Clinical presentation and genetic analyses of neurofibromatosis type 1 in independent patients with monoallelic double de novo closely spaced mutations in the NF1 gene
Abstract
Neurofibromatosis type 1 (NF1) belongs to RASopathies, a group of syndromes caused by germline mutations in Ras/MAPK pathway genes. Most NF1 patients exhibit single inactivating pathogenic variants within the NF1 gene. We performed extensive genetic analyses in two NF1 families disclosing the first two cases of double de novo monoallelic NF1 variants. Both index patients described in this study had classical NF1. Probands were born from fathers in their late 30s and presented closely spaced double mutations (<100 bp) in NF1 regions showing an excess of somatic mutations. Closely spaced multiple mutations have been reported in RAS/MAPK signaling genes but never in NF1. Mutagenesis is a quasi-random process in humans, therefore two causative variants in the same gene, moreover in the same allele are exceptional. Here, we discuss possible mechanisms for this ultrarare event. Our findings confirm the possibility of a higher risk of concurrent de novo variants in NF1.
Neurofibromatosis type 1 (NF1 [OMIM: 162200]) is one of the most common genetic diseases with an estimated prevalence of 1 in 3000 (Friedman, 1993). Inherited as autosomal dominant, it is caused by mutations in the large NF1 gene spanning 58 exons encoding for neurofibromin, a 2839 amino acids protein. NF1 is clinically diagnosed in accord with the recently revised diagnostic criteria (Legius et al., 2021).
In 50% of cases, NF1 mutations arise de novo, and diagnosis is frequently suspected in prepuberal years, thus a correct variant interpretation is crucial for a reliable NF1 assessment. In diagnostic laboratories, variant pathogenicity is assessed in accord with the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) (Richards et al., 2015). NF1 pathogenic variants (PVs) can occur across the entire coding region and include virtually the full range of various types from multiexons deletion/duplication, including whole gene deletions, to missense mutations (Messiaen, 2020). In addition, most missense variants are still classified as variants of uncertain clinical significance (VUS), and little has been reported on the correlation between variants’ position and their pathogenic effects (Accetturo et al., 2020; Koczkowska et al., 2020, 2018).
A second NF1 hit, and consequent loss-of-function, has been documented in several NF1-associated cancers (Boudry-Labis et al., 2013; Uusitalo et al., 2016), in cutaneous and plexiform neurofibromas as well as in tissues from pigmentary nonneoplastic lesions such as cafè-au-lait macules (CALMs) (De Schepper et al., 2008). A phenotype mimicking NF1 is caused by homozygous mutations in mismatch repair genes in a novel clinical entity known as constitutional mismatch repair deficiency (Alotaibi et al., 2008; Raevaara et al., 2004; Urganci et al., 2015).
Here, we present the first two independent cases of NF1 patients with de novo in cis double mutations in the NF1 gene with a classical NF1 phenotype.
The first patient was a male individual in his 50s, displaying several cutaneous neurofibromas, bilateral axillary freckling, and no CALMs. Therefore, he was diagnosed with NF1 in accord with National Institutes of Health guidelines. Four years before counseling the patient had a surgically removed gastrointestinal stromal tumor (GIST) in the jejunum and gastric fundus. No adjuvant therapy was recommended after surgery, and the patient has been undergoing periodic follow-ups since. The patient's parents had no history of NF1 and were deceased in their 90s of natural causes. At the patient's birth, the father was 38 years old while the mother was 32 years old. After genetic counseling, a next-generation sequencing (NGS) multigene panel was used (A2ML1, BRAF, CBL, HRAS, KRAS, MAP2K1, MAP2K2, NF1, NRAS, PTPN11, RAF1, RIT1, SHOC2, SOS1, and SPRED1) to search for the presence of causative mutations in RASopathies associated genes.
The second case was a 6 years old girl who was referred to our unit with >20 CALMs and bilateral inguinal and axillary freckling (Supporting Information: Figure S1a–d). In addition, slight diffuse hypertrichosis was present (Supporting Information: S1e,f). Both parents did not show any clinical features suggestive of NF1. The paternal age at the daughter's birth was again 38 years old. while the mother was in her late 20s. After genetic counseling NGS gene testing was performed with the same multi-gene panel mentioned previously.
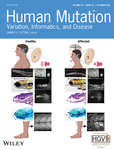
In Case 1, the NGS analysis disclosed two PVs in the NF1 gene (reference sequence: NM_000267.3): a c.3198-1G>A splicing mutation (genomic coordinates in GRCh38/hg38: chr 17: 31232072G>A) and a c.3295A>T p.(lys1099*) nonsense mutation in the 3’-adjacent exon 25 (genomic coordinates in GRCh38/hg38: chr 17: 31232170A>T). Both variants were confirmed with Sanger sequencing (Figure 1a). After gene testing, the patient's only daughter, in her late 20s, was invited to a genetic counseling session during which several CALMs >1.5 cm, diffuse cutaneous neurofibromas, bilateral axillary freckling, and a plexiform neurofibroma on her left calf were detected. Sanger sequencing of NF1 exon 25 confirmed the presence of both variants in the patient's daughter suggesting they were in cis. The proband's DNA was amplified with primers flanking the two variants and the PCR product was cloned into the TA vector. Sequencing of 20 recombinant clones had invariably either the normal sequence or both variants confirming that they were in cis on the same allele (Figure 1b). The c.3198-1G>A nucleotide change is predicted to disrupt the splicing acceptor (SA) site according to all predictors in the Alamut® Splicing module (Figure 1c). To confirm the variant's effect on splicing the complementaryDNA obtained from the patient's and his daughter's blood samples was sequenced and, in both, revealed an aberrant mRNA containing the r.3198_3199delAG mutation predicted to cause a p.(Asp1067Phefs*21) at protein level (Figure 1d). Therefore, the c.3198-1G>A mutation cannot be considered a compensatory mutation, since the novel splicing site created offsets the normal open reading frame resulting in a similarly truncated NF1 protein.
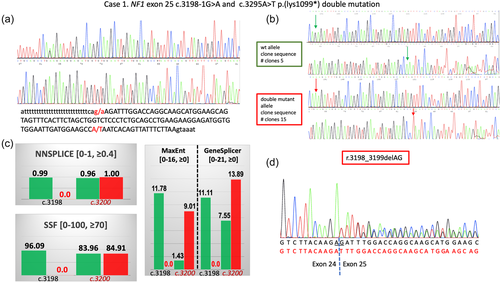
In Case 2, NGS disclosed the presence of two NF1 variants in exon 21: a frameshifting mutation c.2546del p.(Gly849GlufsTer29) (genomic coordinates in GRCh38/hg38: chr 17: 31229161del), and the missense variant c.2548G>A p.(Val850Met) (genomic coordinates in GRCh38/hg38: chr 17: 31229163G>A). This second missense variant has never been reported before and is classified as likely pathogenic by the Varsome variant impact assessment tool (accessed November 4th, 2021), which implements the ACMG/AMP rules. Specifically, the Val850Met lies in a region of 17 amino-acids length which has 14 non-VUS missense/in-frame variants (14 pathogenic and 0 benign, ACMG/AMP rule PM1), and therefore is considered a mutation hotspot. Furthermore, it is not present in gnomAD (ACMG_AMP rule PM2), and is predicted to be pathogenic by 9 of 12 variant predictions (ACMG/AMP rule PP3). Also, the c.2548G>A variant had scores equal to or above the cutoff values for pathogenicity, as previously defined (Accetturo et al., 2020), for 5/5 of cutoff performance indicators (ClinPred), and for 3/5 (REVEL and VEST4).
The two variants were again confirmed with Sanger sequencing (Figure 2a). Since the first of these two variants is located in a run of five consecutive guanines, the exact position of this nucleotide change cannot be precisely determined and may lie anywhere from −2 to −7 with respect to the subsequent missense variant. Sequencing analysis on the girl's parent DNA, and an allele-specific PCR confirmed the presence of the two variants only in the proband (Figure 2b). Finally, as previously described, the girl's DNA was amplified with primers flanking the exon and the PCR product cloned in the TA vector. The sequencing of 20 recombinant clones had again invariably either wild-type sequence or both variants (Figure 2c).
Next, we inspected the sequence surrounding the two double variants with nonB-DB (https://nonb-abcc.ncifcrf.gov/apps/site/default), since it has been reported that non-B DNA structures could favor the generation of multiple mutations (Chen et al., 2009). However, no non-B DNA structures were identified in both regions. Then, we used the UNAFold web server (http://www.unafold.org/) to verify whether the two regions might assemble secondary structures. UNAFold returned one structure for exon 21 (Supporting Information: Figure S2) and five for exon 25 (Supporting Information: Figure S3a–e). In all structures, variants were close to stem-loop switch regions. Interestingly, in one of the five structures built by UNAFold for exon 25, the two variants spaced 98 nucleotides apart were in close proximity (Supporting Information: Figure S3e).
To our knowledge, the two patients described here are the first reported carriers of double de novo mutations in the same NF1 allele. The three patients presented in this study had a rather classical NF1 phenotype. However, in Case 1, a GIST was previously diagnosed. GIST has been occasionally reported in NF1 and is present in 6%–8% of NF1 patients (Vargas Ávila et al., 2021). The patient's daughter was diagnosed with NF1 at the time of the genetic counseling session since she presented typical NF1 clinical signs. In Case 2, the presence of cutaneous symptoms only (CALMs and axillary freckling) is in line with the age-dependent penetrance of more easily recognizable NF1 stigmata such as cutaneous or plexiform neurofibromas. As previously mentioned, the girl also had mild diffuse hirsutism which has been sporadically reported in NF1, and mostly colocalizing with cutaneous neurofibromas (Friedrich & Hagel, 2018; Ortonne et al., 2018; Praxedes et al., 2010).
Interestingly, despite the large coding sequence of the NF1 gene, in both cases, the concurrent in cis variants were closely spaced (98 nucleotides in Case 1 and 2–7 nucleotides in Case 2) and in the same exon (exons 21 and 25, respectively). Sequence inspection did not reveal a clear mutational hotspot excluding the long poly-T stretch (28 nt) immediately preceding exon 25. However, the two de novo variants identified in exon 25 were both single nucleotide substitutions rather than indel changes as expected if variants were the result of DNA polymerase slippage errors caused by the poly-T sequence.
The presence of multiple mutations in close proximity has been found in several human genes and dubbed as closely spaced multiple mutations (Chen et al., 2009). In particular, pair of mutations separated by less than 100 bp, as in our two cases, have been hypothesized to represent a signature of transient hypermutability in human genes. While no obvious mutation hotspots have been identified in NF1, in COSMIC (https://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=NF1#distribution), accessed on January 13th, 2022) there were 22 nucleotide substitutions targeting the SA site of exon 25 (intronic position −1 to −3), of which three were c.3198-1G>A. In contrast, only three substitutions were listed in ClinVar at exon 25 SA (c.3198-1G>A not present, assessed on September 13th). We focused on the last three positions of the preceding intron since these positions are significantly associated with splicing deregulation (Stella et al., 2018). Similarly, the exon 21 c.2546del, is present twice in COSMIC, and together with the c.2548G>A lies in a stretch of 21 coding nucleotides hosting 26 mutations in COSMIC (NF1 coding sequence from c.2525 to c.2546). This would result in a local mutation ratio of 1.24 mutations/nucleotide compared to 0.39 mutations/nucleotide for the remaining 417 nucleotides of exon 21. In this 21-nucleotide sequence, there are contiguous GG, TT (2), CC, TTT, CCC, and the final GGGGG repeats that may render the region particularly prone to replication or misalignment errors. Two de novo mutations occur in two different NF1 families with two NF1 PVs lying 80 and 3 nucleotides, respectively, upstream of our doublet de novo c.2546del, c.2548G>A have been recently reported (Garcia et al., 2022) (Supporting Information: Figure S4). This finding might confirm that the region could be frequently targeted by de novo NF1 variants.
Thus, it is possible that the two variants were the outcome of a simultaneous or a quasi-simultaneous mutational event in regions prone to replication errors. Considering that the closest of the five consecutive guanines lies two nucleotides upstream of the following c.2548G>A, the possibility of single mutational events generating a complex allele cannot be ruled out. It should be added that, differently from exon 25 splicing variants, 20 variants in the same 21 nucleotide stretch were also present in ClinVar.
Recently, combining ultradeep sequencing with a low-frequency variant prioritization strategy, 61 different variants were identified in the testes of five aged men (Maher et al., 2018). Eighty percent of variants were found in genes encoding for a protein involved in the RAS/MAPK pathway. Furthermore, a clonal expansion of already mutated male reproductive precursor cells, i.e. spermatogonia, has been described in achondroplasia, Apert, Noonan, and Costello syndrome (Goriely & Wilkie, 2012). This phenomenon has been named “selfish spermatogonial selection” and is associated with high rates of de novo mutations and strong paternal age effects (PAE). A further feature of selfish selection is the frequent occurrence of multiple nucleotide substitutions that have been documented in most PAE disorders (reviewed in Goriely & Wilkie, 2012). NF1 shares overlapping symptoms with Costello and Noonan syndromes, and similarly encodes for a key regulator of the RAS/MAPK pathway. In NF1, as in other RASopathies, although yet debated, increasing paternal age has been associated with a higher risk for de novo mutations (Adel Fahmideh et al., 2018; Dubov et al., 2016). Interestingly, in both our cases, fathers were 38 years old at probands’ birth, therefore at the first peak of the curve correlating the incidence of new mutations in NF1 and parental age in one of the first studies analyzing this correlation (Risch et al., 1987). In the abovementioned report, both Apert syndrome and neurofibromatosis showed an increase up to age 37, a sudden drop at age 42, and a new increase at age 47. Recently, the co-occurrence of two independent biallelic NF1 changes has been documented in five NF1 families (Pacot et al., 2019). Also, a very recent work reports four different families where two different NF1 disease-causing variants were detected. In all families, a de novo mutation was present in the offspring of an affected NF1 male, and in two, the paternal origin of the de novo mutation could be demonstrated (Garcia et al., 2022). However, multiple de novo mutations occurring in the same NF1 allele have never been described. Differently from all known PAE disorders which are caused by activating mutations, NF1 acts as a classical tumor suppressor gene with most PVs causing a low protein abundance (Long et al., 2022). Thus, different lines of evidence point to a crucial role of RAS/MAPK/ERK pathway dysregulation in spermatogonial stem cells homeostasis. Being part of the RAS signaling pathway, implicated in oncogenesis, and highly expressed in the testis (https://gtexportal.org/home/gene/NF1) could target NF1 for having a role in this phenomenon.
It has to be said that the double de novo NF1 variants we report, being on the same allele, are unlikely to confer an additive growth advantage in spermatogonial cells. Rather, they could represent the result of serendipitous events in a gene locus with a high mutation rate (Garcia et al., 2022).
Three of the NF1 variants identified in our two families are novel while the c.2546del has been already reported. None of the four variants was present in GnomAD (last accessed on October 30th, 2021). The c.3198-1G>A, the nonsense c.3295A>T, and the c.2546del are all clearly pathogenic whereas, as discussed before, several lines of evidence show that also the c.2548G>A could be pathogenic.
The two double de novo variants are both localized in regions of the NF1 gene which appear to represent a mutation hotspots in oncogenesis, a process somehow recapitulated by the selfish selection in the testis. However, as mentioned previously, the concurrent double changes could also be the result of a single event targeting regions prone to replication errors.
Finally, the possibility of a first NF1 mutation followed by a quick second mutational event in the same cell clone has been proposed to explain isolated segmental monoclonal two-hit mosaicism in NF1 (Torrelo & Happle, 2021).
Our study has the following limitations. First, given the available biological samples, we cannot formally rule out that in one or both cases the double in cis, de novo variants could be originated in an early post-zygotic stage. Second, we could not unequivocally determine the parental origin of the mutations even though this topic was analyzed by several experimental means (see Supporting Information Text). Lastly, considering the type of variants we have identified, we cannot formally ascribe a specific underlying mechanism(s) of mutagenesis. In fact, the four variants reported are not homocoordinate mutations (i.e., multiple mutations of the same type occurring in cis at different sites) for which different mechanisms of mutagenesis have been hypothesized (Chen et al., 2009).
These findings could have important consequences in NF1 genetic counseling considering the possibility of an increased risk for de novo mutations associated with paternal age. The de novo doublets reported here, and the recently described four NF1 families with two PVs (two of them paternal in origin), should also alert to the possibility of failure in detecting NF1 causative variants once the presence of parentally derived ones has been excluded (Garcia et al., 2022). While the NF1 doublets we identified, since in cis, have not influenced the clinical phenotype of our patients, it is yet possible that in other genes the same type of situation could explain apparent genotype-phenotype incongruities.
Finally, the two independent cases of double de novo variants in the NF1 gene could lend support to the important role of RAS/MAPK signaling in the spermatogonial homeostasis and the predatory clonal expansion of cells carrying selfish PVs.
ACKNOWLEDGMENTS
The authors are thankful to all patients, families, referring physicians, and specialists for their contribution to this study. This study was partly supported by grant Ricerca Scientifica di Ateneo Università degli Studi di Bari Aldo Moro (#H95E10000710005) and FARB to Alessandro Stella. Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
WEB RESOURCES
LOVD NF1 database: https://databases.lovd.nl/shared/genes/NF1; ClinVar NF1 database: https://www.ncbi.nlm.nih.gov/clinvar/?term=NF1 [gene]; Varsome NF1 database: https://varsome.com/gene/hg38/NF1; Non-B structures database (nonB-DB): https://nonb-abcc.ncifcrf.gov/apps/site/default; UNAFold web server for secondary structures assembly: http://www.unafold.org/; COSMIC NF1 database: https://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=NF1#distribution; GTEX database for NF1 expression: https://gtexportal.org/home/gene/NF1; GnomAD: https://gnomad.broadinstitute.org/gene/ENSG00000196712?dataset=gnomad_r2_1