Dominant TP63 missense variants lead to constitutive activation and premature ovarian insufficiency
Elena J. Tucker and Niklas Gutfreund contributed equally to this work.
Abstract
Premature ovarian insufficiency (POI) is a leading form of female infertility, characterised by menstrual disturbance and elevated follicle-stimulating hormone before age 40. It is highly heterogeneous with variants in over 80 genes potentially causative, but the majority of cases having no known cause. One gene implicated in POI pathology is TP63. TP63 encodes multiple p63 isoforms, one of which has been shown to have a role in the surveillance of genetic quality in oocytes. TP63 C-terminal truncation variants and N-terminal duplication have been described in association with POI, however, functional validation has been lacking. Here we identify three novel TP63 missense variants in women with nonsyndromic POI, including one in the N-terminal activation domain, one in the C-terminal inhibition domain, and one affecting a unique and poorly understood p63 isoform, TA*p63. Via blue-native page and luciferase reporter assays we demonstrate that two of these variants disrupt p63 dimerization, leading to constitutively active p63 tetramer that significantly increases the transcription of downstream targets. This is the first evidence that TP63 missense variants can cause isolated POI and provides mechanistic insight that TP63 variants cause POI due to constitutive p63 activation and accelerated oocyte loss in the absence of DNA damage.
1 INTRODUCTION
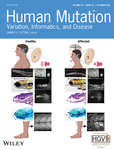
The TP63 gene encodes the transcription factor p63, which has varied roles from ectodermal development to the maintenance of genomic integrity and female fertility. The varied roles are achieved via regulated and cell-specific expression of alternative isoforms. The p63 protein can have one of four different N-termini, denoted TA, TA*, GTA, and ΔN and one of five different C-termini denoted α, β, ɣ, δ, and ε (Figure 1). The longer isoforms, such as TAp63α or TA*p63α, have a transactivation domain (TAD) in their N-terminus and a transactivation inhibition domain (TID) in their C-terminus. These domains are pivotal for the controlled activation of the corresponding p63 isoforms (Krauskopf et al., 2018; Pitzius et al., 2019). The TAD and TID interact with each other to generate an inactive dimer conformation that has reduced affinity for DNA, as well as coactivators such as p300 (Deutsch et al., 2011; Krauskopf et al., 2018). In certain physiological conditions, a series of phosphorylation events can break this interaction, forcing p63 into an active tetramer conformation that can then activate downstream gene transcription.
Although the shorter ΔN isoforms have been shown to have a role in the development of ectodermal structures, such as the skin, hair, teeth, and nails (Koster et al., 2009; Medawar et al., 2008), the TAp63α isoform is best known and characterised for its role in the genomic integrity of oocytes and female reproduction (Suh et al., 2006). This isoform is almost exclusively expressed in female germ cells with a tightly regulated temporal expression that reflects its role in the management of DNA damage (Suh et al., 2006). It is not expressed until after homologous recombination, which involves orchestrated and permissible DNA double-strand breaks. Expression of TAp63α remains high throughout the entire dictyate arrest and only ceases when oocytes resume meiosis (Suh et al., 2006). In resting oocytes TAp63α adopts the inactive dimeric form (Coutandin et al., 2016; Deutsch et al., 2011). DNA damage leads to the phosphorylation of TAp63α by the priming kinase CHK2 followed by phosphorylation by the executioner kinase CK1 (Tuppi et al., 2018). These phosphorylation events alter the conformation of TAp63α into an active tetrameric form that induces expression of NOXA and PUMA culminating in the apoptosis of oocytes that have experienced DNA damage (Kerr et al., 2012). This maintains genomic integrity, thereby protecting female fertility (Suh et al., 2006).
In contrast to TAp63α, the alternative isoform GTAp63α is highly and exclusively expressed in spermatogenic precursors of human testes (Beyer et al., 2011). Here, its role is analogous to TAp63α in human ovaries, protecting the genomic integrity of male germ cells. Upon DNA damage it is activated by caspase cleavage of its C terminus, resulting in the induction of an apoptotic program that eliminates damaged germ cells (Beyer et al., 2011).
The role of the TA* isoform remains elusive. It has been shown to have greater activation and greater repression due to its extended N terminus (Pitzius et al., 2019) but its relevance to human biology is yet to be established. Recently a TP63 variant affecting only this isoform of p63 was found to cause furrowed tongue, implicating a role of TA*p63 in development (Schmidt et al., 2021), but whether it also has a role in oocyte integrity remains to be established.
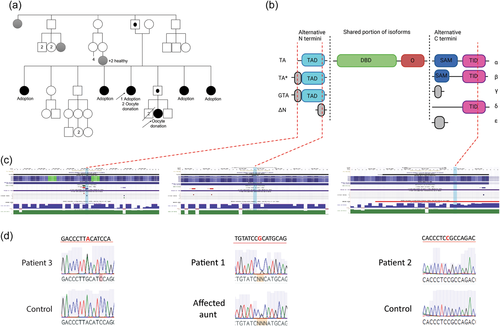
Not surprisingly, given its integral role in development, variants in TP63 have been shown to cause syndromes with variable features including, but not limited to, ectodermal dysplasia, split-hand/foot malformation/syndactyly, lacrimal duct obstruction, hypoplastic breasts and/or nipples, ankyloblepharon filiforme adnatum, and cleft lip/palate (Sutton & van Bokhoven, 1993). Causative variants tend to cluster in the DNA binding domain and the downstream SAM domain (Figure 2). There is some genotype:phenotype correlation with missense variants in the DNA binding domain tending to cause Ectrodactyly-Ectodermal Dysplasia-Clefting (EEC) Syndrome characterised by missing or irregular fingers and/or toes (ectrodactyly or split hand/foot malformation) as well as cleft lip/palate (Celli et al., 1999; Russo et al., 2018) (Figure 2). In contrast, mutations in the SAM domain and frameshift variants in the C-terminal tend to cause the more severe phenotype of Ankyloblepharon-ectodermal defects-cleft lip/palate (AEC) syndrome characterised by fusion of eyelids (ankyloblepharon), mild to severe skin erosions, abnormal hair and nails, and cleft lip/palate (Figure 2) (Russo et al., 2018).
Specific TP63 variants have recently been found to cause premature ovarian insufficiency (POI) without syndromic features (Bestetti et al., 2019; Tucker et al., 2019). POI is defined as loss of ovarian activity associated with elevated follicle-stimulating hormone (FSH) before the age of 40. Previously reported POI variants are either truncating variants in the terminal exon of TP63 predicted to generate a protein lacking the TID, or an intragenic duplication that causes an extra TAD. Both variants are likely to promote p63 activation, leading to accelerated oocyte death. However, this theory has not been tested via functional studies.
Despite TP63 C-terminal nonsense and N-terminal duplication variants being a known cause of POI, the genetic basis of most POI patients remains elusive. There are over 80 causative genes and these affect a variety of processes (Tucker et al., 2016). Understanding the genetic basis of POI is important because it helps to minimise the risks of comorbidities and enables cascade screening for potentially affected family members who can benefit from early intervention.
2 MATERIALS AND METHODS
2.1 Ethics
Written informed consent was obtained from all participants. All procedures were approved by the Ethics Committee of Rennes University Hospital and the French law (CCTIRS Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé) or the Human Research Ethics Committee of the Royal Children's Hospital, Melbourne (HREC/22073).
2.2 Participants
Patients were recruited after clinical consultation as part of our ongoing research program investigating the genetics of POI. Family and personal medical history was collated and is included in Table 1. All patients had POI, defined by menstrual disturbance and elevated FSH (>20 mIU/ml) measured twice at least 1 month apart as per the European Society of Human Reproduction (ESHR) guidelines (https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency.aspx) (European Society for Human Reproduction and Embryology (ESHR) et al., 2016). There was no history of ovarian surgery, infection, or gonadotoxic therapy that could explain their condition. Karyotyping or microarray was performed to confirm normal 46,XX chromosomal complement, and to exclude patients with causal chromosomal rearrangements. All included cases were negative for FMR1 premutation and negative for ovarian auto-antibodies.
2.3 General molecular techniques
Genomic DNA was extracted from EDTA-blood manually with the NucleoSpin® Blood XL kit (Macherey-Nagel) or with an automated system, Hamilton Microlab STAR and Nucleospin® Blood L kit (Macherey-Nagel), and were assessed by NanoDropTM 1000 spectrophotometer and Qubit dsDNA BR Assay (Thermo Fisher Scientific). Selected variants were validated by Sanger sequencing using BigDye v3.1 Terminators (Applied Biosystems) and ABI 3130X. Primer sequences are available on request.
2.4 Whole-exome sequencing (WES)
DNA underwent WES at the Victorian Clinical Genetics Service (VCGS) or CHU-Rennes with exome capture using SureSelect QXT Clinical Research Exome V1 (Agilent) or SureSelect Human All Exon V7 (Agilent) respectively and sequencing performed on the HiSeq 4000 (Illumina) or NextSeq 500/550 (Illumina). WES data were processed using Cpipe (Sadedin et al., 2015) or C-GeVarA pipeline (Constitutional Genetc Variant Analysis) and deposited into SeqR for analysis (https://seqr.broadinstitute.org/).
We performed two phases of analysis as previously described (Tucker et al., 2016)—the first was gene-centric using a candidate POI gene list and the second was variant-centric. Variant-centric analysis focused on high-priority variants (frameshift, nonsense, or splice site variants) in any gene and with any inheritance, or potentially bi-allelic moderate-high priority variants (missense, in-frame indels, frameshift, nonsense, or splice spite). Only variants with MAF <0.005 in 1000 genomes and gnomAD (https://gnomad.broadinstitute.org/) and with high-quality scores (Q>50 and allele balance >25) were considered. Variant pathogenicity was predicted in silico using Mutation Taster (http://www.mutationtaster.org/), Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/), SIFT/Provean (http://provean.jcvi.org/), and CADD (Combined Annotation-Dependent Depletion) (https://cadd.gs.washington.edu/snv). The conservation of affected residues was assessed by Multiz Alignments of 100 vertebrates (UCSC Genome Browser https://genome.ucsc.edu/).
2.5 Cell culture, BlueNative-PAGE, and transactivation assays
The non-small cancer cell line H1299 (p53 null) was cultured in RPMI medium 1640 (Gibco) with 10% fetal bovine serum (Capricorn Scientific) and 1% Penicilin/Streptomycin (Gibco) at 37°C and 5% CO2. BlueNative-PAGE and Transactivation (TA) assays were performed as described before (Pitzius et al., 2019).
For TA assays, 0.8 × 105 cells were seeded in a 12-well plate 24 h before transfection. Afterwards, equal amounts (267 ng) of p63 pCDNA3.myc, pBDS2-Luc, and pRL-CMV plasmids were transfected using Lipofectamine®2000 and grown for another 24 h. Quantification of relative luciferase activities was performed using the Promega Dual-Glo® luciferase reporter assay kit according to the manufacturer's instructions. Empty pCDNA3.myc vector served as control. Significance was calculated with one-way analysis of variance using GraphPad Prism.
For oligomeric state analysis of p63 isoforms and mutants via BlueNative (BN)-PAGE, 0.8 × 105 H1299 cells were transfected in a 12-well plate with 800 ng pCDNA3.myc p63 constructs and harvested in lysis buffer (20 mM Tris, 150 mM NaCl, 2 mM MgCl2, 20 mM CHAPS, 1 mM DTT, 1× PhosSTOP [Roche], 1× cOmplete [Roche]). Cell lysis was supported by multiple freeze-thaw cycles. Subsequently, 1 µl Benzonase (Merck) was added to each sample and further incubated for 1 h on ice. The supernatant was separated by BN-PAGE (3%–12%; Thermo Fisher) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (4%–12%, Mini-PROTEAN TGX; Bio-Rad) before blotting on polyvinylidene fluoride membranes. Membranes were blocked with 5% skim milk in TBS-T for 1 h at room temperature and probed with anti-myc primary antibody (4A6; Merck) overnight at 4°C. Signal detection was performed using goat anti-mouse HRP (A9917; Sigma Aldrich).
For stability determination of N-Terminal TA*p63α mutants via urea assay, H1299 cells were transfected in 10 cm dishes with 12 µg pCDNA3.myc p63 constructs, grown for 24 h and harvested in lysis buffer without CHAPS. The supernatant was split and incubated with different urea concentrations (0–4 M) for 1 h on ice. Equal volumes of lysis buffer containing 40 mM CHAPS was added to the samples and incubated for another 10 min on ice. BN-PAGE and immunoblotting was performed as described. Signal intensities were quantified with ImageJ.
3 RESULTS
3.1 Clinical history of three patients presenting with premature ovarian insufficiency
The first patient experienced menarche at 13 years of age, with one period at age 13 and one period at age 15, before commencing oral contraceptive pill (OCP). At 24 years of age, she experienced secondary amenorrhea when ceasing OCP. At this time, her FSH was elevated at 78 IU/L and estradiol was low at 20 pg/ml. Repeat hormonal assessment at 29 years of age showed high FSH (69.9 IU/L) and LH (30.5 IU/L), low estradiol (<25 pg/ml), and AMH (<0.10 ng/ml). Ultrasound examination showed atrophic ovaries and a small uterus. She was overweight, and had mild hirsutism including facial hairiness. She obtained one pregnancy through oocyte donation. She had a similarly affected paternal aunt who experienced menarche at 13 years of age with only one period then secondary amenorrhea and had a unique right atrophic ovary and small uterus on ultrasound. Exploratory endoscopy did not visualize any ovary. She also experienced facial hirsutism. She obtained two pregnancies through oocyte donation and adopted one child (Table 1). Familial history found other members in the paternal family branch with infertility, including four additional paternal aunties with atrophic ovaries and other members with infertility but without information on ovarian status (Figure 1a). None of the men of the family are reported to have infertility.
| Patient | Age at diagnosis | Karyotype | Amenorrhea | Secondary sex characteristic | US | FSH (IU/l) | LH (IU/l) | Estradiol (pg/ml) | AMH (ng/ml) | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1PROBAND | 24 | 46,XX | Early secondary | Menarche at 13 | Atrophic ovaries streak gonads | 78 | 30.5 | 20 | <0.10 | 5 Affected aunts |
| 69.9 | <25 | |||||||||
| Hirsutism | ||||||||||
| 1AUNT | Early adulthood | 46,XX | Early secondary | Menarche at 13-14 | Atrophic unique ovary | - | - | - | - | Aunt of Patient 1 |
| 4 Affected sisters | ||||||||||
| Hirsutism | ||||||||||
| 2 | 27 | 46,XX | Early secondary | Menarche at 13 | Atrophic ovaries without follicles | 82.67 | 100.23 | <5 | - | - |
| 3 | 21 | 46,XX | Primary | Delayed puberty | No detectable ovaries | 83 | 20 | 5 | 0.4 | Mali descent |
- Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone.
The second patient experienced menarche at 13 yo with regular menstruation from 15 to 17 years of age, and OCP from 17 to 27 years with secondary amenorrhea when ceased. Hormonal assessment at this time showed high FSH (82.67 IU/L) and LH (100.23 IU/L) and low estradiol (<5 pg/ml). Ultrasound examination showed atrophic ovaries devoid of follicles and a small uterus (Table 1). Personal history includes allergy and eczema, but no familial history of infertility.
The third patient experienced delayed puberty and primary amenorrhea (Table 1). Her FSH was elevated at 84 IU/L and AMH was low at 0.4 ng/ml. No ovaries could be observed on ultrasound and she had an atrophic uterus and short vagina. Clitoral hypertrophy was also noted.
Given all three patients presented only with ovarian dysfunction and related hormonal abnormalities/symptoms without other (syndromic) comorbidity, they received a diagnosis of isolated POI.
3.2 WES identified heterozygous TP63 missense variants
Given the number of genes potentially causing POI, the optimal strategy for genetic diagnosis is WES. As part of our larger study using WES to investigate the genetic basis of POI, we identified three rare heterozygous missense variants in TP63 in three unrelated POI patients (Figure 1).
Patient 1 and her affected aunt shared a novel heterozygous c.290G>C, p.Arg97Pro variant (Figure 1). This variant is absent from gnomAD and alters a highly conserved residue within the N terminal TAD of TAp63α (Figure 1 and Supporting Information: Figure S1) and is predicted pathogenic based on most online algorithms (Table 2). Consistent with this site having a critical role for TP63, an alternative missense mutation at this site (p.Arg97Cys) is associated with isolated ectrodactyly (Zenteno et al., 2005).
| Patient | gDNA variant (GrCH38) | cDNA variant | Protein variant | gnomAD | Polyphen | Mutation Taster | CADD | SIFT | Provean | Functional support |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | chr3:189738740G>C | c.290G>C | p.Arg97Pro | 0 | Possibly damaging | Disease-causing | 23.5 | Damaging | Neutral (score -1.12) | Yes |
| (score 0.922) | (score 0.997) | (score 0.007) | ||||||||
| 2 | chr3: 189894398C>T | c.1939C>T | p.Arg647Cys | 0 | Probably damaging | Disease-causing | 32 | Damaging | Deleterious (score 3.33) | Yes |
| (score 1.000) | (score 1.000) | (score 0.000) | ||||||||
| 3 | chr3:189631568A>G | c.53A>G | p.Tyr18Cys | 0 | Benign | Disease-causing | 22.3 | Damaging | Neutral (score -0.24) | No |
| (score 0.000) | (score 0.741) | (score 0.004) |
- Note: Variants described according to NM_003722.5 and NP_003713.3.
- Abbreviations: cDNA, complementary DNA; gDNA, genomic DNA.
Patient 2 harbored a paternally inherited novel heterozygous c.1939C>T, p.Arg647Cys variant (Figure 1), also absent from gnomAD. This variant altered a highly conserved residue within the C terminal TID and was consistently predicted pathogenic using online algorithms (Table 2 and Supporting Information: Figure S2).
In Patient 3, WES identified a novel heterozygous c.53A>G, p.Tyr18Cys variant (Figure 1), absent from gnomAD. This variant affected a residue within the unique TA* isoform of TP63. The residue is moderately conserved (Supporting Information: Figure S3), and pathogenicity predictions using online algorithms were conflicting (Table 2).
3.3 TP63 missense variants can alter protein oligomerisation
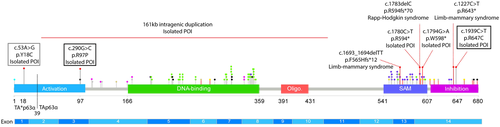
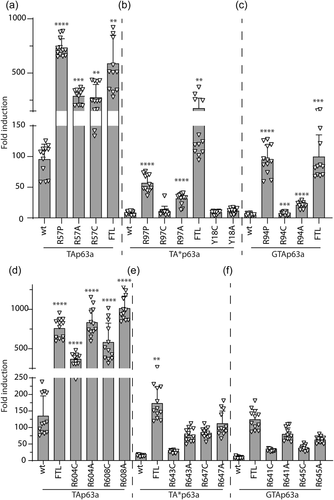
To investigate the potential causation of these three rare novel TP63 variants, we analysed their influence on protein structure and function. We first assessed complex conformation using BN-PAGE. Using this method, dimeric and tetrameric p63 proteins can be distinguished. As a positive control, we used p63 isoforms with mutation of three residues (Phe605Ala/Thr606Ala/Leu607Ala, FTL>AAA) that disrupt dimerization and lead to constitutive open conformation, as previously described (Straub et al., 2010). We found that wild-type (WT) p63 isoforms exist mostly in the dimeric state (Figure 3).
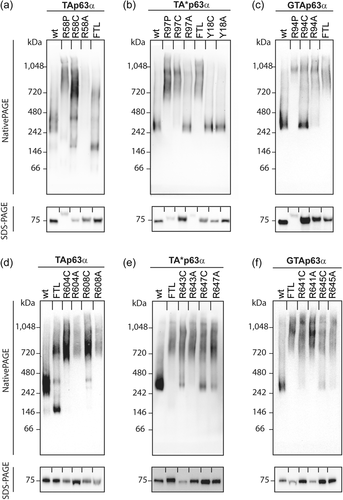
Two of the patient variants disrupted p63 complex conformation. The p.Arg58Pro/Arg97Pro/Arg95Pro variant found in Patient 1 causes an open tetramer conformation of the TAp63α, TA*p63a, and GTAp63α isoforms, respectively (Figure 3). In contrast, changing this site to a cysteine to model the previously reported pathogenic variant at this site (Zenteno et al., 2005) or alanine, leads to significant disruption of only the TAp63α isoform. Similarly, the p.Arg608Cys/Arg647Cys/Arg644Cys variant found in Patient 2 causes open active tetramer conformation of TAp63α and to a lesser extent of TA*p63a and GTAp63α. An alternative residue at this site, alanine, similarly induced the active conformation, as did alteration of a nearby arginine, p.Arg604/Arg643/Arg640 (Figure 3).
The third variant of interest, p.Tyr18Cys, alters a residue previously predicted to be important for the potential helical conformation of the N-terminal extension of TA*p63 (Pitzius et al., 2019). In contrast to the other variants investigated, the p.Tyr18Cys variant and an alternative variant affecting the same residue (p.Tyr18Ala) had no detectable impact on the conformation of TA*p63α, which is the only isoform in which the residue is present (Figure 3). To determine whether the p.Tyr18Cys variant might cause a more subtle defect in protein dimerization, we performed a urea titration followed by analysis on BN-PAGE. We have previously shown that low amounts of urea can convert TA*p63α into the open tetrameric state (Pitzius et al., 2019). In this experiment, we found no difference in the concentration of urea required to disrupt the WT or variant TA*p63α dimers (Supporting Information: Figure S4).
3.4 TP63 missense variants can alter transcriptional activity
To confirm that the tetrameric mutant protein complexes containing the p.Arg58Pro/Arg97Pro/Arg95Pro (Patient 1) or p.Arg608Cys/Arg647Cys/Arg644Cys (Patient 2) variants have enhanced transcription of target genes, we performed luciferase reporter assays. These assays demonstrated that the p.Arg58Pro/Arg97Pro/Arg95Pro variant and the p.Arg608Cys/Arg647Cys/Arg644Cys variant cause a significant increase in transcriptional activity on a promoter with an optimized p63 binding site compared to the corresponding WT p63 isoforms (Figure 4). In contrast, and in keeping with the retained dimeric state of TA*p63 harboring the p.Tyr18Cys variant, this variant caused no significant change in transcriptional activity (Figure 4b).
4 DISCUSSION
4.1 TP63 missense variants are a novel cause of POI
Studies in mice have demonstrated the requirement of the TAp63α p63 isoform for maintenance of oocyte integrity (Suh et al., 2006). More recently, the identification of heterozygous TP63 C-terminal truncation variants in human POI patients, demonstrated the likely requirement of functional TP63 for human ovarian function (Tucker et al., 2019). The proposed mechanism was that truncation variants remove the C-terminal repression domain leading to constitutive activation and excessive oocyte death. A N-terminal duplication has also been reported in a POI patient (Bestetti et al., 2019). Although not demonstrated, it can be hypothesized that duplication of the N terminal activation domain could similarly lead to constitutive activation and subsequent oocyte depletion. The finding of rare heterozygous missense variants in three independent POI patients, raised the possibility of missense variants also being causative. We used functional validation to prove the deleterious impact of two of these novel variants and to provide conclusive evidence that TP63 missense variants can cause POI in humans.
4.2 POI-associated TP63 variants disrupt oligomerisation and transcriptional activity
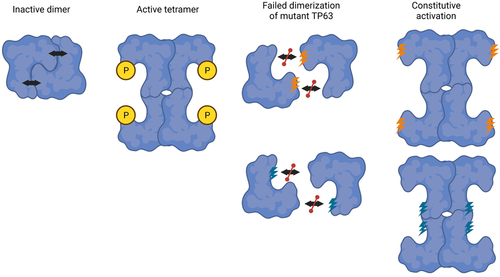
To investigate the potential causation of the three rare novel TP63 variants, we analysed their influence on protein structure and function. Via blue-native page, we demonstrated that the missense variant within the N-terminal activation domain and the missense variant within the C-terminal repression domain both disrupted TP63 dimerisation leading to an active tetrameric confirmation. We coupled this finding with luciferase reporter assays that concordantly demonstrated a corresponding increase in transcriptional activity of the mutant p63 proteins. These experiments demonstrated a clear deleterious impact on TP63 structure and function, and supports our hypothesis that POI-related variants lead to constitutive TAp63α activation, increasing expression of downstream targets that can initiate the apoptotic pathway in oocytes (Figure 5).
4.3 The role of TA*p63 remains unknown
Although prior studies have demonstrated the critical role of the TAp63α isoform for ovarian function (Suh et al., 2006), less is known about the role of the TA*p63α and GTAp63α isoforms. These isoforms have been shown to have tighter transcriptional regulation than TAp63α due to their extended N termini (Pitzius et al., 2019). It has been proposed that GTAp63α may be the TAp63α equivalent in testes, with a role in the maintenance of germ cell integrity (Beyer et al., 2011). Whether the TA*p63α isoform has an important role in development remains to be elucidated, however, the recent identification of a variant affecting the unique N-terminus of this isoform that associated with familial cleft tongue indicates a biological role (Schmidt et al., 2021). Although we identified a rare heterozygous missense variant affecting this isoform alone in a POI patient, our functional studies were not able to demonstrate any deleterious impact on TA*p63α conformation or activity. Our study, therefore, has failed to provide further insight into the role of this isoform and further work is required to establish its biological function.
4.4 Functional experiments allow accurate variant curation
WES is increasingly being used for the management of patients with a wide range of genetic conditions, such as POI. Following WES variants have to be carefully filtered and appropriately curated to determine those that are clinically relevant. After WES, we applied American College of Medical Genetics (ACMG) criteria to the three heterozygous TP63 missense variants identified in the patients of this study, each of which was curated as a variant of uncertain significance (Supporting Information: Tables S1-3). With the inclusion of functional validation, however, two of the variants could be upgraded to “likely pathogenic.” This demonstrates the importance of combining experimental data with genomic data for accurate diagnoses and optimal clinical outcomes.
5 CONCLUSION
In conclusion, we have identified three novel TP63 variants in individuals with isolated POI. One variant was in the N-terminal TAD, one was in the C-terminal TID and one was present only in the N-terminal region unique to the TA*p63 isoform. Using functional analyses, we provide evidence that two of the three variants, promote an active tetrameric conformation and an increase of transcriptional activity. We conclude that the c.290G>C, p.Arg97Pro and c.1939C>T, p.Arg647Cys novel variants are likely pathogenic, disrupting the interaction of the activation domain with the inhibition domain and rendering the protein constitutively active, signalling for excessive oocyte death in the absence of DNA damage (Figure 5). This is the first evidence that human heterozygous missense variants can disrupt dimeric p63 conformation and cause isolated POI. The third variant remains a variant of uncertain significance. It is possible that our assays are not sensitive enough to detect the impact of the variant, or that the variant is benign, and another cause of POI exists in the patient. Whether the TA*p63 isoform has a role in ovarian development or oocyte maintenance remains elusive. This study provides better understanding of POI pathogenesis as well as the structure and function of the transcription factor, p63.
ACKNOWLEDGMENTS
This study was supported by a CHU Rennes grant (Appel à Projets Innovations 2019 to SJ), an Australian National Health and Medical Research Council (NHMRC) program grant (1074258 to AHS), NHMRC fellowships (1054432 to EJT, 1062854 to AHS), a Suzi Carp postdoctoral scholarship (to EJT) and the Deutsche Forschungsgemeinschaft (DO 545/18-1). The research conducted at the Murdoch Children's Research Institute was supported by the Victorian government's operational infrastructure support program. We thank the Bioinformatic department of Rennes University Hospital (Pr M. De Tayrac and Dr W. Carré) for technical assistance. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
WEB RESOURCES
https://gnomad.broadinstitute.org/
http://www.mutationtaster.org/
http://genetics.bwh.harvard.edu/pph2/
Open Research
DATA AVAILABILITY STATEMENT
Described variants are submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/, SUB11348440). Further data generated during and/or analysed as part of the current study are available from the corresponding author on reasonable request.