retains its nanosheet structure (Figure 2A). Transmission electron microscope (TEM) images and corresponding selected area electron diffraction (SAED) patterns of Co9Zn1 and p-Co9Zn1 are presented in Supporting Information S1: Figure S5b,c and Figure 2B–D. Both Co9Zn1 and p-Co9Zn1 nanosheets exhibit a polycrystalline structure. The high surface area of the nanosheet structure could increase the accessibility and exposure of active sites, thus improving the overall catalytic activity. Additionally, the open nanosheet architecture may facilitate mass transport of reactants and products, reducing diffusion limitations. High-resolution TEM (HRTEM) analysis of Co9Zn1 (Supporting Information S1: Figure S5d) reveals its poor crystallinity. Notably, the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image, along with corresponding elemental mapping images of Co9Zn1 (Supporting Information S1: Figure S5e,f), indicates a uniform distribution of Co and Zn elements throughout the nanosheet. The results from inductively coupled plasma mass spectrometry (ICP-MS) analysis of Co9Zn1 reveal mass fractions of 20% for Zn and 33.3% for Co. This observation underscores a notable Zn content in the Co-based catalysts. The difference between the feeding ratio and the measured ICP-MS ratio of Co and Zn in the electrodeposited catalysts can be attributed to the distinct chemical properties of each metal and the specific electrodeposition conditions employed. Upon plasma reduction, the phase undergoes transformation to cobalt (PDF# 15-0806) and zinc oxide (PDF# 65-0523) (Supporting Information S1: Figure S4c). The crystallinity of p-Co9Zn1 is improved (Figure 2E–G), revealing clear lattice fringes with interplanar distances of 0.098, 0.123, 0.106, and 0.106 nm, corresponding to ZnO (331), ZnO (222), Co (311), and Co (311) planes, respectively. Such polycrystalline array structure exhibits high surface area, offering abundant catalytic active sites. Furthermore, the homogeneous distribution of Zn, Co, and O elements throughout the entire nanosheet confirms the successful construction of a heterostructure composed of metallic Co and ZnO (Figure 2H,I).
X-ray photoelectron spectroscopy (XPS) was employed to probe the changes in the chemical states of Co(OH)2, p-Co(OH)2, Co9Zn1, and p-Co9Zn1, as illustrated in Figure 3. Distinct Co2+ signals are observed in the XPS spectra of Co(OH)2, whereas dominant signals of Co0 are evident in p-Co(OH)2 (Figure 3A). The peak positions align with the Co 2p3/2 signals of Co2+ (781.1 eV) and Co0 (777.9 eV) species. An additional Co 2p3/2 peak in p-Co(OH)2 at a lower binding energy (780.4 eV) originates from the surface oxidation of Co in the air. The primary O 1s peak at 531.4 eV (Figure 3B) corresponds to metal hydroxides (531–532 eV). After plasma reduction, the O 1s peaks at 533.0, 531.4, and 529.38 eV are attributed to adsorbed water molecules, residual hydroxyl groups, and zinc oxide, respectively (Figure 3B,D). The change in valence states for Co (Figure 3C) and O (Figure 3D) in Co9Zn1 and p-Co9Zn1 is basically consistent with the variations observed in Co(OH)2 and p-Co(OH)2, affirming the transformation of the Co2+ in Co9Zn1 to Co0 in p-Co9Zn1, consistent with the XRD results.
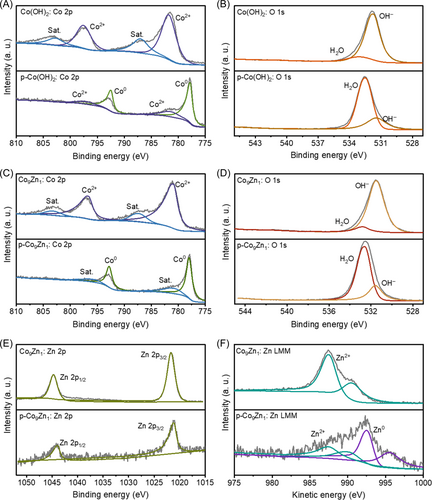
Concerning the valence states of Zn, analysis employing Zn 2p and Auger Zn LMM XPS spectra was conducted. The XPS spectra of Zn 2p generally remain unaltered after plasma treatment (Figure 3E). Auger electron spectroscopy of Zn (Figure 3F) reveals that Co9Zn1 exhibits only Zn2+ signals, while p-Co9Zn1 shows both Zn2+ and Zn0 signals. However, the XRD pattern exclusively indicates the existence of the ZnO phase, implying that the Zn0 in p-Co9Zn1 results from the reduction of a small proportion of surface Zn atoms. The XPS results elucidate that the surface composition of p-Co9Zn1 predominantly comprises Co0, Zn0, and Zn2+.
2.3 Electrochemical Performance of Catalysts for HzOR
The HzOR performances of the synthesized catalysts were meticulously assessed using a standard three-electrode configuration, with a Hg/HgO electrode as the reference electrode and a graphite rod as the counter electrode, immersed in a 1.0 M KOH electrolyte containing 0.1 M N2H4. LSV curves, presented in Figure 4A, were measured to evaluate the electrocatalytic activity towards HzOR, and the corresponding Tafel plots are depicted in Figure 4B.
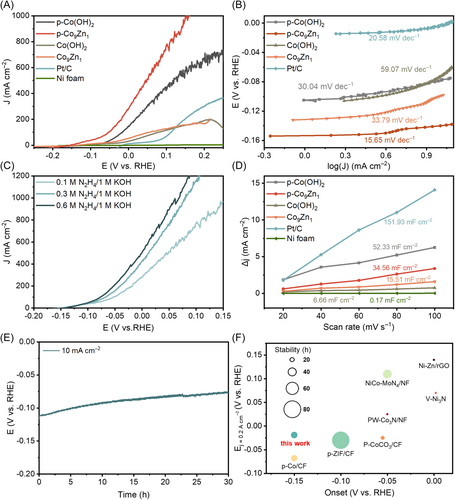
In this electrode configuration, the Ni foam substrate exhibited negligible catalytic activity, while Pt/C catalyst displayed a relatively high overpotential for HzOR (10 mA cm−2 at ~ −0.003 V vs. RHE). Intriguingly, both Co(OH)2 and Co9Zn1 demonstrated a lower overpotential than Pt/C (10 mA cm−2 at ~ −0.07 V vs. RHE). However, they exhibited lower current density at high overpotential, which is probably attributed to the suboptimal conductivity and insufficient active sites inherent in hydroxide-based catalysts. Note that hydrazine hydrate in the electrolyte would simultaneously reduce hydroxides to certain degree, thus the LSV curves of Co(OH)2 and Co9Zn1 do not fully reflect their intrinsic catalytic activity.
Remarkably, after plasma treatment, p-Co(OH)2 and p-Co9Zn1 exhibited a notable improvement in catalytic activity. Specifically, p-Co(OH)2 displayed a overpotential of −0.1 V (vs. RHE at 10 mA cm−2) and a lower Tafel slope (30.04 mV dec−1) compared to Co(OH)2. In the case of p-Co9Zn1, the overpotential shifted significantly to a negative value of −0.15 V (vs. RHE) at 10 mA cm−2, and the anodic current density rapidly increased to sub-ampere per cm2 as the overpotential rose. An exceptionally small Tafel slope of 15.65 mV dec−1 was obtained, indicating rapid HzOR kinetics on p-Co9Zn1. The superior performance of p-Co9Zn1 than p-Co(OH)2 underscores the pivotal role of Zn incorporation and plasma reduction in generating catalytically active sites for HzOR, revealing the significance of these factors in optimizing electrocatalytic activity.
The HzOR activities of the p-Co9Zn1 electrode in electrolytes with varying hydrazine concentrations are presented in Figure 4C. The recorded LSV curves in different electrolyte concentrations overlap at low overpotential (−0.15 ~ −0.1 V), indicating comparable reaction kinetics under small current densities irrespective of hydrazine concentration. However, at higher overpotential, the p-Co9Zn1 electrode in electrolytes with high hydrazine concentrations exhibited significantly enhanced current density, which suggests the mass-transfer limitation of HzOR at high current densities exceeding 50 mA cm−2.
To evaluate the intrinsic catalytic activity of the catalysts, the electrochemical active surface area (ECSA) was determined by measuring the double-layer capacitance (Cdl), as shown in Figure 4D and Supporting Information S1: Figure S6. Notably, the Cdl of p-Co9Zn1/NF (34.56 mF cm−2) is substantially higher than that of Co9Zn1/NF (15.51 mF cm−2). Similarly, p-Co(OH)2 also exhibits a higher Cdl (52.33 mF cm−2) compared to Co(OH)2 (6.66 mF cm−2), probably due to the formation of porous structure during the plasma treatment. The large Cdl of Pt/C is likely attributed to the high specific surface area of carbon support. The ECSA-normalized current density, displayed in Supporting Information S1: Figure S7, highlights the superior intrinsic electrocatalytic performance of p-Co9Zn1 among the investigated catalysts. Hence, the H2 plasma treatment and the incorporation of Zn are pivotal factors in constructing abundant highly active catalytic sites for HzOR.
The stability of p-Co9Zn1 electrode was systematically assessed through a chronopotentiometry test conducted at a current density of 10 mA cm−2, as depicted in Figure 4E. Following a continuous 30-hour measurement, a small potential drop of 30 mV was observed, indicative of the decent stability of the electrode. After electrolysis, the XPS spectra of p-Co9Zn1 (Supporting Information S1: Figure S8a,b) unveiled a subtle shift in the Co 2p3/2 peak. This shift implies a minor oxidation of Co on the surface, leading to the formation of Co(OH)x/CoOx during the HzOR [26, 27]. Notably, the valence states of O and Zn in p-Co9Zn1 remained unchanged after long-term test. The loss of catalytic active Co0 species as well as detachment of active component from the electrode might be the main reason for the performance degradation.
When compared with previously documented HzOR catalysts (see Figure 4F and Supporting Information S1: Table S2), p-Co9Zn1/NF demonstrates notably superior characteristics. Notably, it displays the lowest onset potential at 10 mA cm−2 (−0.15 V vs. RHE), as well as a low overpotential at 200 mA cm−2 (−0.02 V vs. RHE), coupled with a remarkable electrolysis stability. The outstanding performance of the Co-Zn bimetallic catalyst underscores the effectiveness of our catalyst design strategy.
2.4 Mechanistic Insights of Co-Zn Catalysts for HzOR
We conducted comprehensive DFT calculations to elucidate the HzOR process on Co-Zn electrocatalysts. Two pathways, namely alternating (path 1) and distal (path 2) configurations of adsorbed N2H2*, were considered in our simulations. Experimental results reveal the presence of Zn2+ and Zn0 on the surface of p-Co9Zn1 catalyst, both of which might participate in forming the catalytic active sites. Considering the dominant ZnO phase in the catalyst, a simplified model is used to represent the structure of p-Co9Zn1 for theoretical calculations, where a ZnO (111) surface with a cobalt cluster was constructed and denoted as Co/ZnO (see computational model in Supporting Information S1: Figure S9a,b). For comparison, DFT calculations of the HzOR process were also performed on Co (111) and Pt (111) surfaces (see computational model in Supporting Information S1: Figure S10a,h).
The complete free energy diagrams for the HzOR were calculated to investigate the reaction thermodynamics on dual-site Co-Zn catalyst (Figure 5A). Supporting Information S1: Tables S3 and S4 provide the free-energy changes of the elementary reactions, while Supporting Information S1: Figures S9 and S10 present the configurations of intermediates on catalyst surfaces. Notably, the intermediate species were found to bond with both Co and Zn atoms on the dual-site Co/ZnO surface. Our results indicate that Co/ZnO exhibits a preference for the alternating pathway, Pt (111) favors the distal pathway, and Co (111) demonstrates similar overpotential for both pathways. The dehydrogenation of N2H3* is identified as the potential-determining step on both Co/ZnO and Pt (111), with energy barriers of 0.28 and 0.48 eV, respectively. As for Co (111), the dehydrogenation of N2H3* and N2H2* exhibits identical energy barriers of 0.44 eV. Thus, Co/ZnO with the lowest energy barrier for the dehydrogenation of N2H3* emerges as the most active catalyst for HzOR. The comparison between theoretical and experimental onset potential, presented in Figure 5B, confirms the reliability of our simulations. The theoretical onset potential of Co/ZnO at −0.05 V (vs. RHE) is lower than that of both Co (111) and Pt (111), consistent with our experimental results. This discrepancy in onset potential arises from the varying energy barriers of dehydrogenation of N2H3* on different surfaces.

The N2H3* adsorption energy on Co/ZnO was calculated as −2.51 eV, lower than that on Co (111) (−2.63 eV) and ZnO (111) (−3.23 eV). This aligns with our hypothesis that the incorporation of Zn in Co-based catalysts could attenuate the strong bonding between Co and N2H3*. Importantly, the strong adsorption energy of N2H3* on ZnO (111) excludes the possibility of HzOR active sites on the surface of ZnO. The differential charge density in Supporting Information S1: Figure S11 further indicates a significant electron transfer between Co and Zn at the interface, optimizing the adsorption energy of N2H3*. The adjacent ZnO sites then modify the desorption of the N2H3* intermediate of Co sites with a significant electron transfer between Co and ZnO at the interface. In contrast, on a pure metal catalyst, the adsorption and desorption of the N2H3* intermediate could be more challenging. Our Gibbs energy calculations elucidate that the dual-site Co-Zn sites at the Co/ZnO interface serve as the active sites for HzOR, with an optimal N2H3* adsorption energy. The consistency of experimental and calculated results validates our catalyst design strategy and prediction, opening avenues for the development of highly active catalysts.
2.5 Assembly and Performance of DHzHPFC
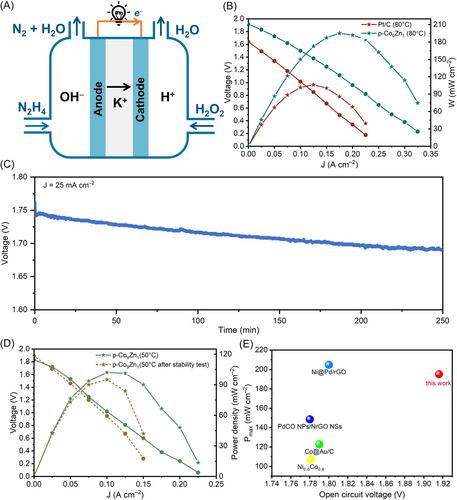
DHzHPFC was assembled using p-Co9Zn1/NF as the anode and Pt/C-coated carbon paper as the cathode. Pt/C was also tested as the anode catalyst for comparison (see Methods for preparation of electrodes). In these experiments, mixtures of (0.5 M H2SO4 + 2.0 M H2O2) and (2.0 M KOH + 1.0 M N2H4) served as the catholyte and anolyte, respectively. Current-voltage (I-V) characteristics at different operation temperatures were shown in Figure 6B and Supporting Information S1: Figure S12, illustrating temperature-dependent variations. Notably, the open-circuit voltage (OCV) of DHzHPFCs remained consistent at different temperatures, while the power density increased with rising temperature. This behavior is attributed to enhanced kinetics of anodic and cathodic reactions and improved electrolyte conductivity at higher temperatures [29, 30]. The OCV of the cell using p-Co9Zn1 (1.916 V) exceeded that of Pt/C (1.64 V), highlighting the high intrinsic activity of p-Co9Zn1. At 80°C, p-Co9Zn1 exhibited a significantly higher maximum power density (195 mW cm−2) compared to Pt/C (106 mW cm−2), indicating superior reaction activity and kinetics for p-Co9Zn1-assembled DHzHPFC.
To assess the electrochemical stability of the p-Co9Zn1-assembled DHzHPFC, the cell output voltage was investigated over time at 25 mA cm−2 and 50°C (Figure 6C). The results showed fluctuations in the cell potential due to the production of oxygen and hydrogen from H2O2 and N2H4 decomposition in the cathodic and anodic compartments, respectively. The output voltage of the DHzHPFC remains generally stable with slight degradation during the operation. The polarization and power density curves revealed a decrease in maximum power density from 102 to 95 mW cm−2 (50°C) after over 4 h of stability test (Figure 6D). While acknowledging the need for further investigation and improvement in the long-term durability of the as-prepared catalyst and DHzHPFCs, our results suggest that the catalytic activity of p-Co9Zn1 for N2H4 electrooxidation remains relatively stable in DHzHPFCs.
Comparisons with literature reports of DHzHPFCs operated under similar conditions (Figure 6E and Supporting Information S1: Table S6), our work demonstrates the excellent performance of the DHzHPFC assembled with a p-Co9Zn1 electrode, particularly in terms of OCV and maximal power output. The OCV of our p-Co9Zn1-assembled DHzHPFC surpasses previously reported DHzHPFCs, indicating the excellent intrinsic HzOR activity of our p-Co9Zn1 catalyst.
3 Conclusion
This study presents a rational catalyst design strategy for enhancing the HzOR activity in DHzHPFCs. Guided by DFT calculations, zinc was incorporated into cobalt-based catalysts to weaken the binding strength of the critical N2H3* intermediate, which limits the rate-determining N2H3* desorption step. The synthesized p-Co9Zn1 catalyst exhibits the optimal performance, displaying a remarkably low overpotential of −0.15 V (vs. RHE) at 10 mA cm−2 and an impressive Tafel slope of 15.65 mV dec−1. Experimental and computational analyses suggest that both Zn and Co atoms on the Co/ZnO interface serve as catalytically active sites for HzOR. The successful incorporation of Zn plays a pivotal role in balancing the adsorption energy of N2H3*. DHzHPFC using p-Co9Zn1 as anode catalyst achieved an exceptional open circuit voltage of 1.916 V, underscoring the intrinsic activity of Co-Zn catalyst. This work validates the strategy of tuning the N2H3* binding energy through dual-site interactions, advancing the fundamental understanding of hydrazine electrooxidation on cobalt catalysts.
Acknowledgments
Hao Bin Wu acknowledges the funding support from National Key R&D Program of China (2023YFB4203900) and the Fundamental Research Funds for the Central Universities (226-2024-00075). The authors would like to thank Yupeng Lou from Shiyanjia Lab (http://www.shiyanjia.com) for the XPS and TEM analysis.
Conflicts of Interest
The authors declare no conflicts of interest.




