Polarized trafficking and copper transport activity of ATP7B: A mutational approach to establish genotype–phenotype correlation in Wilson disease
Authors have followed the journal's recommendations for the use of in silico prediction software, as published in the article by Vihinen ( 2013).
Abstract
Mutation in ATP7B gene causes Wilson disease (WD) that is characterized by severe hepatic and neurological symptoms. ATP7B localizes at the trans-Golgi Network (TGN) transporting copper to copper-dependent enzymes and traffics in apically targeted vesicles upon intracellular copper elevation. To decode the cellular underpinnings of WD manifestation we investigated copper-responsive polarized trafficking and copper transport activity of 15 WD causing point mutations in ATP7B. Amino-terminal mutations Gly85Val, Leu168Pro, and Gly591Asp displayed TGN and subapical localization whereas, Leu492Ser mislocalized at the basolateral region. The actuator domain mutation Gly875Arg shows retention in the endoplasmic reticulum (ER), Ala874Val and Leu795Phe show partial targeting to TGN and post-Golgi vesicles. The nucleotide-binding domain mutations His1069Gln and Leu1083Phe also display impaired targeting. The C-terminal mutations Leu1373Pro/Arg is arrested at ER but Ser1423Asn shows TGN localization. Transmembrane mutant Arg778Leu resides in ER and TGN while Arg969Gln is exclusively ER localized. Cellular Cu level does not alter the targeting of any of the studied mutations. Mutants that traffic to TGN exhibits biosynthetic function. Finally, we correlated cellular phenotypes with the clinical manifestation of the two most prevalent mutations; the early onset and more aggressive WD caused by Arg778Leu and the milder form of WD caused by mutation His1069Gln.
1 INTRODUCTION
Copper (in Cu1+ oxidation state) is an essential micronutrient for all eukaryotic life forms. It serves as a cofactor for a multitude of enzymes like ferroxidase ceruloplasmin (Cp), dopamine-β-hydroxylase, tyrosinase (Lutsenko, 2016; Schmidt et al., 2018; Setty et al., 2008). However, excess copper is toxic as it triggers oxidative stress in the cell (Gupta & Lutsenko, 2009). The mammalian copper homeostasis pathway is regulated by the synchronized functioning of multiple key proteins. Copper (I) uptake is primarily regulated by the high-affinity copper transporter-1 (CTR1), which is then transferred to metallochaperons, ATOX1, CCS, and COX17 for its delivery to specific proteins, Copper-ATPases, SOD, and the mitochondrial copper-dependent respiratory chain proteins, respectively (Bhattacharjee et al., 2017; Lutsenko, 2021). The copper-ATPases, ATP7A, and ATP7B are primarily responsible for transporting the copper in the biosynthetic pathway that is critical for maturation of copper-dependent proteins in the Golgi lumen and post-Golgi vesicles (PGV). Besides biosynthesis function, the two Cu-ATPases are also pivotal in exporting the unutilized copper out of the cell (Muchenditsi et al., 2017; Telianidis et al., 2013).
Mutations in the ATP7B gene (MIM# 606882) cause Wilson disease, WD (OMIM# 277900), first described as a hepatolenticular disorder by Alexander Kinnear Wilson in 1912 (Compston, 2009; Wilson, 1912). It follows a monogenic Mendelian autosomal pattern of inheritance (Gupta et al., 2003). The gene is located on the 13th chromosome (13q14.3). (https://www.omim.org/entry/277900). WD is the most frequent disorder of copper metabolism whose frequency varies from 1/5000 to 1/30,000 live births (Gupta et al., 2005). Though classified as a rare genetic disease, WD is a major health challenge due to its high frequency is some inbreeding populations and also for manifesting with a wide spectrum of symptom that does not faithfully correlate with any specific ATP7B mutation (Gupta et al., 2018; Yahata et al., 2017). The disease primarily manifests with hepatic symptoms with jaundice and associated symptoms and neurological symptoms that includes but not exclusive to tremors, dystonia, open jaw, drooling, dysarthria, and other movement disorders (Rosencrantz & Schilsky, 2011). A major fraction of WD patients exhibit symptoms atypical to classical manifestations in WD. Though the age of disease onset of the disease usually ranges from 8 to 16 years (Gupta et al., 2018), the detection age has been reported as late as in 70 years (Ala et al., 2005).
The ATP7B protein performs two key functions pertaining to copper homeostasis and metabolism, (a) biosynthetic function and (b) excess copper export via the late secretory pathway and lysosomal exocytosis (Das et al., 2020; Lutsenko, 2021; Polishchuk et al., 2014). In its biosynthetic function, ATP7B mediates maturation of the ferroxidase Ceruloplasmin (Cp) synthesized in the liver by transporting 6 coppers to Cp at the trans-Golgi network (TGN). Cp carries more than 95% of the total copper in a healthy human plasma (Bernevic et al., 2018). Cp exhibits a copper-dependent oxidase activity, which is associated with oxidation of Fe2+ (ferrous iron) into Fe3+ (ferric iron), therefore assisting in its transport in the plasma in association with transferrin, which can carry iron only in the higher oxidation state (Hellman & Gitlin, 2002). Apo-Cp is unstable and is quickly degraded if not converted into its holo-form by the ATP7B-dependent copper transfer as observed in WD patients (Gitlin et al., 1992). In the copper homeostatic pathway, upon high copper accumulation in the hepatocytes, ATP7B exits the TGN in vesicles and traffics vis the endo-lysosomal pathway to export excess copper in the bile (Das et al., 2020). Lack of mature Cp (holo-Cp) and copper accumulation in the liver are the major diagnostic symptoms of WD. High urinary copper and non-Cp bound serum copper are also the distinguishing features of the disease (Hermann, 2019).
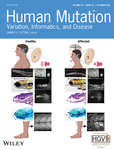
The WD protein, ATP7B (Accession no. NP_000044.2) is ~160 kDa, traverses the membrane by its 8 transmembrane (TM) helices and has multiple conserved cytosolic domains containing signature sequences of P-type ATPases. The N-terminal metal-binding domain (MBD) domain-containing six conserved MXCXXC motifs binds copper and plays a critical part in regulation of the protein. The nucleotide-binding and the phosphorylation domain are responsible for binding and hydrolysis of ATP respectively. The Actuator domain (A-domain) situated between TM2 and TM3 plays a role in dephosphorylation and continuing the ATP-dependent copper transport cycle. The C-terminus plays a role in TGN localization and retrograde trafficking of the protein (Jain et al., 2015; Lalioti et al., 2016; Shanmugavel & Wittung-Stafshede, 2019; F. Wu et al., 2015).
WD mutations have been found to encompass the entire length of the ATP7B gene. The mutations can be broadly classified into major subgroups on the basis of cellular localization and copper-induced trafficking: (a) majority of the ATP7B mutant protein fails to exit endoplasmic reticulum (ER) with a small fraction localizing at the TGN (misfolded mutant) (b) the mutant protein localizes at the TGN but fails to vesicularize upon copper treatment (trafficking mutant) (c) exhibits constitutive trafficking even in copper depleted conditions. There are major gaps in studies on functional characterization of the ATP7B mutations. First, correlative studies on copper-induced trafficking and copper transport activity at the TGN that eventually affects the WD phenotype in a combinatorial fashion is absent. Second, baring a few studies by the Hubbard group, most of the studies on ATP7B trafficking has been conducted in unpolarized cells, for example, HepG2 and HEK293 cells or fibroblast cell line (de Bie et al., 2007; Payne et al., 1998). Unlike the Menkes disease protein, ATP7A, that is present in most cell types, ATP7B has been primarily detected in polarized epithelia that includes hepatocytes and cells of proximal tubules of the kidney (Barnes et al., 2009). Ideally, trafficking studies of ATP7B should be conducted in polarized epithelial cell where it is naturally expressed as an endogenous protein. In the present study, we have selected 15 WD-causing ATP7B mutations that encompasses the entire length of the protein and are present in the functional and conserved domains (Figure 1i). These are as follows: c.254G>T (Gly85Val), c.503T>C (Leu168Pro), c.1475T>C (Leu492Ser), c.1769G>A (Gly591Asp), c.2382>C (Leu795Phe), c.2621C>T(Ala874Val), c.2622G>C (Gly875Arg), c.2333T>G (Arg778Leu), c.2930C>T (Thr977Met), c.2906G>A (Arg969Gln), c.3207C>A (His1069Gln), c.3247C>T (Leu1083Phe), c.4118T>C (Leu1373Pro), c.4118T>G (Leu1373Arg), c.4202G>A (Ser1423Asn). The Refseq sequence NM_000053.4 was used as the wild-type reference sequence. The amino acid residues are well conserved in the course of evolution as determined by sequence alignment of the ATP7B proteins from different organisms (Figure 1ii). We have characterized these mutations on the basis of copper transport property and polarized trafficking upon copper treatment. Further, we have tried to correlate our findings for two most frequent mutations, His1069Gln and Arg778Leu in Caucasian and East-Asian populations, respectively with patient phenotype as a meta-analysis study.
2 MATERIALS AND METHODS
2.1 Plasmids and reagents
The GFP–ATP7B construct was available in our laboratory. We have constructed the 15 mutations by substitutions using side-directed mutagenesis (SDM) following Q5® Site-Directed Mutagenesis Kit (#E0554) protocol. The tyrosinase plasmid pcTYR was a kind gift from Prof. Svetlana Lutsenko, Johns Hopkins University, USA. The following antibodies were used for experiments: mouse anti-P230 (TGN marker, Immunofluorescence, IF dilution 1:800) and sheep anti-TGN46 (IF dilution 1:400) were a kind gift from Prof. Carolyn E. Machamer, Johns Hopkins University, USA; mouse anti-sodium potassium ATPase (Na-K ATPase) #ab7671, IF dilution 1:400; mouse anti-GP135 (podocalxyin), #3F2/D8, IF dilution 1:300; rabbit anti-calnexin (ER marker) #ab22595, IF dilution 1:600, Phalloidin (actin marker) Alexa 405 #ab176752, IF dilution 1:1000; rabbit anti-GFP antibody #BB‑AB0065, Immunoblot dilution 1:5000; donkey anti-mouse IgG (H+L) Alexa Fluor 568 #A10037, IF dilution 1:1000; donkey anti-mouse IgG (H+L) Alexa Fluor Plus 647 #A32787, IF dilution 1:1000; Goat anti-Rabbit IgG (H+L) Alexa Fluor 568 #A-11011, IF dilution 1:1000; Donkey anti-Sheep IgG (H+L) Alexa Fluor 647 #A-21448, IF dilution 1:1000; ProLong® Gold Antifade Reagent #9071S mounting media without 4′,6-diamidino-2-phenylindole (DAPI); Fluoroshield™ with DAPI mounting media #F6057. Endotoxin-free DNA plasmid isolation was performed using an EndoFree Plasmid Maxi Kit (NucleoBond Xtra Midi EF, Midi kit, #740420.50).
2.2 Cell lines and culture
HEK293T cells (a kind gift from Prof. Arindam Mukherjee's laboratory, Indian Institute of Science Education and Research Kolkata) and YS Menke's fibroblast cells (a kind gift from Prof. Svetlana Lutsenko's laboratory, Johns Hopkins University) were grown and maintained in complete medium containing Dulbecco's modified Eagle′s medium-high glucose (DMEM) (#D6429-500ML; Sigma-Aldrich) supplemented with 10% fetal bovine serum (#10270-106; Thermo Fisher Scientific), 1× penicillin-streptomycin (#A001; HIMEDIA) and 1× amphotericin B (#15290026; Thermo Fisher Scientific). Madin Darby Canine Kidney (MDCK, a kind gift from Prof. Enrique Rodriguez-Boulan's laboratory, Weill Cornell Medical College) cell line was grown and maintained in the same DMEM supplemented with 10% fetal bovine serum (#26140079), 1× penicillin-streptomycin and 1× amphotericin B. For transfection in HEK293T, for immunoblotting, JetPrime (#114-07; PolyplusTransfection) transfection reagent was used. For transfection in MDCK, cells were nucleofected using NucleofectorTM 2b Device, programme T-23 (#AAB-1001; Lonza) in BTX electroporation cuvettes (#45-0125) and grown on Trans-well (#3401; Corning) and allowed to polarize for 3–4 days.
2.3 Copper and ammonium tetrathiomolybdate (TTM) treatments
For studying the trafficking of GFP-ATP7B in MDCK, copper chloride (CuCl2) at 50 μM was used. Similarly, TTM (#323446-1G), a copper chelator, was used at 25 μM concentration. Both the reagents were prepared fresh in autoclaved miliQ-water before treatment.
2.4 Immunofluorescence
Any treatment was performed after complete polarization attended. MDCK cell on trans-well was washed in ice-cold 1× phosphate-buffered saline (PBS), pH 7.4 for 2 min. Cell was fixed with 2% paraformaldehyde (PFA) for 20 min at room temperature (RT) followed by quenching with 50 mM ammonium chloride for another 20 min at RT and subsequently washed with 1× PBS for 2 min ×3 times. After fixation, permeablization and blocking was done with 1% BSA in 1× PBSS (S, 0.075% Saponin) for 20 min at RT. Post blocking, primary antibody (1°) incubation was done at RT in a moist chamber for 2 h. After 2 h incubation, cells were washed with 1× PBSS three times and again reincubated with corresponding secondary antibodies (2°) for 1.3 h at RT. This was followed by further washing with 1× PBSS three times and finally with 1× PBS twice. The membrane from trans-well was stripped off using a blade and mounted on glass coverslip using mouthing media. The solvent for antibody suspension was 1% BSA in 1× PBSS.
2.5 Tyrosinase assay
YS cells grown on glass coverslips were transfected with either 0.1 µg of pcTYR or 0.25 μg of GFP-ATP7B (wt or mutants) or both expression plasmids using JetPrime (#114-07; PolyplusTransfection) transfection reagent. Twenty-four hours later, cells were washed twice in PBS and fixed for 30 s in cold acetone-methanol (1:1). These fixation conditions do not interfere with tyrosinase activity. The cells were then incubated for 4 h at 37°C in 0.1 M Na-phosphate buffer (pH 6.8) containing (0.4 mg/ml) levo-3,4-dihydroxy-l-phenylalanine (l-DOPA), freshly prepared. Coverslips were mounted on slides, and the formation of the black l-DOPA chrome pigment was detected by phase-contrast microscopy.
2.6 Immunoblotting
For studying ATP7B mutant's stability, immunoblotting was performed. Briefly, HEK293T cell was transfected at 40% confluency with 0.75 µg of either wt or mutant GFP-ATP7B using JetPrime (#114-07; PolyplusTransfection) transfection reagent. The GFP-ATP7B (WT and the mutants) protein was expressed for 12 h following transfection. Whole-cell lysate was prepared with RIPA lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA, 0.5 mM EGTA, 1.0% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 140 mM NaCl, and 1× protease inhibitor cocktail) and incubated on ice for 30 min with intermittent tapping. The solution was then sonicated with a probe sonicator (3–4 pulses, 5 s, and 100 mA) and rested for 15 min. Following this, centrifugation at 3000g for 10 min at 4°C was done to pellet cellular insoluble debris, and supernatant was collected. Protein sample preparation was done by adding 4× loading buffer (Tris-HCl pH 6.81, 4% SDS, 10% β-mercaptoethanol, 20% glycerol, 0.02% Bromophenol Blue, and urea 8 M) to a final concentration of 1× and run on SDS-PAGE (6%) gels to separate proteins according to molecular mass. This was further followed by wet transfer of proteins onto nitrocellulose membrane (1620112; BioRad). After transfer, the membrane was blocked with 3% BSA in 1× Tris-buffered saline (TBS) buffer pH 7.5 for 2 h at RT with mild shaking. Primary antibody (rabbit anti-GFP, dilution 1:4000) incubation was done overnight at 4°C following blocking and then washed with 1× TBST (0.01% Tween-20) for 10 min (three times). HRP-conjugated anti-rabbit secondary, dilution 1:5000 (#BB-SAB01A) incubation was done for 1.3 h at RT, further washed and signal was developed by ECL developer (170-5060, BioRad/1705062; BioRad) through chemiluminescence by Chemi Doc (BioRad). Densitometric analysis of immunoblot was done in ImageJ software following an early protocol (Peterson, 1997).
2.7 Microscopy
All images were acquired with Leica SP8 confocal platform using an oil immersion ×63 objective and deconvoluted using Leica Lightning software. Images for MDCK was taken in Z-stacks (average gap between two stacks was 0.3 μm).
3 IMAGE ANALYSIS
Macro used in ImageJ is available in (https://github.com/saps018/polarised-MDCK-colog).
Graphs were plotted using GraphPad Prism version 8.0.2 for Windows (GraphPad Software), www.graphpad.com
3.1 Transcript analysis of MDCK-II cell-specific ATP7B and GAPDH
Total RNA was isolated from untransfected MDCK and MDCK transfected with GFP-hATP7B, using TRIzol reagent (Invitrogen #15596026). DNA contamination was removed with DNaseI (Invitrogen #18047-019) treatment. Verso complementary DNA (cDNA) synthesis kit (Thermo Fisher Scientific #AB1453A) was used for cDNA preparation from 1 μg of total RNA. Semi-quantitative PCR (Marone et al., 2001) was run for both the samples. The relative transcript level of MDCK-specific ATP7B gene was normalized using MDCK-specific GAPDH gene as an endogenous control. For statistical analysis and plotting, GraphPad Prism version 8.0.2 for Windows was used. Statistical analyses were performed by Student's t test. Primers used for semi-qPCR are as follows: dogGAPDH Forward: 5′-CCTGCCGCCTGGAGAAAGC-3′, dogGAPDH Reverse: 5′-TGGAAGAGTGGGTGTCACTGTTG-3′, dogATP7B Forward: 5′-GCCAGTGCCCGAGGAGG-3′ dogATP7B Reverse: 5′-GTGGCCGTCTGAGGAGGG-3′.
3.2 In silico study and homology modelling
The homology model of ATP7B (Accession no.AF-P35670) was derived from the alpha fold, an AI-based protein structure prediction method (Jumper et al., 2021; Varadi et al., 2022) and the structures corresponding to each mutation were made using the mutagenesis function in PyMol Version 2.0 (https://pymol.org/2/). Out of the several side-chain orientations (rotamers) possible, the ones with the highest frequencies of occurrence in protein (indicated with the highest percentage at the mutation object) in each case were selected.
DeepDDG, a neural network-based server was further used to predict the stability change of the mutations (Cao et al., 2019). The homology model of ATP7B was fed as the input and the DeepDDG network model was selected for all possible mutations for the entire structure to predict the stability of mutant protein.
ConSurf (consurf.tau.ac.il/) server was used to analyse the evolutionary conservation of amino acid positions in the protein to identify the conserved regions and were scored and subsequently divided into separate nine-grade scales (Ashkenazy et al., 2016). Multiple Sequence Alignment was built using MAFFT and the Homologues were collected from UNIREF90 with HMMER Homolog search algorithm (Mayrose et al., 2004). Conservation scores between 1 and 4 are classified as variable, 5 and 6 intermediate, and between 7 and 9 as conserved. Homology model of the ATP7B modelled using MODELLER was used to predict the solvent accessibilities of the amino acids and the relative solvent accessible surface areas of the amino acids calculated using NACCESS was used to make a buried/exposed prediction for each amino acid (Šali & Blundell, 1993).
Authors have followed the journal's recommendations for the use of in silico prediction software, as published in the article by Vihinen (2013).
3.3 Meta-analysis
A systematic search using Clinvar, Genomenon, HGMD, Pubmed, and Science direct was performed (Landrum et al., 2018; Stenson et al., 2003). The search strategy yielded 18 eligible articles and were thoroughly screened and over 80 patients with ATP7B mutations of our interest included in the study.
4 RESULTS
4.1 Most WD-causing ATP7B mutations shows abrogated local intradomain interactions
At the outset, we used homology modeling based on ATP7B structure sourced from Alphafold (Accession no. AF-P35670) to study the polar interactions at each residue of our interest using Pymol. Polar contacts at a radius of 3.5 Å were modelled and H bonds were labelled and measured. Generally, H bonds of length between 2.2 and 2.5 Å are considered as strong and those above 2.5–3.2 Å are regarded as moderate and anything above 3.2 Å is classified as weak interaction (Jeffrey, 1997). Hence the cut-off length of H bonds was selected as 3.2 Å for detection. The structures corresponding to each mutation were made using the mutagenesis function in Pymol and the same process was repeated to study the effect of mutation on the local residue interactions. Further, the homology model was submitted to DeepDDG, a deep neural network-based server to predict the stability change upon mutation in terms of differences between Gibbs free energy (ΔΔG) of WT and mutant where predicted ΔΔG value > 0 represents stable mutant, and value < 0 an unstable mutant protein (Cao et al., 2019). Bioinformatics server ConSurf was used to analyze the conservation status and to predict the role of each residue based on solvent-accessible surface area. The alterations in the local environment, ΔΔG values and conservation scores, and predicted role of residues are enlisted in Supporting Information: Table S1. The homology models depicting the intra-domain interactions of the wild-type residue in its local peptide environment is compared with the mutants and is shown in panel i for figures that illustrates characterization of the mutants (in the following results sections).
4.2 GFP-ATP7B exhibits copper-induced polarized trafficking in MDCK-II cells
MDCK-II cell is an ideal model of polarized epithelia to study apical and basolateral trafficking (Mendes et al., 2005; Rodriguez-Boulan & Macara, 2014; Wang et al., 2000). To utilize MDCK-II cells as model to study trafficking of ATP7B, we first tested endogenous levels of ATP7B in MDCK cells. Since MDCK cells are derived from canine kidney, anti-human ATP7B cannot detect the endogenous dog protein. Moreover, the commercially available antibody is raised against the C-terminus peptide of the human ATP7B (Abcam #ab124973). Specifically human and canine ATP7B differs majorly at the carboxy terminus sequence. Hence to detect endogenous ATP7B, we tested the presence of its transcript in MDCK cells and found that these cells express ATP7B. Further, we found that heterologous expression of GFP-tagged ATP7B (human) in MDCK cells does not affect the transcript levels of the endogenous protein (Supporting Information: Figure S1Ai and S1Aii). Hence, we utilized GFP-ATP7B to characterize the WD mutations in MDCK cells.
We tested trafficking of the wild type and the 15 WD-causing mutations in these polarized MDCK cells. Fourteen of the 15 N-terminally GFP-tagged mutant proteins expressed well in these cells as determined by immunoblot. However, their level of expression varied in comparison to the wild-type ATP7B and loading control of the house-keeping protein GAPDH (Figure 1iii) and (Supporting Information: Figure S1B). Copper transport activity of these mutant and the wt proteins were tested by their capacity to activate tyrosinase in cells lacking both the endogenous copper ATPases ATP7A and ATP7B (Gupta et al., 2011; Roy et al., 2020). Formation and precipitation of the eumelanin pigment in the cells were utilized as a read-out of active ATP7B that only occurs if the Cu-ATPase transports copper to tyrosinase hence turning-on the pigmentation cascade.
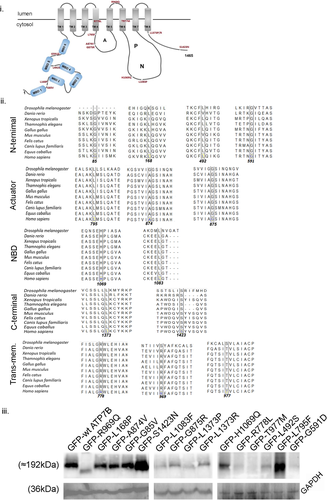
The GFP-ATP7B wild-type construct (GFP-wt-ATP7B) was transfected in unpolarized MDCK-II cells and the cells were polarized for next 96 h till they attained a minimum height of 12 µM and distinct apical (marked with podocalyxin protein, GP135) (Supporting Information: Figure S2i and S2ii) and basolateral polarity (marked with Na-K ATPase) (Supporting Information: Figure S3i and S3ii). The entire lateral cell boundary is marked with cortical actin, stained using phalloidin (Supporting Information: Figure S3). The ER (marked by Calnexin) and actin were costained to determine the apposition of the two organelles in polarized MDCK cells (Supporting Information: Figure S4i and S4ii). In copper chelated condition (25 µM ammonium tetrathiomolybdate or TTM, 2 h), wtATP7B localized at the TGN as determined by its overlap with the TGN GRIP-domain marker, P230 (Figure 2i). Upon copper treatment (50 µM CuCl2, 2 h), ATP7B localized to the apical membrane marked by GP135 (Figure 2ii). Figure 2iii illustrates the localization of ATP7B with the markers of TGN and apical membrane in copper depleted and treated conditions.
To test copper transport capacity of the protein, we transfected the construct in Menkes patient fibroblast that lacks copper ATPases. Pigmentation was recorded upon treating the cells with l-DOPA. We observed black pigments of mature eumelanin in multiple cells indicating that the GFP-wt-ATP7B is capable of transporting copper (Supporting Information: Figure S5).
4.3 N-terminus (M1-L655) mutations exhibit normal copper transport activity but impaired trafficking
We selected four nonsynonymous point mutations spanning the N-terminal domain, Gly85Val, Leu168Pro, Leu492Ser, and Gly591Asp (de Bie et al., 2007; Guttmann et al., 2018; Hamza et al., 1999; Huster et al., 2012; Van Den Berghe et al., 2009; Vanderwerf et al., 2001) for characterization on the basis of copper transport in the biosynthetic pathway and copper-induced polarized trafficking.
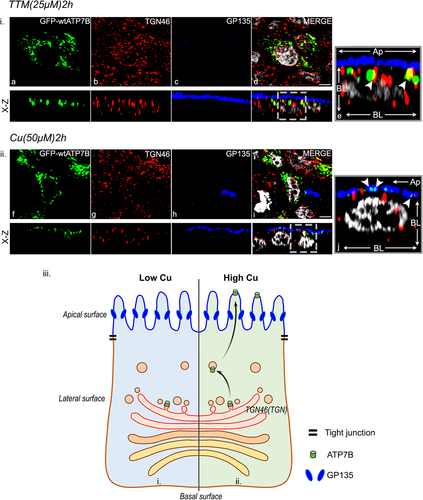
The mutation Leu168Pro was modeled using PyMOL and the predicted changes in local protein interaction is shown in Figure 3i. We conducted the trafficking study in MDCK-II, in copper-depleted condition. ATP7B-Leu168Pro primarily localizes at the TGN as evident from colocalization with p230 and in ER as evident from its overlap with ER marker, Calnexin. Upon copper treatment, we observed partial trafficking of the mutant protein to vesicles with some retention in the TGN as evident from trafficking assay and Mander's overlap coefficient (represented as fraction of GFP-ATP7B colocalization) (Figure 3ii,iii). In polarized MDCK cells the ER lies in close juxtaposition to the apical membrane (Supporting Information: Figure S4i and ii). To resolve whether the mutant protein is truly arrested at the ER or it reaches the apical membrane upon copper treatment, we costained the cells with ER and apical membrane marker (Supporting Information: Figure S6). Upon copper treatment, the GFP-ATP7B puncta were found to be nonoverlapping and in close apposition with the apical marker (GP135) (Supporting Information: S6a and S6b). However, some overlap of the mutant protein was observed with the ER marker Calnexin. In both depleted and high copper conditions we observed post-Golgi vesicular GFP-ATP7B that were concentrated along the lateral side of cell (Figure 3iid,iil). Interestingly, as determined by black pigment deposition in fibroblasts in tyrosinase assay, the mutant protein retains its ATPase activity and hence is capable of copper transport (Figure 3iv).
The mutation Gly85Val was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S7i. Interestingly, ATP7B-Gly85Val exhibits similar phenotype as of Leu168Pro with localization at the ER and TGN in both copper conditions. In addition, the mutant protein localized to vesicles that are situated towards the apical membrane in both depleted copper and high copper conditions as evident from both trafficking assay and fraction of ATP7B localization analysis (Supporting Information: Figure S7ii and S7iii). Interestingly, the fraction of the protein localizing on these subapical vesicles fails to reach the apical membrane as determined by its noncolocalization with GP135 even in high copper condition (Supporting Information: Figure S6c and S6d). Gly85Val is capable of copper transport as it exhibits a range of pigmentation of l-DOPA precipitates, varying form light grey to dark pigments (similar to the wt protein) (Supporting Information: Figure S7iv).
The mutation Leu492Ser was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S8i. The mutant protein, ATP7B-Leu492Ser exhibits similar phenotype to Gly85Val with regard to localization and copper-induced trafficking. In addition, in high copper a fraction of ATP7B was found to be basolaterally concentrated as evident from both trafficking assay and fraction of ATP7B localization analysis (Supporting Information: Figure S8ii and S8iii). ATP7B-Leu492Ser exhibited weak copper transport activity to tyrosinase as revealed by lighter l-DOPA pigment precipitate (Supporting Information: Figure S8iv).
The mutation Gly591Asp was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S9i. ATP7B-Gly591Asp exhibits TGN localization in copper chelated conditions and post-TGN vesicles restricted to subapical region along with ER localization as evident from trafficking and fraction of ATP7B localization analysis (Supporting Information: Figure S9ii, a–h and S9iii). Upon copper treatment, the mutant protein is partially retained in the TGN. A fraction of the protein traffics out of TGN and localizes in vesicles in subapical as well as lateral region of cell. Similar to other N-terminal mutants, it fails to reach the apical membrane (Supporting Information: Figure S9ii, i–p and S9iii). The mutant is capable of transporting copper to the biosynthetic pathway as observed by pigmentation of eumelanin (Supporting Information: Figure S9iv).
Gly85Val, Leu168Pro, G491S, Gly591Asp are mutations at the linker regions of the MBDs possibly affecting the kinase-mediated phosphorylation at the N terminal (Pilankatta et al., 2011).
4.4 A-domain (L780-Y923) mutations exhibits impaired copper transport and partial Golgi retention upon copper treatment
The A-domain (or the Actuator domain) regulates the dephosphorylation step of the ATPases. The glutamic acid of TGEA motif in A-domain mediates dephosphorylation of invariant aspartic acid residue in DKTG motif of P-domain (F. Wu et al., 2015). Mutations on the A-domain possibly freezes the protein in the dephosphorylated step preventing it from continuing to the next step of the cycle. Mutation of TGE>AAA caused constitutive trafficking independent of copper level (Cater et al., 2007). We characterized the A-domain mutations Ala874Val, Gly875Arg, and Leu795Phe. The third most common mutation in Chinese population, Ala874Val accounts for a 9.4% of all ATP7B mutations, impairs ATP hydrolysis coupled with Cu translocation across TM (Dong et al., 2016; Yoo, 2002).
The mutation Ala874Val was modeled using PyMOL and the predicted changes in local protein interaction is shown in Figure 4i. Upon testing copper-dependent trafficking, ATP7B-Ala874Val was found to overlap with both calnexin (ER) and P230 (TGN) in both copper depleted and high conditions (Figure 4ii,iii). Additionally, in high copper, basolateral localization of the mutant protein was observed as evident from trafficking assay (Figure 4iii). Its apical localization was also tested in both low and high copper conditions. However, we did not observe any appreciable colocalization with GP135, the apical marker (Supporting Information: Figure S6e and S6f). Tyrosinase assay revealed moderate copper transport capability of the mutant as compared to wt-ATP7B (Figure 4iv).
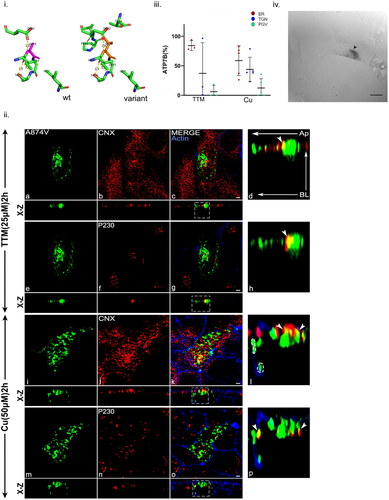
The mutation Gly875Arg was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S10i. The Gly875Arg mutant has been previously characterized as an ER arrested mutant that could be rescued by copper treatment (Gupta et al., 2011). However, we observed that the mutant primarily localized at the ER both in high and low copper conditions (Supporting Information: Figure S10ii). Tyrosinase assay revealed that the protein was completely unable to copper transport (Supporting Information: Figure S10iii).
The third A-domain mutation studied was Leu795Phe, found within a cohort of western India and associated with late-onset and neurological symptoms (Aggarwal et al., 2013; Chandhok et al., 2016). The mutation Leu795Phe was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S11i. ATP7B-Leu795Phe showed TGN localization as evident from its codistribution with P230 and presence of post-Golgi GFP-ATP7B vesicles in copper conditions (Supporting Information: Figure S11ii and iii). From the fraction of ATP7B localization analysis it can be inferred that there is a shift in the pool of ATP7B-Leu795Phe from TGN to PGV in high copper condition (Supporting Information: Figure S11iii). However, this mutant failed to reach the apical membrane upon copper treatment. Leu795Phe showed almost similar copper transport activity like wild-type as observed from tyrosinase assay (Supporting Information: Figure S11iv).
4.5 Nucleotide-binding domain (NBD) (L989-I1323) mutations exhibits mislocalization irrespective of copper treatment but displays normal biosynthetic activity
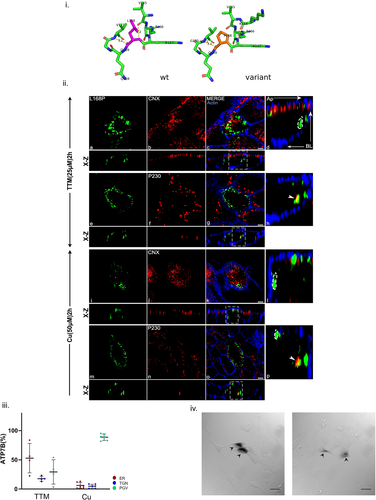
ATP binding followed by phosphorylation is a prerequisite in copper transport activity for a P-type ATPase like ATP7B. This function is served by NBD (Lutsenko et al., 2007). The most prevalent disease-causing variant His1069Gln, found in Caucasian population is situated in the SEHPL motif of this domain. The mutation His1069Gln was modeled using PyMOL and the predicted changes in local protein interaction is shown in Figure 5i. Previous studies on unpolarized cells have shown that the mutant is primarily retained at the ER fails to localize to the TGN (Payne et al., 1998). Interestingly, besides ER localization, we observed consistent vesicular localization of the mutant. This phenotype is observed in both copper-depleted and copper-treated condition. Upon copper treatment ATP7B-His1069Gln shows a mixed phenotype mostly concentrated at sub apical region beneath the cortical actin stained by phalloidin and localizes within TGN even in high copper as evident from GFP vesicular punctate and colocalization with P230 (Figure 5ii,iii). This mutant also retains its copper transport activity as determined by tyrosinase assay further supporting its TGN localization (Figure 5iv).
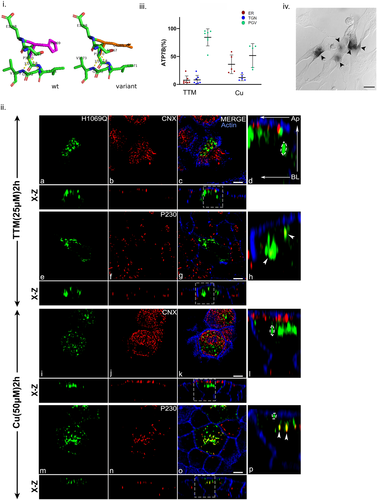
The mutation Leu1083Phe was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S12i. ATP7B-Leu1083Phe shows similar phenotype like His1069Gln in all copper conditions. Furthermore, it also exhibits near-basolateral localization in both TTM and high copper reflecting its copper irresponsive mislocalization (12ii and iii). However, it did not colocalize with basolateral membrane marker Na, K-ATPase (data not shown). Previous study on HEK293 cell has shown ER localization but normal catalytic activity (Huster et al., 2012). This disparity is resolved in our polarized cell model where ATP7B-Leu1083Phe shows positive for tyrosinase assay as it localizes to TGN and post-Golgi compartments. Leu1083Phe shows eumelanin pigmentation similar to wt-ATP7B (Supporting Information: Figure S12iv) however, His1069Gln display a range from grey to dark pigmentation demonstrating its instability in copper transport activity.
Except for the disordered loop between residues 1114–1142, the nucleotide-binding N-domain is generally well folded and compact as evident from high polar interactions and strong bonds suggesting mutations in this region would disrupt its folding, reduce protein stability and or interfere with the domain's ability to bind ATP (Dmitriev et al., 2011).
4.6 Carboxy-terminal (L1371-I1465) mutations exhibits mixed phenotype
The WD patient mutation database WilsonGen (https://clingen.igib.res.in/WilsonGen/) has reported few missense mutations on the Carboxy terminal domain of ATP7B that interfere with the structure or regulatory function of ATP7B (Kumar et al., 2020). Further, Braiterman et al. (2011) has shown two disease-causing variants at C-terminal Leu1373Pro and Leu1373Arg to have pathological impact owing to its mislocalization in WIF-B cell (Braiterman et al., 2011). Leu1373Pro and Leu1373Arg belonging from Japanese and Brazilian populations respectively incur both hepatic and neurological symptoms (Gupta et al., 2018).
The mutation Leu1373Arg was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S13i. We found that ATP7B-Leu1373Arg expression is restricted to ER as evident from its colocalization with calnexin in both low and high copper (Supporting Information: Figure S13ii). Its complete absence from TGN or vesicles is further supported by tyrosinase assay where it fails to deliver copper to tyrosinase and hence no pigmentation is observed (Supporting Information: Figure S13iii). Also, its reduced level of expression as evident from immunoblot suggests reduced stability of the protein and degradation by ER-mediated degradation pathway (Figure 1iii and Supporting Information: Figure S1B). Leu1373Pro was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S14i. Similar trafficking phenotype was observed for ATP7B-Leu1373Pro as of Leu1373Arg. However, besides its ER localization, this mutant also localizes at vesicles that increases with copper treatment (Supporting Information: Figure S14ii). Interestingly, it does not localize at all in the TGN. This observation can be attributed to the fact that its dwell time on TGN is much less and is a possible reason for it being negative in tyrosinase assay (Supporting Information: Figure S14iii) since steady-state localization on TGN is required for appropriate copper delivery to TGN lumen. Though the two C-terminal mutations substitute the same amino acid, Leucine, Arg being a bulkier group might causes the misfolded protein to be entirely retained at the ER as compared to the Pro variant that shows partial rescue from the ER to vesicular compartments.
A third mutation located at end of C-terminal selected for functional characterization is ATP7B-Ser1423Asn. This disease-causing variant was reported as compound heterozygous with Leu168Pro in a child with mild neuropsychiatric symptoms without hepatic manifestation (Guttmann et al., 2018). The mutation Ser1423Asn was modeled using PyMOL and the predicted changes in local protein interaction is shown in Figure 6i. When trafficking study was conducted, in addition to ER localization, the mutant localizes on the TGN and traffics to PGV mostly positioned laterally in both copper conditions as evident from its trafficking assay and fraction of ATP7B localization analysis (Figure 6ii,iii). ATP7B-Ser1423Asn shows tyrosinase assay positive similar to wt-ATP7B (Figure 6iv).
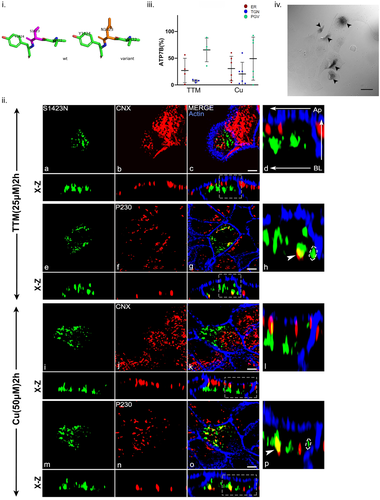
Homology modelling studies suggest the C terminal region of ATP7B to be mostly unstructured with several existing Serine/Threonine phosphorylation sites.
4.7 TM and luminal loop mutations exhibit distinct phenotypes
We selected three TM mutations that are in TM4 (Arg778Leu), TM6 (Thr977Met), and at the luminal–membrane junction of TM6 (Arg969Gln). Arg778Leu mutation is the highest disease variant mostly concentrated in the Asian population like China, Japan, and South Korea with severe hepatic symptoms at a nearly early stage of life (Li et al., 2019). The mutation Arg778Leuwas modeled using PyMOL and the predicted changes in local protein interaction is shown in Figure 7i. In cellulo, this mutant shows a diffuse cytosolic staining in HepG2 cell line and relatively unstable (Chandhok et al., 2016). We found ATP7B-Arg778Leu specifically localizes in TGN as evident from its colocalization with P230 in both copper chelated and high copper conditions. Interestingly, the mutant completely fails to traffic upon copper treatment (Figure 7ii,iii). The mutant protein also shows ER localization as evident from fraction of ATP7B localization analysis (Figure 7iii). This mutant is positive for tyrosinase assay however the pigmentation is lighter and diffused (Figure 7iv). Arg778Leu was found to be the only stable mutation among our study with a positive ΔΔG value for free energy change (Supporting Information: Table S1).
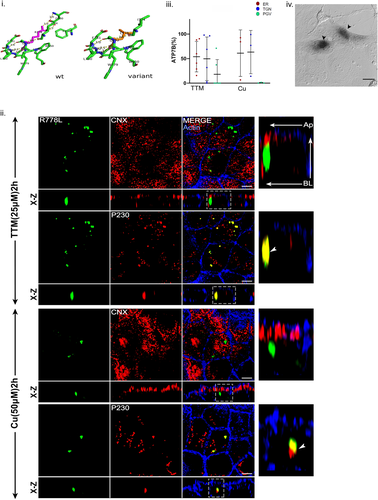
Huster et al. (2012) have shown the Arg969Gln mutant to be located in TGN in HEK293 cells (Huster et al., 2012). The mutation Arg969Gln was modeled using PyMOL and the predicted changes in local protein interaction is shown in Supporting Information: Figure S15i. In cellulo, this mutant however showed ER restricted localization as evident from colocalization with CNX irrespective of copper conditions in polarized MDCK (Supporting Information: Figure S15ii). The mutant fail to localize at the TGN since no colocalization was observed with P230. Its ER arrest was further confirmed by tyrosinase assay where its fails to develop any eumelanin pigment inferring its copper transport across TGN membrane is impaired (Supporting Information: Figure S15iii). Immunoblot reveals a low expression compared to wt-ATP7B indicating its instability and possible degradation by the ER-mediated degradation pathway (Figure 1iii).
All the mutants tested in this study exhibited defects in localization and trafficking to a varying degree. To confirm that any alteration(s) introduced in the wt-GFP-ATP7B does no impair its trafficking, we further selected a non-disease variant (single-nucleotide polymorphism; rs732774), c.2855G>A (K952R) and characterized its trafficking phenotype in MDCK cells. The K952 variant localized on the TGN under basal copper and trafficked to the apical membrane upon copper treatment, completely mimicking the wt-GFP-ATP7B (Supporting Information: Figure S16).
TM residues showed high polar interactions and numerous H bonds of varying strengths and conserved exposed residues suggesting mutations in this region to possess effects in protein functions (Supporting Information: Table S1).
4.8 Genotype–phenotype correlation of prevalent mutations detected in homozygous condition in WD patients
The onset of clinical manifestation of WD occurs in liver accounting for (40%–60%) of cases (Czlonkowska et al., 2018). Being the primary site of copper overload, hepatic symptoms may show with recurrent jaundice, cirrhosis, or even acute liver failure (Rodriguez-Castro et al., 2015). The pathophysiology in brain in WD is an extension of hepatic copper toxicity and does not lead to obvious clinical manifestation (Dusek et al., 2019). Neurological symptoms primarily include movement disorders like dystonia, tremor, drooling, and dysarthria. Since WD-causing mutations span the entire length of the ATP7B gene and most of the WD patients contains ATP7B mutations in compound heterozygous mutations, it is difficult to ascertain any specific phenotype or symptom to a specific mutation. The analysis of genotype–phenotype correlationship is easier if the mutations are present in homozygous condition. Due to the Founder effect, WD patients usually harbor prevalent mutations and not rare mutations in homozygous condition. We selected the two prevalent point mutations, Arg778Leu (Chinese and Eastern Asian population) and His1069Gln (Caucasian) population mostly from patients that harbor them in homozygous condition.
We observed that the age of onset for the patients with Arg778Leu/Arg778Leu lies between 10 and 16 with the mean age of onset being 15 years (Figure 8i) (Z. Y. Wu et al., 2001; Yang et al., 2021) and serum ceruloplasmin (Cp) being drastically reduced varying from 0.4 to 10 mg/dl (Figure 8ii); a health adult individual has values in the range of 14–40 mg/dl (Geng et al., 2013; Wiernicka et al., 2017). Interestingly, most of these patients manifests with hepatic symptoms and high urine copper (Okada et al., 2000) (Figure 8ii). High urinary copper corresponds with the nontrafficking phenotype of the mutant protein observed in MDCK cells upon copper treatment. Pigmentation in tyrosinase assay correlated well with ceruloplasmin levels that is not much below the normal levels as observed in normal individuals.
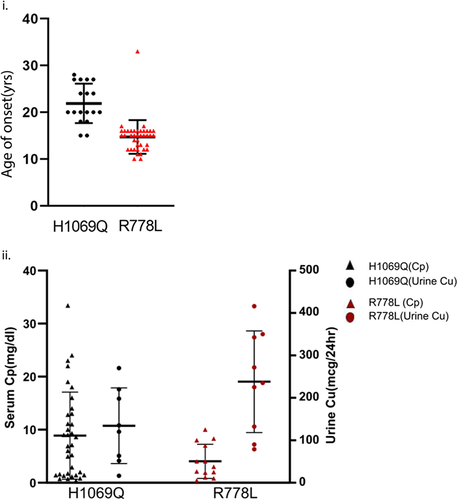
His1069Gln homozygous patients on other hand primarily manifest late-onset neurological symptoms (mean age of onset calculated = 20 years) (Figure 8i). Interestingly, unlike for ATP7B-Arg778Leu we observed trafficking of ATP7B-His1069Gln upon copper treatment in MDCK cells. These vesicularized ATP7B- His1069Gln though failed to reach the apical membrane upon copper treatment is possibly responsible for some copper export, therefore, reducing the copper load in cells (Figure 8ii).
5 DISCUSSION
WD patients manifest the disease primarily with hepatic, neurological or mixed symptoms. Usually, neurological symptoms are late-onset with disease appearing in the second decade of life. Hepatic symptoms primarily appear in the first decade of life and can progress to severe jaundice and cirrhosis of liver. In the present study we tried to deconstruct WD phenotype on the basis of two overarching symptoms, copper accumulation due to absence of copper-induced trafficking of the ATP7B protein (secretory function) and loss of copper transport activity of the ATP7B protein to copper-dependent enzymes (biosynthetic function).
To date, barring studies on WIF-B and Can10 cells, most of trafficking studies of ATP7B has been conducted in unpolarized cells, HEK293, HeLa, and HepG2 to name a few (de Bie et al., 2007; Huster et al., 2012; Payne et al., 1998). We used MDCK-II, a well characterized model of polarized epithelial cells. Moreover, ATP7B has been shown to be endogenously expressed in kidney epithelia (Barnes et al., 2009). Unlike WIF-B cells where short cell length (TGN to apical surface) is a disadvantage, MDCK cells upon polarization attains a height of 12–14 µM. The increased height of the cells adds to an advantage to track protein trafficking (endocytic as well as secretory pathway) along the entire height of the cell (Rodriguez-Boulan & Macara, 2014). At the outset, we ensured that GFP-ATP7B is expressed in MDCK cells and localizes at the TGN and traffics to the apical surface upon copper treatment marked by GP135.
Upon testing the mutant proteins for copper-induced trafficking study we noticed that some of the mutants either fails to traffic out of the TGN (Arg778Leu, Ala874Val) or despite exhibiting copper-induced trafficking, fails to localize at the apical membrane (Gly85Val, Leu168Pro, Leu492Ser, Gly591Asp, His1069Gln, Leu1083Phe, Leu795Phe, Ser1423Asn). We infer that this non-trafficking cellular phenotype of these disease-causing mutations to apical surface may be the causal reason for copper accumulation in liver and subsequent elevation in urinary copper. Interestingly, a few mutations (Gly875Arg, Arg969Gln, Leu1373Pro/Arg) fails to exit the ER and localize at the TGN. Since ATP7B functions at the biosynthetic pathway at the TGN membrane, these ER localizing mutations show no pigmentation in the tyrosinase assay besides copper accumulation due to its nontrafficking phenotype. Table 1 summarizes the intracellular localization and copper transport activity of all the mutants and the WT-ATP7B.
| Domain | Mutation | Intracellular localization and Cu-induced trafficking | Copper transport activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −Cu | +Cu | |||||||||||||
| ER | TGN | A | SA | V | BL | ER | TGN | A | SA | V | BL | |||
| - | WT | ✓ | ✓ | +++ | ||||||||||
| N- terminus | Gly85Val | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ++ | ||||||
| Leu168Pro | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | +++/++ | ||||||
| Leu492Ser | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | + | ||||||
| Gly591Asp | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | +++ | ||||||
| A-domain | Leu795Phe | ✓ | ✓ | ✓ | ✓ | +++ | ||||||||
| Ala874Val | ✓ | ✓ | ✓ | ✓ | ✓ | ++ | ||||||||
| Gly875Arg | ✓ | ✓ | − | |||||||||||
| TM | Arg778Leu | ✓ | ✓ | ✓ | ✓ | + | ||||||||
| Luminal | Arg969Gln | ✓ | ✓ | − | ||||||||||
| NBD | His1069Gln | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | +++/++ | ||||||
| Leu1083Phe | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | +++ | |||||||
| C-terminus | Leu1373Pro | ✓ | ✓ | − | ||||||||||
| Leu1373Arg | ✓ | ✓ | − | |||||||||||
| Ser1423Asn | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ++/+ | |||||||
| Lys952Arg | ✓ | ✓ | ||||||||||||
- Abbreviations: +, tyrosinase assay positive; no. of +, degree of pigmentation; −, tyrosinase assay negative; A, apical; BL, basolateral/Lateral; A-domain, actuator; ER, endoplasmic reticulum; NBD, nucleotide-binding domain; ✓, positive for corresponding column; SA, subapical; TGN, trans-Golgi network; V, vesicles.
The most common mutations in three major world populations are His1069Gln in Caucasian, Arg778Leu in Chinese, Japanese, and Korean (East Asian) and C271X in population from the Indian subcontinent. We selected His1069Gln and Arg778Leu for our genotype–phenotype meta-analysis as C271X will form a truncated protein and will quickly degrade even before localizing on the TGN from the ER. This Indian ATP7B mutation lacks all the conserved cytosolic and the TM domains except the first metal-binding MXCXXC-containing domain (Aggarwal et al., 2013). As more than 350 mutations are found on the ATP7B gene, most WD patients are compound heterozygotes for the mutations. Since disease symptoms and other manifestations will be an outcome of phenotypic participation from two different mutations, we consciously did not select compound heterozygous individuals for genotype–phenotype correlation. Homozygous patients for ATP7B mutations Arg778Leu and His1069Gln revealed very interesting phenotypic delineation with cell biology and biochemical determinations in this study. Early-onset in WD usually runs parallel with severity of the disease marked essentially with elevated copper accumulation and low ceruloplasmin levels. Interestingly, for Arg778Leu homozygous patients that exhibit early-onset disease, we found completely defective trafficking and showed low-level pigmentation in tyrosinase activity of the mutant protein. On the other hand, GFP-His1069Gln exhibits partially defective trafficking and partial tyrosinase activity that is consistent with later age of onset and less disease severity of the homozygous patients.
We agree that our study lacks a large cohort of patients that harbors a wide spectrum of different homozygous mutations. However, the importance of our study lies in our success to delineate phenotype in polarized epithelium cell for the 15 WD disease variants including the two most common mutations, His1069Gln and Arg778Leu of two major world populations using two simple and cost-effective in-cell assays. The two assays that we utilized in this study can serve as preliminary benchmark to determine genotype-phenotype correlation for other WD mutations in homozygous condition in WD patients.
ACKNOWLEDGMENTS
This study is supported by DBT-Wellcome Trust India Alliance Fellowship (IA/I/16/1/502369), Early Career Research Award (ECR/2015/000220), and Core Research Grant (CRG/2021/002150) from SERB, Department of Science and Technology (DST), Government of India, and IISER-K intramural funding to AG. SM is supported by a predoctoral fellowship from Council of Scientific and Industrial Research (CSIR), India. The predoctoral fellowship for Ruturaj is supported by Intramural Institute funding (IISER-K). TM is supported by KVPY fellowship from the Govt. of India.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
WEB RESOURCES
DeepDDG: http://protein.org.cn/ddg.html
Consurf: https://consurf.tau.ac.il/
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.