Marfan Syndrome and Related Disorders: 25 Years of Gene Discovery
Contract grant sponsors: University of Antwerp (Lanceringsproject); Fund for Scientific Research, Flanders (FWO, Belgium, G.0221.12); The Dutch Heart Foundation (2013T093); Fondation Leducq (MIBAVA – Leducq 12CVD03); European Research Council (ERC- StG-2012-30972-BRAVE).
For the 25th Anniversary Commemorative Issue
ABSTRACT
Marfan syndrome (MFS) is a rare, autosomal-dominant, multisystem disorder, presenting with skeletal, ocular, skin, and cardiovascular symptoms. Significant clinical overlap with other systemic connective tissue diseases, including Loeys–Dietz syndrome (LDS), Shprintzen–Goldberg syndrome (SGS), and the MASS phenotype, has been documented. In MFS and LDS, the cardiovascular manifestations account for the major cause of patient morbidity and mortality, rendering them the main target for therapeutic intervention. Over the past decades, gene identification studies confidently linked the aforementioned syndromes, as well as nonsyndromic aneurysmal disease, to genetic defects in proteins related to the transforming growth factor (TGF)-β pathway, greatly expanding our knowledge on the disease mechanisms and providing us with novel therapeutic targets. As a result, the focus of the developing pharmacological treatment strategies is shifting from hemodynamic stress management to TGF-β antagonism. In this review, we discuss the insights that have been gained in the molecular biology of MFS and related disorders over the past 25 years.
Introduction
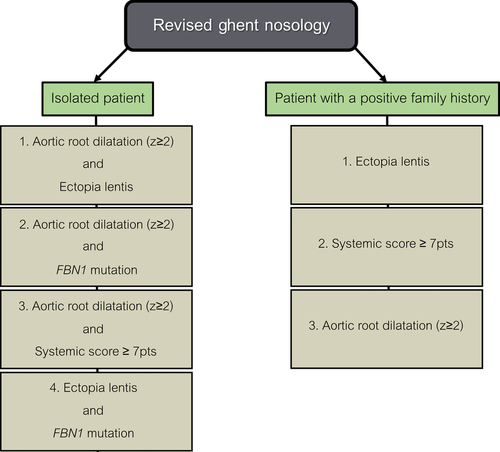
In 1896, the French pediatrician Antoine-Bernard Marfan first described the skeletal abnormalities (i.e., overgrowth and joint laxity) that characterize a hereditary connective tissue disorder that eventually was named after him, Marfan syndrome (MFS; MIM #154700) (Marfan, 1896). Meanwhile, numerous other clinical manifestations have been linked to the disease, including eye (ectopis lentis, myopia), skin (striae), and cardiovascular phenotypes (aortic root aneurysm, mitral valve prolapse), with the latter being the major culprits for high morbidity and mortality in MFS patients (Cook et al., 2015). Estimated disease incidences range between 1:5,000 and 1:10,000 live births. No gender or ethnicity biases have been reported. MFS is clinically heterogeneous and other presentations of systemic connective tissue disease, such as Loeys–Dietz syndrome (LDS), Shprintzen-Goldberg syndrome (SGS) and the MASS phenotype among others, display a significant clinical overlap. Approximately 10 years ago, LDS (MIMs #609192, #610168, #613795, #614816, #615582) was first recognized as a separate disease entity (Loeys et al., 2005). Characteristic LDS features include hypertelorism, craniosynostosis, bifid uvula, cleft palate, and arterial tortuosity as well as aneurysms and ruptures. Compared with MFS and SGS, cardiovascular manifestations are more severe, with dissections and ruptures occurring at smaller aortic diameters and younger ages (Williams et al., 2007). Ectopia lentis is not a feature of LDS. SGS (MIM #182212) was described in the early 1980s by, evidently, Shprintzen and Goldberg (1982). The disease presents with common learning difficulties, craniosynostosis, and skeletal muscle hypotonia, in addition to many of the craniofacial, skeletal, skin, and cardiovascular symptoms of MFS and LDS. Most affected individuals have only mild vascular pathology though, with no arterial tortuosity or risk of aneurysm development or dissection other than at the aortic root (Greally et al., 1998). In the 1980s, a fraction of the adults who were referred to the clinic for suspected MFS but did not completely fulfill the criteria of the MFS diagnostic nosology, were, for the first time, classified as having MASS syndrome (MIM #604308) (Glesby and Pyeritz, 1989). MASS syndrome stands for, and is characterized by, myopia, mitral valve prolapse, mild and nonprogressive aortic dilation, and nonspecific skin and skeletal marfanoid features. This phenotypic designation is only valid in the absence of ectopia lentis. As MASS syndrome is not easily distinguished from "emerging" MFS during childhood, the diagnosis can only be established for individuals aged 20 or older. In 2010, new diagnostic MFS criteria, known as the revised Ghent nosology, were defined to more accurately deal with this overlap in clinical manifestations (Fig. 1) (Loeys et al., 2010). Compared with the previous guidelines, they give more weight to the presence/absence of aortic dilatation and ectopia lentis. Furthermore, they aim at reaching a higher diagnostic sensitivity and specificity by integrating genetic testing into the diagnostic criteria.
More Than a Decade of Genetic Breakthroughs
MFS, LDS, MASS syndrome, and SGS are multisystem genetic disorders that segregate in an autosomal-dominant manner. Whereas in about 25% of the MFS probands disease arises de novo, a clear family history is apparent in the remaining, and thus vast majority, of patients. Because of the latter observation, McKusick et al. (1955) suggested, already in the 1950s, a contribution of genetic factors in the etiology of MFS. Yet, it took several extra decades to pinpoint mutations in the fibrillin 1 gene (FBN1; MIM #134797) as the disease cause (Dietz et al., 1991) (Table 1; Fig. 2). FBN1 (chr15q15-15q21.1) encodes a 350-kDa extracellular matrix (ECM) glycoprotein that takes part in the formation of microfibrils, which are of utmost importance for elasticity and structural support of numerous tissues. The encoded protein has a modular structure consisting of 47 epidermal growth factor (EGF)-like motifs, seven transforming growth factor (TGF)-β-binding domains as well as a cysteine-rich and proline-rich module. The EGF-like and TGF-β-binding domains, respectively, contain six and eight highly conserved cysteine residues, which have a pivotal role in protein structure and stability through covalent formation of three disulfide bridges.
| Disease | Subtype | Chromosome locus | Gene | Identification method | Reference | Key variant database |
|---|---|---|---|---|---|---|
| MFS | - | 15q21.1 | FBN1 | Linkage analysis | Dietz et al. (1991) | http://www.umd.be/FBN1/ |
| MASSa | - | 15q21.1 | FBN1 | Candidate gene approach | Dietz et al. (1993) | http://www.umd.be/FBN1/ |
| LDSa | LDS1 | 9q22.33 | TGFBR1 | Candidate gene approach | Loeys et al. (2005) | http://www.umd.be/TGFBR1/ |
| LDS2 | 3q24.1 | TGFBR2 | Candidate gene approach | Loeys et al. (2005) | http://www.umd.be/TGFBR2/ | |
| LDS3 | 15q22.33 | SMAD3 | Linkage analysis | Van de Laar et al. (2011) | http://www.umd.be/SMAD3/ | |
| LDS4 | 1q41 | TGFB2 | Microarray analysis | Lindsay et al. (2012) | - | |
| LDS5 | 14q24.3 | TGFB3 | Linkage analysis + WES | Bertoli-Avella et al. (2015) | - | |
| NA | 18q21.1 | SMAD2 | Candidate gene approach | Micha et al. (2015) | - | |
| SGS | - | 1p36.33 | SKI | WES | Carmignac et al. (2012) Doyle et al. (2012) | http://www.lovd.nl/SKI |
- aIndicates that more genes remain to be identified.
- WES, whole-exome sequencing; NA, not yet determined.
Over the past 10 years, six LDS genes have been identified: TGFBR1 (MIM #190181) (Loeys et al., 2005), TGFBR2 (MIM #190182) (Loeys et al., 2005), SMAD2 (MIM #601366) (Micha et al., 2015), SMAD3 (MIM #603109) (van de Laar et al., 2011), TGFB2 (MIM #190220) (Lindsay et al., 2012), and TGFB3 (MIM #190230) (Bertoli-Avella et al., 2015) (Table 1; Fig. 2). These breakthroughs significantly improved our insights in both the clinical and genetic heterogeneity of LDS and urged the subdivision of the clinical LDS continuum in multiple disease classes (LDS1-5). LDS1 and 2 have now been allocated to patients with mutations in TGFBR1 and TGFBR2 (Maccarrick et al., 2014), respectively, the two genes of which the identification coincided with definition of the clinical manifestations of LDS (Loeys et al., 2005). They encode serine-threonine kinases that form a heteromeric receptor complex upon binding of the TGF-β cytokines to TGFBR2 (Franzen et al., 1993). TGFBR2 was considered a strong candidate gene for LDS as cleft palate, calvaria, and outflow tract defects were observed in conditional Tgfbr2 knockout mice (Sanford et al., 1997; Ito et al., 2003). Additionally, TGF-β signaling had been shown to be key for normal vascular and craniofacial development. As a consequence of the functional and structural relationship between TGFBR1 and TGFBR2, the identification of TGFBR2 mutations in LDS patients also instigated mutation analysis of TGFBR1 (Loeys et al., 2005). The latter analysis confidently pinpointed TGFBR1 as the second LDS gene. SMAD3 mutations were identified in the aneurysm-osteoarthritis syndrome, which is now also termed LDS3, using linkage analysis in a large Dutch family followed by mutation analysis of TGF-β-related genes that were located within the linked region (van de Laar et al., 2011). Very recently, a candidate gene approach also identified mutations in SMAD2 in syndromic aneurysm patients (Micha et al., 2015). Being the first intracellular effectors that are activated by ligand binding to the TGF-β receptors, SMAD2 and SMAD3 are considered key regulators in the TGF-β pathway. As TGF-β signaling starts from, and depends on, proper binding of TGF-β cytokines to their receptors and the ligand encoding genes (TGFB1, TGFB2, and TGFB3) are highly expressed in the cardiovascular system, TGFB genes represent strong candidates for aneurysmal syndromes. It took, however, until 2012 and 2015 before mutations in TGFB2 and TGFB3, respectively, were identified in LDS patients (LDS4/5) (Lindsay et al., 2012; Bertoli-Avella et al., 2015). Interestingly, individuals with discriminating features of LDS without a mutation in any of the six known genes exist. Additional LDS genes therefore remain to be identified.
Besides to MFS, FBN1 mutations have been confidently linked to several phenotypically overlapping conditions, including the MASS phenotype (Table 1) (Dietz et al., 1993). Implication of FBN1 as well as TGFBR1/2 mutations in the genetic etiology of SGS had been advocated for as well (Sood et al., 1996; Ades et al., 2006; Kosaki et al., 2006). Over time, however, either the presumed SGS-causing mutations were found to be benign polymorphisms, or the mutation carriers’ diagnoses were shifted to probable MFS or LDS (Cannaerts et al., 2015). Hence, SGS remained genetically unexplained until 2012, when two research groups independently reported on the identification of heterozygous mutations in the SKI proto-oncogene gene (SKI; MIM #164780) in SGS patients using a trio-based or family-based exome sequencing approach (Table 1; Fig. 2) (Carmignac et al., 2012; Doyle et al., 2012). Few large replication studies have been published so far, but it is estimated that in about 90% of the SGS probands a, mostly de novo, mutation in SKI can be identified (Schepers et al., 2015). Functionally, the SKI family of proteins represent negative regulators of SMAD-dependent TGF-β signaling (Prunier et al., 2003).
In Search for Strong Genotype–Phenotype Correlations
Uncovering genotype–(endo)phenotype correlations facilitates the diagnostic process, genetic counseling, as well as more personalized surgical and/or pharmacological patient management strategies. Evidently, such analyses require in-depth knowledge on the genetic and phenotypic variability that associates with a particular disease. In case of FBN1, about 1,850 different mutations that are widely spread all over the protein have been described so far (http://www.umd.be/fbn1/), of which the majority is unique to each family. The mutational spectrum encompasses nonsense, frameshift, splice altering, insertion/deletion, and missense variations as well as whole-gene or multiexon deletions. It has been a longstanding matter of debate, however, whether these mutations cause marfanoid syndromes through haploinsufficiency, a dominant-negative mechanism or a combination of both (Dietz et al., 1993; Judge et al., 2004; Matyas et al., 2007). Lately, particular the latter hypothesis is gaining momentum in the MFS field (Colovati et al., 2012; Franken et al., 2015). Despite significant progress in the understanding of the molecular defects underlying MFS, a limited number of convincing genotype–phenotype correlations has emerged. Cysteine-destroying or cysteine-creating mutations are commonly associated with ectopia lentis (Schrijver et al., 1999). Haploinsufficient mutations, on the other hand, more frequently correlate with aortic events at young ages as well as pectus carinatum, dural ectasia, and skin striae (Baudhuin et al., 2015; Franken et al., 2015). Additionally, the vast majority of the mutations in exons 24–32 are linked to either neonatal MFS or other severe marfanoid presentations (Tiecke et al., 2001). Although MFS mostly inherits in an autosomal-dominant manner, rare cases with recessive homozygous or compound heterozygous FBN1 mutations have been described (Hogue et al., 2013). They exhibit a more severe phenotype compared with their heterozygous relatives, who are usually asymptomatic or only mildly affected. Taken together, the nature of the implicated FBN1 defect can, at least to some extent, explain phenotypic variability. Yet, also related individuals carrying an identical FBN1 mutation vary widely with respect to onset age, organ-system involvement, and disease severity. Over the past few years, evidence suggesting that differences in wild-type FBN1 expression levels partially explain this intrafamilial clinical variability has emerged (Hutchinson et al., 2003; Aubart et al., 2015). Low levels of wild-type mRNA have been shown to associate with increased risk for both ectopia lentis and pectus abnormalities (Aubart et al., 2015). A trend toward an increased risk for aortic dilatation was reported as well, but should be replicated. Alternatively, genetic studies in very large MFS families with discrete intrafamilial phenotypic variability are being conducted to identify other genetic modifiers.
Even fewer genotype–phenotype correlations have been described for LDS. Clinically, TGFBR1 and TGFBR2 mutation carriers do not differ significantly from each other (Van Hemelrijk et al., 2010), and definition of LDS endophenotypes based on the nature of the underlying TGFBR1/2 mutation has proven challenging. In both receptor genes, mostly missense mutations affecting the serine/threonine kinase domains have been identified (Loeys et al., 2005; Loeys et al., 2006). Nonetheless, also rare truncating (nonsense, frameshift, and splice site) mutations causing LDS have been reported (Renard et al., 2014), but most of these, if not all, are predicted to escape nonsense-mediated decay. However, over the past couple of years, it has become evident that diverse TGFBR1/2 mutation types do lead to surprisingly dissimilar clinical presentations. Whereas mostly missense mutations in TGFBR1 cause LDS, complete loss-of-function mutations have been linked to multiple self-healing squamous epithelioma (MIM #132800), an autosomal-dominant skin cancer that is characterized by multiple squamous-carcinoma-like locally invasive skin tumors (Goudie et al., 2011). Additionally, a somatically mosaic deletion of TGFBR2 has been described in a 20-month-old female presenting with microcephaly and developmental delay, but without cardiovascular features of LDS (Campbell et al., 2011). In families segregating SMAD3 mutations, a high incidence of early-onset osteochondritis dissecans and osteoarthritis affecting the spine, knees, and hands has been reported (van de Laar et al., 2012). To date, more than 20 pathogenic SMAD3 variations have been identified (Zhang et al., 2015). The mutation spectrum encompasses both truncating and missense mutations, with the latter clustering within the MH2 protein domain (van de Laar et al., 2011). Interestingly, also the currently reported SMAD2 mutations locate in the MH2 domain (Micha et al., 2015). LDS-related SMAD2/3 mutations have therefore been proposed to impair oligomerization with SMAD4, which is a prerequisite to initiate SMAD-dependent transcriptional activation of the key players of the TGF-β signaling pathway. Although genetic defects in TGFBR1, TGFBR2, SMAD3, and possibly also SMAD2 predominantly associate with syndromic presentations, rare mutations have also been described in isolated familial thoracic aortic aneurysm and dissection (Bradley et al., 2015; Micha et al., 2015). Thus far, it is not known why some mutations cause LDS, whereas others account for the mild end of the disease spectrum. In case of the most recently identified LDS genes (TGFB2, TGFB3, and SMAD2), detailed genotypic and phenotypic spectra are yet to emerge, currently hampering deduction of confident genotype–phenotype correlations. Various TGFB2/3 mutation types, that is, missense, frameshift, nonsense, and splice-site mutations, have been reported, most likely resulting in haploinsufficiency for their respective gene (Boileau et al., 2012; Ritelli et al., 2014; Bertoli-Avella et al., 2015). Though replication is definitely warranted, preliminary findings suggest that families segregating TGFB2/3 mutations present with a higher degree of nonpenetrance as well as a less severe cardiovascular phenotype compared with TGFBR1/2 and SMAD3 mutation carriers (Ritelli et al., 2014; Kuechler et al., 2015).
With respect to SGS, remarkably little variability as to the type or location of the reported SKI mutations exists. Except for a few in-frame deletions that affect the Dachshund-homology domain, almost all (73%) are de novo missense mutations located in the R-SMAD-binding domain affecting one of four adjacent amino acid residues (p.S31–p.P35) (Schepers et al., 2015). The R-SMAD domain of SKI, which is involved in TGF-β signaling, has therefore been nominated a mutational hotspot for SGS.
Pathomechanisms Converge on a Paradoxical Increase in TGF-β Signaling
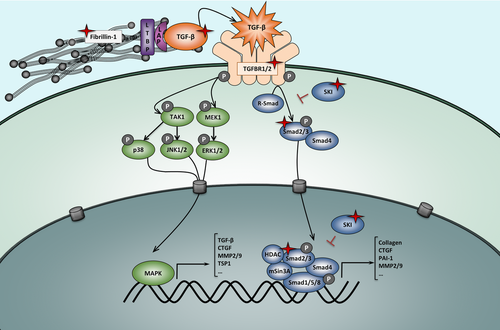
Fibrillin 1 assembles into microfibrils that serve a critical role in the maintenance of the structural integrity of the aortic wall, as well as the ciliary apparatus supporting the ocular lens (Hayward et al., 1997). Hence, FBN1 mutations were initially thought to lead to tissue fragility, exclusively through disintegration and fragmentation of the connective tissue fibers. As such, weakening of the aortic wall would increase susceptibility to ruptures, whereas weakening of the ciliary apparatus would cause ectopia lentis (Fleischer et al., 1997; Pereira et al., 1997). The skeletal overgrowth that is characteristic for MFS patients, however, is very difficult to attribute to pure structural tissue abnormalities. A revolutionary shift in thinking about the pathomechanisms of MFS occurred by studying lung disease in fibrillin 1-deficient mice. In the developing mouse lungs, increased levels of free TGF-β in addition to the downstream effectors of TGF-β signaling (pSMAD2/3) coincided with primary failure of distal alveolar septation (Neptune et al., 2003). In vivo TGF-β antagonism using a pan-specific neutralizing anti-TGF-β antibody restored the lung phenotype. Similarly, involvement of dysregulated TGF-β signaling in the etiology of MFS was later on also established for the myopathy, mitral valve prolapse, as well as the aortic aneurysmal phenotype (Ng et al., 2004; Habashi et al., 2006; Cohn et al., 2007). Interaction between latent TGF-β-binding proteins (LTBP) and fibrillin 1 formed the basis of these experiments (Isogai et al., 2003). Because of this interaction, TGF-β bioavailability is meticulously controlled by cytokine sequestration into the ECM as a large latent complex (LLC) consisting of, obviously, the mature TGF-β ligand, the TGF-β propeptide, and a LTBP isoform (Fig. 2). Fibrillin 1 deficiency, due to FBN1 mutations, impairs ECM targeting of the LLC, resulting in an unrestrained release of TGF-β ligands (Neptune et al., 2003; Franken et al., 2013). Under normal physiological circumstances, TGF-β ligands that have been dissociated from the LCC bind to their respective type II (TGFBR2) and type I (TGFBR1) serine/threonine protein kinase receptors, initiating the downstream TGF-β canonical signal transduction cascade. More precisely, the TGFBR1 kinase domain becomes activated via transphosphorylation by TGFBR2, enabling recruitment and phosphorylation of receptor-regulated SMAD transcription factors (R-SMADs; i.e., SMAD2 & SMAD3). Next, SMAD2 and SMAD3 form a trimeric protein complex with SMAD4, translocate to the nucleus and initiate transcription of TGF-β target genes. Cooperation of the SMAD2/3/4 complex with multiple transcriptional (co)activators or (co)repressors ensures preservation of TGF-β homeostasis. The SKI protein is an example of such a corepressor. It binds the trimeric SMAD protein complex, inducing a negative feedback loop of TGF-β signaling by preventing translocation of the complex to the nucleus (Luo et al., 1999).
Over the last 10 years, a central role for dysregulated TGF-β signaling in aneurysm formation became even more apparent due to the identification of loss-of-function mutations in multiple LDS (TGFBR1, TGFBR2, SMAD2, SMAD3, TGFB2, and TGFB3) and SGS (SKI) genes, all encoding gene products involved in the canonical TGF-β pathway (Fig. 2). Loss-of-function of the respective gene products was initially supported by experimental data obtained in cell models showing impaired canonical TGF-β signaling of mutant TGFBR1/2 upon TGF-β stimulation (Mizuguchi et al., 2004; Loeys et al., 2005; Horbelt et al., 2010). Examination of aortic wall tissue or dermal fibroblasts of patients carrying mutations in any of the LDS or SGS genes, however, revealed paradoxical upregulation of TGF-β signaling, as demonstrated by the detection of elevated pSMAD2, pERK1/2, TGF-β1, collagen, and/or CTGF expression levels (Loeys et al., 2005; Boileau et al., 2012; Doyle et al., 2012; Lindsay et al., 2012; Bertoli-Avella et al., 2015). In case of SKI mutations, these findings correlate with what one might expect: loss of repressor function leads to increased TGF-β signaling (Doyle et al., 2012). In case of patients with TGFBR1, TGFBR2, SMAD2, SMAD3, TGFB2, and TGFB3 mutations, however, the paradoxical observation that loss-of-function mutations in TGF-β components lead to a significant upregulation (and not downregulation) of the TGF-β pathway in human tissues, is extremely intriguing. Over time, experiments in animal models and humans have proposed several mechanisms to explain these opposing findings, but all of them still need further supporting evidence (Lindsay and Dietz, 2011). One of the theories that has been raised states that due to malfunction of components (TGFB2/3, SMAD2/3, TGFBR1/2) of the canonical TGF-β signaling pathway, an autoinhibitory feedback loop is interrupted, resulting in excessive TGF-β secretion followed by a disproportionate increase in expression of components of the noncanonical (ERK/JNK1/MAPK) TGF-β pathway (Bolar et al., 2012). Alternatively, since LDS and SGS patients show a markedly increased expression of TGFB1, shifts in TGF-β cytokine usage may play a role too (Doyle et al., 2012; Lindsay et al., 2012). Finally, as sites of developmental field boundaries correspond to the anatomic regions that show a major predisposition for syndromal TGF-β-related aneurysmal disease (i.e., the aortic/pulmonary roots, the juxtaductal aorta, and the suprarenal abdominal aorta), the most recent hypothesis states that paradoxical TGF-β signaling might be driven by non-cell-autonomous effects. As such, relative perturbation of TGF-β signaling would disproportionately affect the most vulnerable cell lineage of the aortic root, that is, the second heart field-derived vascular smooth muscle cells (VSMCs), resulting in a compensatory increase of TGFB1 expression and release. Subsequently, TGF-β signaling would be stimulated in the adjacent, intrinsically less vulnerable, cardiac neural crest-derived VSMCs (Gallo et al., 2014).
Pathomechanistic Advances Paved the Way for Novel Therapeutic Approaches
Cardiovascular disease, progressive dilatation and dissection of the aortic root in particular, was (and unfortunately still is) the leading cause of death in patients with MFS or related disorders. More than a decade ago, virtually any type of drug lowering blood pressure and/or reducing cardiac contractility was considered a plausible remedy for MFS. Theoretically, reduction in hemodynamic stress placed upon an inherently weakened aortic wall should indeed prevent further aortic expansion and delay or even prevent aortic dissection. The first pharmacological therapy, which has been considered the gold standard for a long time, involved administration of β-adrenergic receptor antagonists (β-blockers) to marfanoid patients. Prophylactic β-blockers impact on aneurysm progression by reducing the mean arterial pressure and the systolic heart rate. These drugs came prominently into the picture after publication of promising findings of an open-label randomized trial that revealed a reduced rate of aortic dilatation upon propranolol treatment (Shores et al., 1994). Since the original report of Shores et al. (1994), however, different clinical trials described somewhat variable, or even conflicting, outcomes (Gersony et al., 2007; Ladouceur et al., 2007; Gao et al., 2011). Possibly, β-blockers attenuate progression of aortic root dilatation, whereas more drastic clinical endpoints (e.g., mortality) remain equally frequent (Vaidyanathan, 2008). Angiotensin converting enzyme inhibitors or calcium channel blockers (CCBs) are occasionally prescribed to patients who are intolerant of β-blockers (Williams et al., 2008). In both cases, efficacy and/or beneficence have been under debate. Preliminary data from MFS mouse models even suggest that the cardiovascular phenotype can worsen upon CCB administration (Doyle et al., 2015). Hence, large randomized controlled trials are warranted to check for CCB-related adverse effects in MFS patients.
About 10 years ago, upregulated TGF-β signaling was pinpointed the key culprit in the pathogenesis of MFS, LDS, and SGS, urging in-depth investigation of TGF-β-neutralizing therapeutic approaches. The angiotensin II type I receptor antagonist losartan had been shown to antagonize TGF-β signaling in animal models of chronic renal insufficiency and cardiomyopathy and was already routinely used for treatment of hypertension, which rendered it the number-one drug to test in MFS mice (Lim et al., 2001; Lavoie et al., 2005). Comparing the structural integrity of the aorta as well as the aortic diameter of propranolol-treated and losartan-treated MFS mice, losartan appeared to diminish the aortic growth rate, to prevent progressive elastic fiber fragmentation and to counteract ongoing abnormal aortic dilatation (Habashi et al., 2006). On the molecular level, losartan proved to diminish the accumulation of activated SMAD2, hereby reducing the expression of TGF-β-responsive genes. Currently, uncertainty surrounds the benefit of losartan therapy with regard to aortic disease in MFS patients. While early results and small studies in humans seemed promising (Brooke et al., 2008; Chiu et al., 2013; Pees et al., 2013), most recent studies (Bhatt et al., 2015; Forteza et al., 2015; Milleron et al., 2015), including a large prospective randomized study of the pediatric heart network in about 600 pediatric and young adult MFS patients (Lacro et al., 2014), did not observe statistical significant differences in the dilatation rate of the aortic root between losartan and atenolol- or placebo-treated patient groups. To allow a more powerful estimate of the effects of β-blocker or losartan treatment in MFS patients, however, a meta-analysis combining all individual trials (2,300 patients) is being conducted at the time of writing (Pitcher et al., 2015). Since Franken et al. (2015) recently demonstrated that MFS patients carrying haploinsufficient FBN1 mutations benefit more from losartan therapy compared with dominant-negative FBN1 mutation carriers, definition of patient subgroups based on the type of FBN1 mutation might be advantageous in future individual trials and meta-analyses (Franken et al., 2015). Also, the use of a therapy combining atenolol and losartan might be worthwhile to consider, as they have been suggested to have distinct modes of action (Bhatt et al., 2015). Alternatively, one might consider high dosing of losartan to obtain more optimal blocking of TGF-β signaling.
Although to a lesser extent, treatments that aim at blocking the noncanonical components of the TGF-β signaling pathway are also being investigated, as well as compounds targeting matrix metalloproteinases (MMPs). Elevated protein levels of the latter have been firmly associated with aneurysmal disease (Lindsay and Dietz, 2011). ERK or JNK antagonism (with RDEA119 or SP600125) significantly reduces aortic aneurysm progression in fbn1 transgenic mice (Holm et al., 2011). Also, in a MFS mouse model, inhibition of MMP-2 using doxycycline delays aneurysm rupture, which is demonstrated by the fact that treated mice live longer as compared with untreated mice and present with decreased elastic fiber degradation (Xiong et al., 2008).
When the diameter of the aortic root or the ascending aorta in MFS patients reaches 5 cm, or if the aorta enlarges at an extremely rapid pace, surgery is recommended. In LDS patients, the surgical threshold generally is around 4.0–4.5 cm (Maccarrick et al., 2014). Factors such as the underlying disease gene and/or mutation, family history, severity of systemic findings, and the patient's personal risk/benefit assessment, however, can influence the precise timing. In the early seventies, the so-called Bentall and De Bono surgical procedures, which replace the aortic root, ascending aorta, and/or aortic valve with a Dacron prosthesis and/or mechanical valve, respectively, became the golden standard (Bentall and De Bono, 1968; Gott et al., 1999). More recently, valve-sparing techniques (e.g., David's surgery) are becoming increasingly popular, as they eliminate the need for life-long anticoagulation therapy (Benedetto et al., 2011).
Conclusions
Although evolving medical and surgical approaches have already significantly improved life expectancy as compared with 50 years ago of patients with MFS or related disorders, additional strategies to further decrease aneurysm-related morbidity and mortality are warranted. During the last couple of decades, gene discovery for MFS, LDS, and SGS led to a significant expansion of our knowledge on the disease mechanisms of syndromic aortopathies, paving the way for the development of novel therapeutic strategies that orient toward reestablishment of the TGF-β homeostasis. Interestingly, genetic and histological studies also revealed a role for increased canonical TGF-β signaling in nonsyndromic aneurysmal disease (Gillis et al., 2013), expanding applicability of such TGF-β antagonizing compounds to larger patient populations and, hence, possibly increasing interest from pharma companies. As prophylactic aortic root surgery can ultimately prevent life-threatening aortic dissections or ruptures, lifelong cardiovascular follow-up is advisable for patients with MFS or related conditions. Another major advantage of the described genetic breakthroughs is that they enable genetic testing, in the affected proband in first instance, but later on also in asymptomatic family members enabling pinpointing of those individuals that should be closely monitored throughout life. Furthermore, in large families, combination of the obtained genotypic data with detailed phenotypic information might lead to the identification of genetic disease modifiers, likely accounting for the wide range of phenotypic variability that has been observed within MFS, LDS, and SGS families. In turn, this can open new avenues for therapeutic management of patients.
Clearly, 25 years of gene discovery, and functional characterization of the encoded gene products, has taken MFS research to the next level. Of course many questions remain unanswered, with the most intriguing one being how loss-of-function mutations in components of the TGF-β pathway can lead to a paradoxical increase in TGF-β signaling. Yet, with the current pace of front-line research in aneurysmal disease, near completion of the mechanistic puzzle of MFS, LDS, and SGS might be feasible within the next 10 to 15 years.
Acknowledgment
Dr. Loeys is senior clinical investigator of the Fund for Scientific Research, Flanders.




