Partial loss-of-function variant in neuregulin 1 identified in family with heritable peripheral neuropathy
Daniel E. Lysko, Ana M. Meireles, Chiara Folland made equal contributions.
Gianina Ravenscroft, and William S. Talbot made contributions equal to each other.
Abstract
Neuregulin 1 signals are essential for the development and function of Schwann cells, which form the myelin sheath on peripheral axons. Disruption of myelin in the peripheral nervous system can lead to peripheral neuropathy, which is characterized by reduced axonal conduction velocity and sensorimotor deficits. Charcot-Marie-Tooth disease is a group of heritable peripheral neuropathies that may be caused by variants in nearly 100 genes. Despite the evidence that Neuregulin 1 is essential for many aspects of Schwann cell development, previous studies have not reported variants in the neuregulin 1 gene (NRG1) in patients with peripheral neuropathy. We have identified a rare missense variant in NRG1 that is homozygous in a patient with sensory and motor deficits consistent with mixed axonal and de-myelinating peripheral neuropathy. Our in vivo functional studies in zebrafish indicate that the patient variant partially reduces NRG1 function. This study tentatively suggests that variants at the NRG1 locus may cause peripheral neuropathy and that NRG1 should be investigated in families with peripheral neuropathy of unknown cause.
Charcot-Marie-Tooth disease (CMT) encompasses a group of clinically and genetically heterogenous peripheral sensory and motor neuropathies (Lassuthova et al., 2018). CMT occurs in approximately 1 in 2500 individuals and has been associated with pathogenic variants in >90 genes (Lassuthova et al., 2018; Ravenscroft et al., 2021; Senderek et al., 2020). There are two main CMT sub-types, which are differentiated based on electrophysiological and neuropathological criteria. CMT1 is a demyelinating neuropathy characterised by slow motor nerve conduction velocity and is caused by abnormalities in the myelin sheath due to cell-autonomous defects in Schwann cells, the glia that make myelin in the peripheral nervous system (PNS). CMT2 is an axonal neuropathy, characterised by normal or mildly reduced nerve conduction velocities and chronic axonal degeneration and regeneration. Differentiation between axonal and demyelinating forms can be complicated by secondary axonal degeneration and demyelination in CMT1 and CMT2, respectively. More than 50% of CMT patients remain without a definitive molecular diagnosis following screening using comprehensive gene panels or clinical exomes (Drew et al., 2015; Lassuthova et al., 2018; Ravenscroft et al., 2021; Senderek et al., 2020), suggesting there are additional CMT disease genes to identify. Herein we describe the identification of a rare homozygous missense variant in the neuregulin 1 gene (NRG1) in a consanguineous patient presenting with CMT disease.
Neuregulin signals are essential for Schwann cell development and myelination in vertebrates including mouse and zebrafish (Britsch et al., 1998; Garratt et al., 2000; Glenn & Talbot, 2013; Lyons et al., 2005; Meyer & Birchmeier, 1995; Morris et al., 1999; Perlin et al., 2011). In developing peripheral nerves, Nrg1 on the axonal membrane activates a heterodimeric ErbB2-ErbB3 receptor tyrosine kinase on associated glia, triggering Schwann cell proliferation, migration, and myelination (Nave & Salzer, 2006). Through different promoters and alternative messenger RNA (mRNA) splicing, the Nrg1 gene encodes more than 30 different isoforms, which are further modified by posttranslational processing (Falls, 2003; Hu et al., 2006; La Marca et al., 2011; Willem et al., 2006). In humans, the NRG1 gene has distinct promoters that generate six main classes of isoforms (types I, II, III, IV, V, and VI) that have different N-terminal regions but share a common EGF-related domain that activates ErbB receptors (Chou & Ozaki, 2010). While types IV–VI are not present in all vertebrates, the types I, II, and III isoforms are widely conserved among vertebrates, and mutational studies in mouse and zebrafish have identified Nrg1 type III signals as essential regulators of Schwann cell development and myelination (Perlin et al., 2011; Wolpowitz et al., 2000). In mouse and zebrafish, type III isoform-specific mutants lack Schwann cells in peripheral nerves (Perlin et al., 2011; Wolpowitz et al., 2000). The extent of myelination is exquisitely controlled by the level of Nrg1 type III on peripheral axons: overexpression of Nrg1 type III generates thicker myelin, whereas mice heterozygous for a Nrg1 type III specific mutation have thinner myelin (Michailov et al., 2004; Taveggia et al., 2005). In addition, compound heterozygous mice for mutations in Nrg1 and ErbB2 have significantly thinner myelin and slower nerve conduction velocity (Michailov et al., 2004). Although overexpression of soluble Nrg1 signals can modulate the phenotypes of CMT animal models (Fledrich et al., 2014), previous studies have not reported NRG1 loss-of-function variants in patients with peripheral neuropathy. Our analysis provides evidence that partial loss of NRG1 function may impair nerve conduction in humans. We propose that variants in NRG1 may be responsible for cases of peripheral neuropathy with unknown cause.
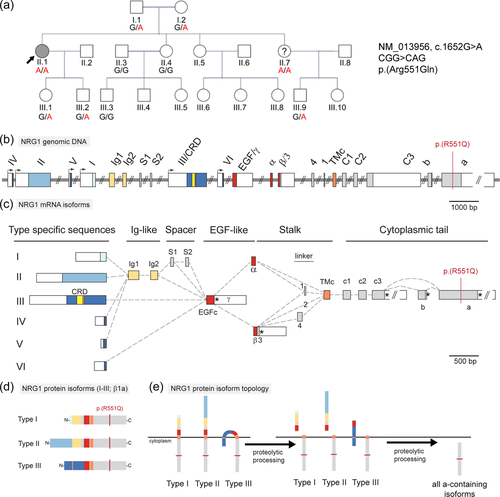
The proband was a female, born to consanguineous parents. She noticed after the birth of her first child, when she was aged 20 years, that she stumbled when walking on uneven surfaces and that her hands were weak, particularly when manipulating small objects like keys. These symptoms gradually worsened over the ensuing 13 years. When examined at the age of 33 years she had normal strength proximally, but distally had wasting of the thenar muscles and the intrinsic muscles of the hand, and the tibialis anterior of the leg. Strength-wise, she had completely lost all thumb opposition, had 3/5 power in intrinsic muscles of the hand, and 4/5 wrist movements. Unusually, her lower limbs were not as affected as her upper limbs. She could still stand on her toes, although not on the toes of one foot only, but could not walk on her heels. All other lower limb movements were within normal limits. Her deep tendon reflexes were slightly augmented, except she had lost her ankle jerks. Plantar reflexes were flexor. Pinprick sensation was reduced to the knees and the mid-forearms. Nerve conduction studies were performed on the proband at the age of 35 years (Table S1). Right peroneal and tibial motor and right sural sensory responses were absent. Right median sensory and motor left median sensory and right radial sensory responses were also absent. Bilateral ulnar motor distal latencies were essentially normal. Motor conduction velocities were slowed, and bilateral ulnar F wave latencies were essentially normal. Limited EMG of left arm and leg muscles showed large motor unit potentials in distal muscles, with fibrillation. In conclusion there is electrophysiologic evidence of a mixed axonal and demyelinating distally prominent neuropathy. Over the ensuing 10 years, the movements about the ankle have worsened to the extent that she cannot walk on toes or heels and has bilateral foot drop.
Whole genome sequencing identified 14 rare (allele frequency <0.001) coding homozygous variants, including a missense variant in the Neuregulin1 (NRG1) gene (ENST00000287842, NM_013956, c.1652G>A, p.(Arg551Gln), referred to here as R551Q; rs141355195). This variant is present on 23 alleles in gnomAD v2.1.1 and is absent from the Greater Middle East Variome Project (Scott et al., 2016). Multiple in silico predictions suggest this variant to have a detrimental functional impact; SIFT: deleterious, PolyPhen: probably damaging, MutationAssessor: medium, MutationTaster: disease-causing, CADD score 21. The substituted arginine is conserved in human, mouse, and frog; in zebrafish the corresponding residue is a serine.
Bidirectional Sanger sequencing confirmed the NRG1 c.1652G>A variant to be homozygous in the proband and showed that all healthy relatives were either carriers or homozygous for the reference allele (Figure 1a). Limited clinical details are available for the youngest sister (II.7), who resides in Iraq. Before genomic testing, she reported cramps and numbness in the legs and feet. She is also homozygous for the variant. We have been unable to obtain further details or formal clinical review of this individual.
Of the 13 other rare homozygous variants identified (Table S2), two had homozygous individuals in gnomAD, and we therefore excluded these. Variants in three other candidate genes (DDX59, GSR, and ST3GAL5) cause diseases in humans that do not include neuropathy (Table S2, Notes) and thus were not regarded as candidates for the disease in this family. We used Sanger sequencing to analyze segregation for the remaining eight variants. By Sanger sequencing six variants did not co-segregate with disease. Two variants co-segregated with disease in the family, including an in-frame indel in ZFHX4 and a missense variant in DMTN. Previously, a de novo balanced translocation of ZFXH4 was identified in a patient with bilateral isolated ptosis (MIM #178300) (McMullan et al., 2002). Studies in a recent Zfhx4 mouse line showed that homozygous Zfhx4 null mouse pups have cleft palate and skeletal malformations and died within a day of birth (Nakamura et al., 2021). DMTN encodes the dematin protein—an actin bundling protein identified in the erythroid membrane skeleton (Derick et al., 1992; Husain-Chishti et al., 1988). Dmtn null mice have anemia and their erythrocytes are osmotically fragile (Lu et al., 2016). Additionally, studies have shown that dematin is critical to the shape and membrane stability of red blood cells (Khanna et al., 2002). Given the different phenotypes associated with loss of ZFHX4 or DMTN, these genes are not good candidates for the phenotype in this family. Taken together, these data and the known role of NRG1 in myelination of the PNS (Meyer & Birchmeier, 1995; Nave & Salzer, 2006; Perlin et al., 2011; Wolpowitz et al., 2000), we conclude that the NRG1 variant remains the most likely plausible candidate in this family.
NRG1 encodes >30 different proteins that share a common EGF-like domain but differ in other regions due to use of different promoters and alternative splicing (Figure 1b,c). The R551Q substitution identified in family 1 affects isoforms with the longest cytoplasmic tail, in which the long “a” exon is included (Figure 1d,e). Transcripts containing the “a” cytoplasmic tail have been detected across multiple isoform classes (Falls, 2003), but NRG1 isoform type III stands out as relevant to peripheral neuropathy because of its essential roles in Schwann cell development and myelination in zebrafish and mouse.
To functionally test the activity of the R551Q variant in peripheral nerve development, we turned to zebrafish, in which peripheral nerve myelination is well studied and easily assessed (detailed Materials and Methods available in Supporting Information). Our previous analysis (Perlin et al., 2011) indicated that Schwann cell proliferation and migration are disrupted in zebrafish mutants homozygous for a missense variant in the type III-specific cysteine rich domain (CRD) of nrg1. In the present study, we used CRISPR-Cas9 to introduce a lesion (designated st153) in the CRD predicted to completely eliminate the function of nrg1 type III but leave the other isoforms intact (Figure S1a,b). This lesion is a 7 bp deletion predicted to cause a frameshift and premature termination of translation, truncating the protein before the transmembrane domain of the CRD (Figure S1c,d). Homozygous nrg1st153/st153 mutants lack myelin basic protein (mbp) mRNA in the posterior lateral line nerve, in accord with a previous study showing that myelinating Schwann cells are absent in nrg1 type III mutants (Perlin et al., 2011) (Figure S1e).
To investigate the functional consequences of the R551Q patient variant, we compared the ability of wildtype and patient variant NRG1 type III proteins to rescue myelination of peripheral nerves in nrg1st153/st153 mutant zebrafish. We constructed Tol2 transposon vectors that express either wildtype human NRG1 type IIIa or the R551Q variant in transient transgenic experiments. The constructs contained pan-neuronal regulatory sequences to drive expression of NRG1 type IIIa in neurons or, as a control, a regulatory element that drives expression in cells of the macrophage lineage. The expression vectors also contained a cmlc2:GFP marker, which allowed the transgene expression efficiency to be assessed by the extent of GFP fluorescence in the heart (Figure 2a).

NRG1 expression vectors and synthetic mRNA encoding Tol2 transpose were injected into embryos from a cross of nrg1st153/+ heterozygotes, and larvae with strong GFP expression in the heart were fixed to detect mbp expression by in situ hybridization at 4.5 days post fertilization (dpf). The extent of mbp expression was assessed, and then the genotypes of all larvae were determined. The ability of the expression constructs to rescue the mutants was assessed by the extent of mbp expression along the posterior lateral line nerve (Figure 2b). Whereas expressing human NRG1 type IIIa in the macrophage lineage did not rescue mbp expression along the lateral line nerve, expressing NRG1 type IIIa in neurons strongly rescued mbp expression in nrg1st153/st153 mutants: 32% of nerves in injected mutants were fully rescued, and only 3% of nerves showed no evidence of rescue (Figure 2c). The R551Q variant had significantly less rescuing activity than wildtype human NRG1 type IIIa in four independent experiments: only 3% of nerves in injected mutants appeared fully rescued, while 22% of nerves showed no evidence of rescue (Figure 2c,d; Chi-square test, p = 0.0277). These results indicate that the R551Q substitution identified in family 1 is a partial loss-of-function variant that reduces the ability of NRG1 type IIIa to direct Schwann cell myelination in peripheral nerves.
Extensive previous studies have demonstrated that Nrg1 signals and their ErbB receptors control Schwann cell development and myelination (Britsch et al., 1998; Garratt et al., 2000; Lyons et al., 2005; Meyer & Birchmeier, 1995; Perlin et al., 2011; Raphael et al., 2011; Taveggia et al., 2005; Wolpowitz et al., 2000). The >30 isoforms encoded by the NRG1 gene share a central EGF-related domain that is flanked by variable N- and C-terminal regions generated via different promoters and alternative mRNA splicing (Falls, 2003). Nrg1 type III signals are prominently expressed in developing sensory and motor neurons in mouse and zebrafish (Honjo et al., 2008; Meyer et al., 1997), and mutational studies provide compelling evidence that these signals are key regulators of myelination in peripheral nerves (Perlin et al., 2011; Wolpowitz et al., 2000). Mice homozygous for a mutation that disrupts all Nrg1 isoforms die during mid-embryogenesis with defects in heart trabeculation and lacking Schwann cell precursors in peripheral nerves (Meyer & Birchmeier, 1995). Several lines of evidence indicate that Nrg1 type III isoforms are the key neuregulins that govern Schwann cell development and myelination. Mice homozygous for a targeted mutation that specifically disrupts Nrg1 type III specific sequences die at the time of birth with a severe reduction in Schwann cell numbers in peripheral nerves (Wolpowitz et al., 2000). Similarly, zebrafish nrg1 type III specific homozygous mutants lack Schwann cells in peripheral nerves (Perlin et al., 2011), because both Schwann cell proliferation and migration are disrupted in the absence of these essential signals. Nrg1 type III signals also control the radial sorting process, during which axons are paired with Schwann cells that will subsequently myelinate them, and it is thought that axons expressing the highest levels of Nrg1 type III are the first to be myelinated (Domènech-Estévez et al., 2016; Goebbels et al., 2010; Raphael et al., 2011; Taveggia et al., 2005). In addition, the level of Nrg1 type III expressed on an axon determines the thickness of the myelin produced by its associated Schwann cells (Michailov et al., 2004). The fact that Nrg1 type III is the rate-limiting signal controlling so many steps of Schwann cell development may explain the loss of function constraint in gnomAD (pLI = 1) and the lack of individuals homozygous for loss of function variants. Not only would individuals entirely lacking NRG1 signaling be expected to have early, lethal abnormalities, even a partial reduction of NRG1 activity might disrupt peripheral nerves and other organs that are finely regulated by the level of NRG1 signaling.
Our analysis has identified a recessive, partial loss-of-function variant in NRG1 that segregates with peripheral neuropathy in a consanguineous pedigree. The variant changes an incompletely conserved Arg residue encoded by one of the alternative 3' exons of the reference sequence to Gln. We provide evidence that this R>Q change partially reduces the function of NRG1 type III signals using our functional tests in zebrafish. Expression of the reference human NRG1 type III-beta1a protein in neurons can rescue Schwann cell development in all but a few percent of zebrafish nrg1 type III mutants. In contrast, about ten times fewer zebrafish mutants were fully rescued when the R>Q variant form was expressed. It is important to emphasize that the R>Q variant does retain significant activity, which likely accounts for the relatively mild clinical phenotype of the patient when compared to the embryonic and perinatal lethality of mice homozygous for complete knockout (Meyer & Birchmeier, 1995) and type III specific (Wolpowitz et al., 2000) Nrg1 mutations, respectively.
There are several possible mechanisms by which an R>Q missense variant in the C-terminal region of NRG1 proteins could reduce function. One possibility is the R>Q substitution might impair trafficking of NRG1 signals through the secretory pathway or proper proteolytic processing (Liu et al., 1998). Consistent with this hypothesis, a previous study reported that the C-terminal region of Nrg1 signals determines the extent to which the proteins are retained in the cytoplasm of sensory neurons (Zhang et al., 2006). Another possibility is suggested by previous work showing that the C-terminal region of Nrg1 type III controls its subcellular localization on the membrane of pyramidal neurons (Exposito-Alonso et al., 2020); thus, the R>Q variant might mislocalize NRG1 signals within peripheral neurons. Finally, it has been reported that a C-terminal fragment of Nrg1 type III proteins is involved in “back signaling”, in which Nrg1—ErbB interactions can have transcriptional (Bao et al., 2003, 2004) and nontranscriptional effects (Canetta et al., 2011; Hancock et al., 2008) on the neurons expressing Nrg1; thus, the R>Q substitution might disrupt such a back signaling function in peripheral neurons.
Our analysis provides evidence that suggests partial loss of NRG1 function may cause peripheral neuropathy in humans. This finding complements prior studies that identified pathogenic variants in ErbB2 and ErbB3, the Schwann cell receptors for axonal NRG1 signals, in patients with peripheral neuropathy and arthrogryposis (Le et al., 2021; Narkis et al., 2007). Analysis of further cases is required to determine the extent to which different types of NRG1 variants may contribute to peripheral neuropathy in humans.
AUTHOR CONTRIBUTIONS
Daniel E. Lysko, Ana M. Meireles, Elyshia McNamara and Chiara Folland: designed, performed and analyzed experiments. Phillipa J. Lamont: performed clinical evaluation. Nigel G Laing, Gianina Ravenscroft, and William S. Talbot: designed experiments and analyzed data. Daniel E. Lysko, Gianina Ravenscroft, and William S. Talbot: wrote the manuscript, with input from all authors.
ACKNOWLEDGEMENTS
The authors thank members of our labs for helpful discussion and critical comment on the manuscript and T. Reyes and C. Hill for fish care. This work was supported by the National Institutes of Health [R35 NS111584 to W.S.T., 1F32NS095466 to D.E.L.]. This work was supported by the Australian National Health and Medical Research Council [APP1122952 to G.R.] and the Australian Genomics Health Alliance [NHMRC GNT1113531].
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




