Setting up of a hospital Covid-19 vaccination center: A descriptive study
Abstract
Background and Aims
The coronavirus pandemic challenged countries worldwide in a race against contaminations and variants. Vaccination campaigns were the answer to such an infectious spread. This descriptive study presents the organizational process of the setting up of a Covid-19 vaccination center in a French University Hospital in January 2021, the issues encountered along the way and assessment of adaptability.
Methods
Three major stakeholders: SARS CoV-2 crisis referent, referring vaccination medical doctor and referring vaccination pharmacist retraced key moments and identified issues encountered during the setting up of the vaccination center and its long term maintenance, threw a series of meetings. Records of crisis and periodic meetings that took place threw out the vaccination campaign were consulted.
Results
A multidisciplinary crisis steering committee with nine different professionals was created January 3. Logistics for the vaccination center opening were discussed: location, informatics, appointment-scheduling, pharmaceutical circuit, internal circuit, human resources, and information communication. The vaccination center was ready to welcome healthcare workers in less than 24 h on January 4. The first month, 2757 1st shots were administered, leading up to a total of 9167 1st shots during 6 months of activity. From January to June 2021, the multidisciplinary group dealt and adapted its processes to challenging and unexpected situations. Indeed, issues encountered with Pfizer BioNTech's and AstraZeneca's vaccine, were: supply shortages, vaccine manipulation, targeted populations, pharmacovigilance, and general communication.
Conclusion
This descriptive study provides an exclusive insight on how a hospital vaccination center was organized and adapted during Covid-19 pandemic to ensure healthcare workers' security and resilience, and to protect high risk patients of severe Covid-19 infection.
1 INTRODUCTION
A reorganization of standard operation procedures (SOP) took place during the SARS-CoV-2 crisis in the French Public Assistance—Hospitals of Paris institution (AP-HP) to adapt human resources (HR) and logistics, with a central crisis medical director supported by administrative directors from each of the 39 AP-HP hospital centers.1 A clear chain of medical and administration responsibilities enabled a reactive operational decision-making process throughout the healthcare institution.
Availability of Covid-19 vaccine made it necessary to rapidly adapt our SOP to vaccinate the population in accordance with national health mandates. Priority targets were mortality and morbidity reduction of at-risk populations as well as healthcare workers who sustain the resilience of the healthcare institution.
The European Medical Agency (EMA) authorized the first Covid-19 vaccine, Comirnaty®, Pfizer and BioNTech laboratories on December 21, 2020.2 On the 24th of December 2020, French approval from the public health authority (Haute Autorité de Santé; HAS) was officially published. Approval came with a national vaccination program parsed out in five scheduled phases, prioritizing target population groups defined by age criteria and comorbidities.3
The first phase, dedicated to nursing homes, started on December 27, 2020. The second phase, for hospital healthcare workers was originally scheduled to begin in February 2021. But, on January 2, 2021, the French government revisited the vaccination program and prioritized all healthcare workers above 50 years old and regardless of age, healthcare workers with a high risk for severe Covid-19 symptoms due to comorbidities (Table 1).
| Chronic heart diseases, hypertension |
| Chronic kidney diseases |
| Chronic lung diseases (interstitial lung disease, pulmonary embolism, pulmonary hypertension, bronchiectasis, chronic obstructive pulmonary disease) |
| Obesity body mass index ≥ 30 |
| Diabetes mellitus, type 1 and type 2 |
| Solid organ or blood stem cell transplantation |
| Cancers and malignant hematology diseases (active in the past 3 years) |
| Down syndrome |
The aim of this run through is to describe the organization process of a Covid-19 vaccination center set up in a French university hospital from AP-HP, the issues encountered along the way, the long term results and the lessons learned.
2 MATERIAL AND METHOD
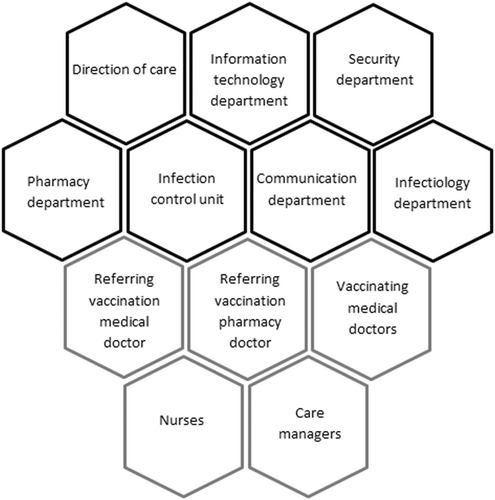
This descriptive study, presented as a case report, was constructed from a retrospective compilation of records from planned and crisis meetings set up threw out the Covid-19 vaccination program. Records retraced the evolution of health recommendations, issues encountered and organization decisions. These multidisciplinary decision-making meetings brought together administrative, medical and paramedical identified key stakeholders (Table 2). A medical crisis referent was designated in March 2020 at the beginning of the SARS-CoV-2 pandemic crisis. In January 2021, with the arrival of Covid-19 vaccines, two referring medical and pharmacist doctors were designated.
| Stakeholders | Tasks for vaccination center opening |
|---|---|
| Infectious diseases department |
|
| Designation of a referring vaccination medical doctor | |
| Infection control unit team |
|
| Pharmacy department |
|
| Designation of a referring vaccination pharmacist doctor | |
| IT department |
|
| Security service |
|
| Direction of care |
|
| Direction of communication |
|
| Nurses |
|
During a series of meetings, referents validated the selection and chronology of the records retained concerning the vaccination center's deployment, logistics set up and workforce cooperation from the time of opening (January 4, 2021) to the center's closing (June 30, 2021).
Vaccination center setting-up data was analyzed from January 4, 2021, to February 26, 2021.
Assessments of adaptability, success and limits over 6 months were discussed.
3 RESULTS
3.1 Covid-19 vaccination center set up
3.1.1 Initiation
To follow new government decisions, a remote AP-HP crisis meeting took place Sunday January 3, 2021 to start the healthcare workers vaccination program. To coordinate the vaccination center opening 24 h later in our hospital, a multidisciplinary crisis meeting was organized by the director on January 4th. The crisis team was composed of the director and heads of departments: information technology (IT), infection control unit, pharmacy department, direction of care, security and Paris emergency services system (SAMU de Paris).
During the meeting, the multidisciplinary team decided on the vaccination center location. The location had to allow easy and direct access without passing through patient care units. The room had to be able to accommodate the necessary equipment, ensure social distancing and allow vaccination surveillance and oversight. The team then discussed the number of vaccination boxes, material, HR and the tasks of each health professional.
Each stakeholder was given tasks to accomplish in 24 h (Table 2). This short notice reorganization required coordination between departments and the adaptability thereof.
A steering committee was then put together with different referring health actors such as the referring vaccination medical and pharmacist doctors.
3.1.2 Environment
Occupational health department was not sufficient in terms of infrastructure and HR to support the vaccination campaign. The vaccination center had to welcome intern healthcare workers with night and day shifts, healthcare workers from other public or private structures and independent healthcare workers. The vaccination center was set up in the SAMU de Paris's meeting room and was opened 7 days a week. The chosen location was not dedicated to patient care, which allowed a simple, direct and close access.
Infrastructure had to be adapted in size to welcome vaccination staff and deploy enough vaccination boxes while guaranteeing a dedicated space for postinjection surveillance and social distancing. Vaccination staff included: three vaccinating doctors, one nurse, one regulator, two clerical personnel and one security guard.
3.1.3 Vaccination boxes and nursing station
Three vaccination boxes and one nursing table for in advance dose preparations were installed. At first, target yield was one vaccination every 15 min per box. Open hours from 8:00 a.m. to 9:00 p.m. with 1 h break allowed a yield of 144 vaccinations per day.
3.1.4 IT and data
An IT platform was implemented to offer appointment scheduling after a few days. A phone line was available in the meantime. Information requested was: name, date of birth, professional identification number, social security number, and cell phone number.
Computers were installed at every box with access to medical software and specific secured database: Orbis® medical record and the national register called “SI Vaccin Covid.” An Orbis® form was created for Covid-19 vaccine traceability (date, arm injected, vaccinator, and vial batch number) which was done through the national register database. A vaccine certificate was generated with: vaccinator's signature, date, vaccine reference, batch number, and injection number.
3.2 Pharmaceutical circuit
3.2.1 Circuit anticipation
In December 2020, pharmaceutical circuits were discussed during remote meetings between central AP-HP drug platform: AGEPS (Agence Générale des Equipements et Produits de Santé) and local AP-HP hospital pharmacy departments.
Covid-19 vaccines came from a national stock. They were regulated and delivered by the national public distributor (Santé Publique France, SPF) to AGEPS and stored at −80°C.
A +4°C circuit between AGEPS platform and hospital pharmacies was decided in December 2020, as already in place for routine drugs. Ordering Comirnaty® with 5-day stability was critical to avoid wasting doses.
3.2.2 Pharmacy department
Vaccine orders had to be made several times a week to allow a 7/7-day vaccination activity. Orders were made to AGEPS before 3:30 p.m. for delivery the next morning. Vial relabeling was done by pharmacy staff with new expiration date and time. A good practice document was created by the referring vaccination pharmacist and validated by the referring vaccination doctor. Nurses were trained by the referring vaccination pharmacist with focus on stability, fragility (mRNA and nanolipids) and asepsis.5
3.2.3 Vaccination center
A qualified fridge was installed in the vaccination center for vaccine day storage. Vials were retrieved at the pharmacy at 7:30 a.m. by the center's nurse, before opening at 8:00 a.m. No vaccine was stored in the center during the night. Unused vials were brought back to the pharmacy for secure storage.
3.3 Surveillance and pharmacovigilance
A postinjection surveillance was required for 15 min. Vaccinated individuals were supervised by staff in case of immediate side effects. Care managers ordered medicines and medical devices for emergency carts and made sure they were functional and available. During vaccination consultation, the medical doctor delivered information on how to declare side effects. For intern hospital professionals, a declaration system was available via OSIRIS® an intern web platform. Declarations could also be made directly to the regional pharmacovigilance centers.
3.4 Steps to get vaccinated
The first step consisted of passing control and regulation. A security guard checked identity on the days' program and regulation verified identity and professional activity. The next step was Orbis® registration and identity label edition.
The shot was injected at the end of the traced consultation, when no contra-indications appeared. Healthcare workers waited 15 min in the surveillance area. Second dose registration was the last step.
3.5 Workforce: Interdisciplinary coordination
- 1.
IT department organized an appointment scheduling platform.
The platform became a tool for the referring vaccination pharmacist, direction of care, care managers, and for specific actions such as transferring appointments from one vaccination center to another. Text messages were generated for appointment confirmations and modifications.
- 2.
Communication department created information posters, established notes on the hospital's internet page offering information on the vaccination program and managed visual communications around the Covid-19 vaccination campaign (webinars, newsletters…).
Good manipulation practice videos for vaccine references where shot with communication department supervision and editing.
- 3.
The referring vaccination doctor oversaw medical coordination of the hospital's vaccination campaign. They supervised the application of national vaccine recommendations, with frequent updates about prioritizing demographic groups, practice guidelines and safety. They participated in daily meetings with referent vaccination doctors from other AP-HP hospitals, referent pharmacists and direction of care personnel.
- 4.
The referring vaccination pharmacist doctor oversaw vaccination program coordination within all vaccinating departments and was in direct coordination with nurses and care managers. Nurse training and good practice instructions were given by the pharmacist. Information pamphlets for each vaccine reference were created by the pharmacist and validated by the referring vaccination doctor. Supply chain management consisted of: vaccine orders, storage and dispensation. Several issues had to be considered for Comirnaty®: 5-day expiration, doses per vial, supply tensions, 6 h expiration after dilution, and fragility (e.g., UV light, shocks/shaking).
Syringe labels were created containing regulatory information: product name, dosage, batch number, and expiration date and time.
- 5.
Direction of care's role was to coordinate general healthcare. During vaccination deployment, the intern vaccination program was controlled by the direction of care for hospital healthcare workers.
- 6.
Care managers dealt with day-to-day issues such as nurse schedules, nursing table supplies, and appointment schedules. They supervised vaccination activities in their departments.
- 7.
Infection control unit supervised and validated patient and healthcare worker circuit. The team validated nursing table set-up. Their expertize concerned all procedures for infection prevention, cleaning/disinfecting, hand sanitizing and control: implementation of standard and additional precautions during vaccination activity, adequate ventilation distancing at all stations: entrance, screening stages, waiting area and during postvaccination observation. During video shootings for good practice, hygiene staff supervised the environment and assured local good practices.
- 8.
Nurses handled vaccine dilution and dose preparation. They were trained by the pharmacist on: vaccine specificities, manipulation, 6th dose from the Comirnaty® vial, and hygiene requirements for each vaccine reference. Concerning vaccine and syringe manipulation, as doses were counted, pressure was put on nurses so the exact dose required would be administered all while leaving enough volume to vaccinate the exact amount. All nurses involved put together different technics to assure total dose while ensuring six doses per vial. The task became crucial as supply tensions appeared and with one Comirnaty® vial sold based on six doses instead of the five initial doses.
- 9.
Vaccinating doctors assured vaccination consultations and shots. Consultations were essential to detect contra-indications. The doctor registered vaccination data in SI Vaccin Covid platform.
Their schedule was supervised by the referring vaccination medical doctor and they received training before administering a new reference: side effects, contra-indications, type of syringes and target population.
- 10.
Security service filtered the center's entrance according to schedule, as non-eligible individuals tried to counter vaccination program guidelines to benefit from early vaccination.
4 DISCUSSION
4.1 Adaptability and optimization
4.1.1 Vaccination scope
A week after the beginning of healthcare worker vaccination, comorbid patients defined by HAS were able to be vaccinated. A specific circuit was organized to preserve fragile patients. Patient-vaccination was set up in day hospitals for nonhospitalized patients and at patient's bedside. Patients with planned hospital visits received their shot during their scheduled visit and for others; special vaccination-consultations were organized by their medical departments. Current March 2021 with less vaccine shortages, a hospital vaccination center for patients was set up to harmonize patient circuit.
Covid-19 vaccines developed in less than a year without pediatric data. Our hospital having important active files of high-risk pediatric patients: transplant, dialysis, immunodeficiency, and rare genetic diseases, the physicians were in an impasse and requested authorization to vaccinate. In March, the Ministry of Health published a list of very high-risk patients authorized to be vaccinated.6 A mention “out of marketing authorization indications” in the medical file and legal representative consent were necessary. Table 3 retraces patient scope timeline in our hospital throughout the vaccination campaign.6, 8
| Date | Patient scope |
|---|---|
| January 2021 | Beginning of elderly patients >75 years old |
| Beginning of kidney transplant patients and dialysis patients—cohort | |
| Beginning of onco-hematology patients—cohort | |
| March 2021 | Beginning of elderly patients >65 years old |
| Beginning of transplant and dialysis pediatric patients | |
| Beginning of pediatric patients with immunodeficiencies | |
| Beginning of rare genetic disease pediatric patients, ex: APECED7 | |
| Beginning of pregnant women from their 2nd trimester | |
| End of March and April 2021 | Second dose scheduling for cohorts: transplant and dialysis and onco-hematology adult patients |
| May 2021 | Third dose scheduling for cohorts: transplant and dialysis and onco-hematology patients |
| June 2021 | End of patient center vaccination on June 30, 2021 |
Targeted populations evolved frequently with new data, references, and campaign progress.
4.1.2 Vaccine references
After starting patient vaccination, nurses managed to draw six doses from one Comirnaty® vial initially marketed for five doses. On January 8, 2021, Pfizer officially changed its manual mentioning that a vial could contain five or six doses.9 Due to supply tension, pressure to achieve the 6th dose grew. Pharmacists compared manipulation technics. With a new syringe reference limiting dead volume to only the needle volume, a 7th dose was able to be drawn.
Manipulation practices and medical devices impacted the vaccination campaign.
The 2nd vaccine approved in Europe was the Moderna laboratory vaccine, January 6th.10 At first only a few 1000 doses were delivered directly to French regions with the highest contamination rates. The 3rd vaccine approved in Europe was Covid-19 Vaccine AstraZeneca®, an AstraZeneca/Oxford University GMO vaccine (January 29, 2021).11, 12 A separate pharmaceutical circuit was installed to avoid cross contamination between GMO vaccine and RNAm vaccine. For clear identification of circuits, Pfizer's vaccine labels were red while AstraZeneca's vaccine labels were green and an identified vaccination box was dedicated for Covid-19 Vaccine AstraZeneca®.
4.1.3 Vaccine supply tension
Once supply tensions appeared for Comirnaty® at the end of January, our scheduling system had to be adapted. Instead of ordering vaccines according to appointments, appointments were established according to supply availability. End of February, a collegial decision was taken to close 1st dose appointments to ensure all 2nd dose appointments arriving. In addition, 2nd doses were pushed back from 21 to 28 days after 1st shot.
To free ourselves from the constraining 5-day expiration, we organized to retrieve the frozen weekly national attribution of Comirnaty® and store it in our pharmacy's −80°C freezers. We gained in flexibility with a new logistic approach. Shuttles were organized between the group's hospitals to distribute allocations.
4.1.4 Appointment schedules
When there were left over doses at the end of the day, a list of hospitalized high-risk patients permitted their distribution.
When Covid-19 Vaccine AstraZeneca® arrived; the new circuit was set up with the experience of the Comirnaty® deployment. Scheduling was established according to supply and an electronic appointment scheduling was instituted directly
Communication campaigns circulated when Covid-19 Vaccine AstraZeneca® became the only reference available for healthcare workers under 65 years old and was considered a second-choice vaccine. The communication department posted information on its efficacy after real life data was published.13 Gradually, common opinion grew positive.
The first week of May 2021, national guidelines recommended a Comirnaty® 3rd dose for immunodeficient patients such as renal transplant recipients. Appointments for the entire cohorts vaccinated had to be reprogrammed.14
With the diversification of vaccinators: nurses, midwives, pharmacists, and medical students, additional boxes were able to open.15
4.1.5 IT
Several discrepancies occurred at the beginning of the platform launch: new appointment slots opening without authorization, no phone text message confirmation, no cross check of healthcare professional's identification with an official database and no filter on retired healthcare professionals. In addition, individuals quickly understood how to avoid scrutiny and get through the system when not eligible for vaccination. When detected, the issues were corrected making the platform more efficient and an indispensable tool in the vaccination program.
4.1.6 Equipment
Two different nursing tables were required to avoid cross contamination between RNAm and GMO vaccine which allowed bio cleaning after each manipulation of a GMO vial. According to regulatory guidance for GMO, a biological waste with an infectious risk container is required for all unused vaccine and waste material.16 Solid containers were disposed at the identified AstraZeneca box and nursing table.
4.1.7 Positive communication
AP-HP's communication department sent out data every day such as the number of shots injected.
After Comirnaty® arrived and the campaign's first weeks went by, vaccination gained in popularity. In February, with Covid-19 Vaccine AstraZeneca®, pharmacovigilance declarations appeared. Intense and frequent flu-like symptoms mostly in younger individuals caused distrust and suspicions about the vaccine batch used. Communications reassured on side effects to increase compliance and recommended to space out appointments coming from the same medical service to avoid too many absences in medical staff.
In March 2021, pharmacovigilance declarations started to show thrombosis about 10 days after administration. French health ministry decided to postpone vaccinations on March 15th until EMA's decision. On March 19th national guidelines restricted Covid-19 Vaccine AstraZeneca® to individuals above 55 years old after data showing more thrombosis in younger populations.17 The communication department juggled between new target populations and guidelines while trying to reassure healthcare workers reluctant to vaccination.
Tables 4 and 5 sum up the different issues encountered with Pfizer and AstraZeneca vaccines and the adaptive responses put together to continue the vaccination campaign.
| Date | Issue | Adaptive response |
|---|---|---|
| January 8, 2021 | Marketed authorization went from five doses per vial to six doses per vial |
|
| Mid-January 2021 | Complete vaccination dosing for people with previous Covid infection |
|
| End of January 2021 | Supply tensions |
|
| March 29, 2021 | Vaccination strategy for pregnant women |
|
| March 2021 | Possibility of drawing seven doses per vial |
|
| April 2021 | Opening of Comirnaty® to all healthcare workers under 55 years old after AstraZeneca thrombosis pharmacovigilance |
|
| May 6, 2021 | Third dose for certain indications such as immunodeficient patients |
|
| May 10, 2021 | Decrease and then stop of hospital vaccinating centers within sight of the opening of all population vaccination centers across France |
|
| May 18, 2021 | Stability up to 1 month after thaw +4°C (no longer 5 days) |
|
| June 1, 2021 | Residual vaccinating offer for patients and healthcare workers |
|
| Date | Issue | Adaptive response |
|---|---|---|
| January 29, 2021 | General opinion: reluctance in front of efficacity and type of vaccine |
|
| February 2021 | Few volunteers above 50 years old. Important percentage already vaccinated with Comirnaty® |
|
| Mid-February 2021 | Pharmacovigilance declarations of intense flu-like syndrome |
|
| March 15, 2021 | Pharmacovigilance declarations of thrombosis |
|
| March 19, 2021 | Vaccination allowed only above 55 years old |
|
4.2 Success
4.2.1 Vaccination center opening
The vaccination center was ready for opening the next morning after the crisis meeting, January 4th. Three vaccinating boxes were assembled; nursing tables and emergency carts were set up. Information posters were displayed and a postvaccination area installed. Comirnaty® vials arrived at the pharmacy department in the evening of January 4th. A good practice pamphlet was created, and the nurses received their training at 8:00 a.m. January 5th. The 1st dose was injected at 9:00 a.m. January 5th. In less than 24 h, the hospital vaccination center opened after great organization and cooperation of all professionals involved.
4.2.2 Vaccination rates
When vaccinations started there was no supply tension. Shortages began end of January 2021. With supply tensions the vaccination center's opening went from 7 days/7 to 5 days/7. In 1 month, from January 4th to February 1st, 2757 1st doses of Comirnaty® were administered to healthcare workers and patients (Figure 2). Week 1, maximum rate was 165 shots/day and week two's maximum went up to 246 shots/day.
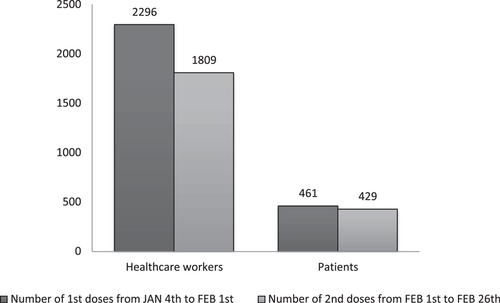
Comirnaty® second dose vaccination started on February 1st. From February 1st to February 26th, 2238 2nd shots were delivered (Figure 2). The smaller number of 2nd shots versus 1st shots can be explained by the number of Covid-19 contaminations between both doses and 2nd shots delivered in other establishments. In addition, local recommendations determined on a single dose if the individual had previously contracted Covid-19.
AstraZeneca vaccinations started on February 9th, for all healthcare workers. From February 9th to 26th, 300 healthcare workers were vaccinated. Rates started around 10 vaccinations per day. After a week, AstraZeneca's vaccine popularity began to rise and reached up to 30 a day.
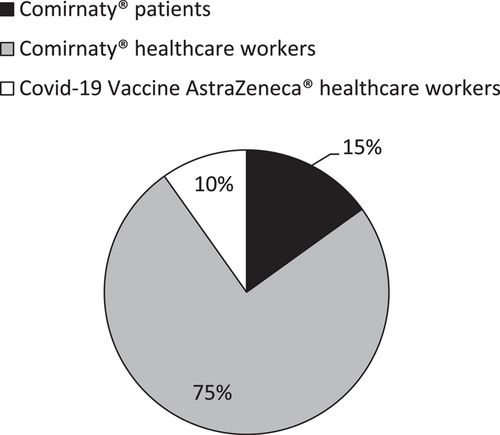
In total, 3057 1st doses were injected from January 4th to February 26th 2021, despite issues such as shortages and appointment schedule discrepancies. Figure 3 shows the distribution of 1st doses between vaccine references and targeted population.
4.2.3 Good manipulation practice
With the official 6 dose per vial announcement by Pfizer and EMA, it became a real challenge to elaborate the best technic to draw six complete syringes. The hospital's method, which implied an air bubble the size of the dead volume (syringe and needle), was chosen to be AP-HP's reference method in all hospitals.18 A good practice video was made with the communication department. The nurse went through every step with pharmacy and hygiene department guidance.
Before Covid-19 Vaccine AstraZeneca® was deployed for general doctors, the pharmaceutical laboratory contacted our hospital to shoot a good practice video. Quality documents, videos and pamphlets were created for staff and external distribution.
4.2.4 Interdisciplinary coordination
Nine different professions came together to make the vaccination campaign possible. Crisis meetings took place several times a day and programmed meetings several times a week to share problems, information, and successful steps.
Meetings also took place between our hospital and two university hospitals from the same group. Sharing and mutual aid occurred during these intense weeks.
Such an organization while facing last minute information and unexpected issues would not have been possible without good communication and mutual support between all stakeholders.
4.3 Limits
4.3.1 Vaccines
During January 2021, the only vaccine available was Comirnaty®. Five day stability caused us to have vials expiring the next day. The first time we managed to send vials to other hospitals across Paris. The second time, less vials were left so we advanced second-dose appointments and vaccinated unscheduled healthcare professionals that had already contracted Covid-19 3 months earlier and who only needed one shot to finish they vaccination program, as recommended by health authorities.19
4.3.2 Supply tension
The vaccination center was first organized around productivity. When hospital vaccination started, there was great pressure from the government to vaccinate high rates to counter the pandemic and emergence of Covid-19 variants. The first yield was one vaccination every 15 min. Doctors then estimated being able to go up to one vaccination every 10 min and adding a fourth vaccination box. This organization lasted for two and a half weeks. Before we could scale up, French authorities declared the opening of vaccination centers in town and a shortage of doses available for hospitals. By the end of February, we did not have enough doses to ensure both 2nd doses and new 1st doses.
4.4 Wrapping up of the hospital vaccination centers
January 2021, at the beginning of the national vaccination campaign, hospitals helped remedy lack of vaccine structures. Five months later, town vaccination centers were organized in gymnasiums and exposition centers throughout the country.
With HAS enlargement of population eligibility, vaccination to general population above 18 years old opened on May 31, 2021. In May, each AP-HP hospital organized a slowing down plan.
Our timing was to stop 1st dose injections on May 28th to close patient vaccination center on June 30th: 28 days between 1st and 2nd dose, and healthcare worker vaccination center on July 8th: 42 days between 1st and 2nd dose.
A residual vaccination procedure to continue guaranteeing access to Covid-19 vaccination was: orienting AP-HP healthcare workers to one unique AP-HP hospital and offer residual patient vaccination in each hospital for specific situations. Vaccine doses were centralized and prepared by the pharmacy department 2 days a week upon a schedule based on a multiple of seven.
After 6 months of vaccination, our balance sheet was 9167 1st dose shots: 4000 intern professionals, 4042 patients and 1125 outside healthcare professionals. Percentage for intern healthcare professionals vaccinated was, for medical professions versus nonmedical professions respectfully 95% (1326 for a target of 1394) and 59% (1835 for a target of 3105). Awareness was crucial for public health. Compliance in this pandemic was a key issue. One can reflect on Covid-19 vaccination becoming mandatory for all healthcare workers, to avoid nosocomial infections.
5 CONCLUSION
- 1.
Efficiency, for how rapidly the center became operational
- 2.
Interdisciplinarity, for the number of different professionals who worked together to make organization possible
- 3.
Adaptability, for last minute decisions in front of supply tensions, evolving target population recommendations, new regulatory information, arriving vaccine references and public opinion.
Finally, coordination and communication helped us in the battle against the Covid-19 pandemic. Vaccination campaigning in our hospital was a privilege and brought hope after a year of lock-downs. The widening of vaccinators helped expand the French vaccination campaign. The national vaccination campaign is now opened to all public in vaccination centers, pharmacies, and general physicians. Public hospitals maintain a low vaccination activity for specific patients on demand.
AUTHOR CONTRIBUTIONS
Scarlett Wise: Writing – original draft; writing – review and editing. Fanny Lanternier: Validation; writing – review and editing. Camille Cotteret: Validation; writing – review and editing. Céline Chasport: Resources. Virginie Juin-Leonard: Resources. Amélie Cantat: Resources. Anne Scemla: Resources. Claire Delage: Resources. Barbara Mantz: Resources. Caroline Telion: Resources. Pierre Carli: Resources. Pierre Frange: Validation; writing – review and editing. Salvatore Cisternino: Validation; writing – review and editing.
ACKNOWLEDGMENTS
Authors thank Mrs Vilayleck and M Lissillour for their help in coordinating with other AP-HP hospitals.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ETHICS STATEMENT
Authors confirm acknowledgment with the Journal's position on ethical publication issues and affirm consistency with guidelines.
TRANSPARENCY STATEMENT
The lead author (Dr Scarlett Wise) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
All data availlable is presented in the manuscript.