Electroacupuncture Improves Sepsis-Induced Acute Gastrointestinal Injury: A Retrospective Propensity Score-Matched Cohort Study
Yi Yu, Bojun Zheng, and Jian Xu contributed equally to this study.
ABSTRACT
Background and Aims
There is limited research on the clinical efficacy of electroacupuncture (EA) for sepsis-induced acute gastrointestinal injury (S-AGI). This study aimed to examine the effects of EA at “Zusanli” (ST36) and “Guanyuan” (RN4) on S-AGI.
Methods
We identified 255 patients with S-AGI from March 2018 to September 2021 who underwent treatment at the Department of Critical Care Medicine, the Second Affiliated Hospital of Guangzhou University of Chinese Medicine. Among these patients, 50 received EA, and 203 did not. After performing 1:2 propensity score matching by sex, age, baseline comorbidity, infection source, laboratory results, and AGI classification, there were 100 patients in the non-EA cohort. In addition to conventional therapies, patients in the treatment group underwent 30 min of EA at ST36-RN4 twice a day for 7 days. The 28-day mortality was recorded.
Results
Our study included 150 participants diagnosed with S-AGI, with an average age of 70.3 years. Kaplan–Meier survival analysis indicated an association between EA treatment and significantly lower 28-day mortality. Adjusted multivariable Cox models consistently suggested a significant reduction in the prevalence of mortality that was associated with the use of EA. After accounting for confounding factors, there was an observed 54% decrease in 28-day mortality among patients who received EA (hazard ratio [HR]: 0.46, 95% confidence interval [CI]: 0.22–0.95, p < 0.05). Subgroup analyses further supported these associations.
Conclusion
There is an indication that EA at ST36-RN4 may be associated with protective effects for patients with S-AGI.
1 Introduction
It is well known that the intestine is cross-linked with many important organs of the body and that its functions affect those of other important organs [1]. Sepsis itself can cause intestinal dysfunction, reduced peristalsis, and damage to the intestinal barrier function, leading to the translocation of intestinal microbes and endotoxins [2, 3]. The digestive tract serves as both a site of bacteremia and a crucial recipient of bacterial products [4]. The gut produces cytokines that ultimately contribute to the induction and exacerbation of multiple-organ dysfunction syndrome (MODS) [5]. The development of MODS aggravates acute intestinal injury and accelerates patient death [6]. Given the significant contribution of the gut to the forecasting of sepsis, what interventions can healthcare providers apply to enhance effectiveness of treatment?
Electroacupuncture (EA) at “Zusanli” (ST36) can reduce the degree of sepsis inflammation [7, 8]. The antiseptic properties of this mechanism may entail mitigating oxidative stress and inflammatory response, enhancing microcirculation, and sustaining dopamine-mediated immune balance [9, 10]. A previous study found that EA improves S-AGI, and the mechanism may involve immune regulation; however, the specific regulatory mechanism remains unclear [11]. A study has shown that EA improves the prognosis of animal models of sepsis by inducing the vagal activation of the aromatic L-amino acid decarboxylase and mediating dopamine production in the adrenal medullary, thus controlling systemic inflammation [10]. EA at ST36 has anti-inflammatory and antioxidant effects, accelerates the recovery of gastrointestinal function in patients with sepsis, and protects the intestinal mucosal barrier function. The index of intestinal injury and quantitative score of intestinal dysfunction in a Traditional Chinese Medicine (TCM) study decreased more significantly than that in the control group [12]. In a study of EA treatment for S-AGI, EA at ST36 increased the expression of intestinal tight junction proteins, reduced serum D-lactic acid production, and improved the permeability of the intestinal mucosal barrier [13]. Thus, EA could be a treatment for S-AGI. Nevertheless, research in this field remains limited, and the clinical effectiveness of this treatment remains uncertain.
We wished to investigate the potential therapeutic benefits of EA in relation to the prognosis of patients with AGI. Thus, here, a retrospective analysis was conducted on a cohort of 150 critically ill patients from 2018 to 2021. We aimed to test the hypothesis that EA use in patients suffering from AGI is associated with decreased mortality.
2 Methods
The preparation of this report adhered strictly to the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology [14].
2.1 Ethical Considerations
Ethical approval for this study was obtained from the ethics committee of Guangdong Provincial Hospital of Chinese Medicine (approval no. AF/04-07.0/10.0). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
2.2 Study Population
This study was conducted in a 19-bed intensive care unit (ICU) of a university-affiliated tertiary-care hospital. Patients admitted to the ICU between March 2018 and September 2021 were evaluated for study inclusion. Sepsis was diagnosed according to the criteria outlined in the Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock [15], and intestinal dysfunction was diagnosed according to the criteria outlined in Gastrointestinal Function in Intensive Care Patients: Terminology, Definitions, and Management [16]. The exclusion criteria were: death within 24 h; patients who needed cardiopulmonary resuscitation; advanced malignant tumor; abnormal coagulation mechanism, such as hemophilia; pregnancy; recent abdominal trauma with the risk of digestive tract perforation; pacemaker implantation. In all, 255 patients were identified with an International Classification of Disease (ICD)-9 discharge diagnosis code of sepsis, and these patients were all diagnosed with acute bowel injury. To reduce confounding factors, 1:2 propensity score matching by sex, age, comorbidity, infection source, laboratory results, and AGI classification through multiple logistic regression analysis was used. The following patients were included after a review of their medical records. Patients (male or female) aged > 18 years and with S-AGI were included. For the EA treatment at ST36-RN4, sessions lasted 30 min and took place twice a day for 7 days. The pattern was set to a continuous wave and the current intensity was adjusted to the maximum tolerable level for the patient.
2.3 Patient Selection for EA
In the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, EA is a routine diagnostic and treatment measure. Clinical doctors assess patients' eligibility for EA treatment, and patients sign a consent form for EA upon admission to the ICU. The selection of EA patients was based on historical medical records, and no active patient selection was carried out during the study.
2.4 Data Extraction
For all patients, the characteristics documented at baseline were age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, comorbidities, infection source, laboratory results, and AGI classification. Data collection was completed by two independent authors.
2.5 Propensity Score Matching (PSM) and Covariate Selection
PSM was employed to enhance the robustness of our results using a 1:1 nearest neighbor-matching algorithm with a caliper width of 0.1. We used Stata module (FILLMISSING) to fill missing values in numeric or string variables. Considering the relatively low percentage of missing data (ranging between 0.3% and 5%), the missing data in vital signs and laboratory parameters (accounting for 5% of the total) were replaced with the median value. We used subgroup analysis for the sensitivity analysis in this study. The principle for selecting covariates was that they were not affected by treatment. Potential outcomes were independent of treatment assignment. Based on the research background, we chose the covariates sex, age, baseline comorbidity, infection source, laboratory results, and AGI classification.
2.6 Statistical Analyses
The patients were monitored for 28 days after enrollment or until death. Their baseline characteristics were assessed within 24 h before enrollment. The independent samples t-test was applied for continuous variables following a normal distribution (checked by the Shapiro–Wilk test with p > 0.05) and independent EA and conventional groups (e.g., age, ICU stay length); the paired t-test was used for paired data like pre- and post-treatment measurements within each patient; the Mann–Whitney U test was for non-normally distributed continuous variables (Shapiro–Wilk p < 0.05) and independent groups; the chi-square test was for comparing categorical variables (e.g., gender, comorbidities); and Fisher's exact test was used when the sample size was small and expected frequencies in contingency table cells were less than 5. Values are shown as mean ± SD. The Kaplan-Meier and log-rank tests were used to estimate differences in the cumulative incidence of mortality between the two cohorts. Cox proportional hazards regression analysis was performed to obtain the hazard ratios (HRs) with 95% confidence intervals (Cis) after adjusting for each covariate. Furthermore, subgroup analyses were undertaken to examine the consistency of our findings across different subgroups. p-values < 0.05 were considered statistically significant. Data were analyzed using SPSS software (version 25.0, USA) and STATA software (version 17.0, USA).
3 Results
3.1 Participants and Baseline Characteristics
Figure 1 shows the screening and matching process for the study participants. A total of 50 S-AGI patients who received EA treatment were enrolled between 2018 and 2021, and a 1:2 propensity score was used to match a cohort of 100 participants as controls. The no-EA and EA cohorts showed no significant differences in the sex, age, baseline comorbidity, infectious source, laboratory results, and AGI classification. The demographic comparisons are presented in Table 1.

| Variable | Total (n = 150) | Conventional (n = 100) | EA (n = 50) | p value |
|---|---|---|---|---|
| Age, years | 70.3 ± 12.9 | 70.6 ± 12.5 | 69.8 ± 13.8 | 0.74 |
| Sex (male) | 107 (71.3) | 70 (70) | 37 (74) | 0.61 |
| Comorbidities (n, %) | ||||
| Cardiovascular disease | 48 (32.0) | 32 (32) | 16 (32) | 1 |
| Cerebrovascular disease | 65 (43.3) | 45 (45) | 20 (40) | 0.56 |
| COPD | 19 (12.7) | 11 (11) | 8 (16) | 0.38 |
| Chronic kidney disease | 35 (23.3) | 23 (23) | 12 (24) | 0.89 |
| Diabetes mellitus | 56 (37.3) | 39 (39) | 17 (34) | 0.55 |
| Malignant tumor | 18 (12.0) | 15 (15) | 3 (6) | 0.11 |
| Infectious source (n, %) | ||||
| Lung | 119 (79.3) | 79 (79) | 40 (80) | 0.89 |
| Urinary | 20 (13.3) | 9 (9) | 11 (22) | 0.03 |
| Abdominal | 17 (11.3) | 13 (13) | 4 (8) | 0.36 |
| Other | 17 (11.3) | 14 (14) | 3 (6) | 0.15 |
| Laboratory results | ||||
| WBC count (×109) | 13.6 ± 7.2 | 13.6 ± 7.4 | 13.6 ± 7.0 | 0.98 |
| Hb (g/dL) | 101.3 ± 27.2 | 100.0 ± 24.6 | 104.0 ± 32.0 | 0.40 |
| Platelets (×109) | 204.3 ± 118.5 | 206.0 ± 122.8 | 200.8 ± 110.3 | 0.80 |
| ALT (U/L) | 20.0 (11.0, 44.8) | 20.0 (9.0, 48.5) | 20.5 (12.0, 32.5) | 0.86 |
| AST (U/L) | 31.0 (18.0, 69.5) | 30.0 (18.0, 68.0) | 37.5 (15.8, 74.2) | 0.73 |
| Blood creatinine (μmol/L) | 107.0 (66.0, 255.8) | 121.5 (68.8, 260.2) | 97.0 (64.0, 223.8) | 0.38 |
| BUN (mmol/L) | 12.5 ± 9.5 | 12.9 ± 9.9 | 11.8 ± 8.5 | 0.51 |
| pH value | 7.40 (7.36–7.45) | 7.41 (7.36–7.44) | 7.40 (7.35–7.45) | 0.97 |
| PaO2 (mmHg) | 120.0 ± 48.9 | 123.9 ± 48.7 | 112.1 ± 49.1 | 0.17 |
| Lactate (mmol/L) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 0.49 |
| Serum iron (µmol/g protein) | 12.63 ± 8.9 | 12.75 ± 9.28 | 12.57 ± 8.75 | 0.90 |
| AGI classification | ||||
| AGI I | 38 (25.3) | 25 (25) | 13 (26) | 0.89 |
| AGI II | 40 (26.7) | 30 (30) | 10 (20) | 0.19 |
| AGI III | 59 (39.3) | 40 (40) | 19 (38) | 0.81 |
| AGI IV | 13 (8.7) | 5 (5) | 8 (16) | 0.03 |
| ICU stay, days | 6.0 (4.0, 12.0) | 7.0 (4.0, 13.0) | 5.5 (4.0, 10.0) | 0.24 |
| APACHE-II | 22.7 ± 7.2 | 23.2 ± 7.4 | 21.6 ± 6.8 | 0.20 |
- Note: For each variable, mean ± standard deviation, median (interquartile range), or number (percent) is reported, as appropriate.
- Abbreviations: APACHE, acute physiology and chronic health evaluation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; EA, electroacupuncture; Hb, hemoglobin; PaO2, arterial partial pressure of oxygen; S-AGI, sepsis-induced acute gastrointestinal injury; WBC, white blood cell.
3.2 Relationship Between EA Use and S-AGI Mortality
In univariate analysis, there was an association between the use of EA and a significantly reduced 28-day mortality compared with the conventional cohort (HR = 0.52, 95% CI: 0.27–1, p = 0.05) (Table 2).
| HR | 95% CI | p value | |
|---|---|---|---|
| Model 1 | 0.52 | 0.27–1 | 0.05 |
| Model 2 | 0.49 | 0.26–0.94 | 0.03 |
| Model 3 | 0.49 | 0.25–0.97 | 0.04 |
| Model 4 | 0.44 | 0.21–0.88 | 0.02 |
| Model 5 | 0.46 | 0.22–0.95 | 0.04 |
- Note: Adjustments: Model 1: No adjustment. Model 2: Age, sex, AGI classification. Model 3: Model 2 + ICU stay days, continuous renal replacement therapy, APACHE-II, ventilation, lactate. Model 4: Model 3 + infectious source, white blood cell count, hemoglobin, platelets, arterial partial pressure of oxygen. Model 5: Model 4 + diabetes mellitus, chronic kidney disease, cerebral vascular disease, cardiovascular disease, chronic respiratory disease, malignant tumor.
- Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 2 shows a significant intergroup difference in the 28-day survival rate: the survival rate of the EA group is significantly higher than that of the conventional group. A log-rank test was conducted to more accurately evaluate the difference between the two groups, and the results still showed a significant intergroup difference (p < 0.05).

We examined the HRs with 95% CIs in univariate and multivariate Cox regression analyses to investigate the potential association of EA with the prevalence of mortality within 28 days (Table 2). We observed that the HRs for EA use were consistently significant in all models (HR range 0.44–0.49, p < 0.05 for all). In comparison with the conventional cohort, patients who received EA treatment showed an association with a lower risk of 28-day mortality (adjusted HR = 0.46, 95% CI = 0.22–0.95, p = 0.04) after adjustment for age, sex, ICU stay days, AGI classification, diabetes mellitus, chronic kidney disease, cerebral vascular diseases, cardiovascular disease, chronic respiratory disease, tumor disease, APACHE II score, continuous renal replacement therapy, ventilation, white blood cell count, hemoglobin, platelets, arterial partial pressure of oxygen, lactate, and infection source.
3.3 Subgroup Analysis and Sensitivity Analysis
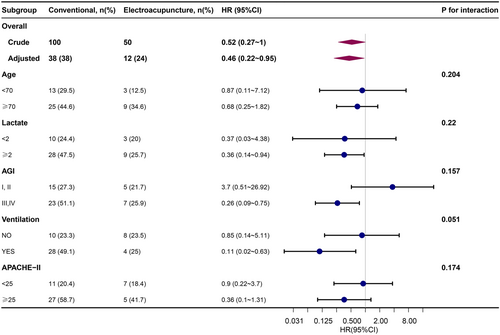
Subgroup analyses indicated the durability and dependability of the association between EA use and the outcome in the ICU. Specifically, the use of EA was found to be associated with a more substantial protective impact among individuals requiring mechanical ventilation and those classified as AGI III or IV. No other significant interactions were observed in the subgroups (Pinteraction > 0.05) (Figure 3).
4 Discussion
4.1 Main Findings
Our study presents a cohort analysis of the effect of EA use on mortality in patients with S-AGI. The findings suggest an association where patients with S-AGI who received EA had a reduced risk-adjusted 28-day mortality rate compared with those who did not receive EA. Notably, these results remained consistent even after adjusting for potential confounders such as demographic factors, laboratory results, infection source, severity-related variables, comorbidities, and treatments. We also analyzed the individual and combined effects of these covariates on the relationship between EA use and mortality, which will help to more comprehensively understand the impact of EA on the prognosis of S-AGI patients. Thus, our study provides compelling evidence supporting the potential advantages of EA use in enhancing outcomes for S-AGI patients.
4.2 Effect of Using EA on Mortality in Patients Suffering From S-AGI
This is the first retrospective trial investigating the effectiveness of a 7-day course of EA treatment in patients with S-AGI. In this study, we found that EA treatment was associated with a significant decrease in patients' mortality on day 28 (p < 0.05). It was also observed that EA use was associated with a reduced 28-day mortality rate among patients with S-AGI. Compared with previous studies [17-19], our study further adjusted for infection source, comorbidities, and treatments and still observed a significant effect of EA on reducing mortality, which provides more comprehensive evidence for the role of EA in S-AGI patients. EA at points ST36-RN4 can regulate the motility of the intestinal tract and promote passage of stool; simultaneously, it can also improve intestinal inflammation. The main therapeutic indication of EA at ST36 and RN4 is digestive system disease, and it performs the functions of dredging excretory organs and immune regulation [20]. EA at the acupoints of the Stomach Meridian at Foot Yangming can reduce the plasma levels of TNF-β and IL-6 in patients with sepsis and inhibit the progression of inflammation. EA at ST36 can shorten the recovery time of bowel sounds, ameliorate defects, and promote the recovery of gastrointestinal function in patients undergoing abdominal surgery [21, 22]. EA at ST36 can improve the gastrointestinal barrier membrane of mucus by improving neuroregulation, regulating the secretion of gastrointestinal hormones, enhancing blood supply to the gastrointestinal mucosa, and removing inflammatory mediators [19]. However, the specific anti-inflammatory mechanism of EA is still unclear. Therefore, our study shows an association where EA may be related to an improvement in the intestinal inflammation of patients with S-AGI and a reduction in the 28-day mortality of S-AGI.
The function of the gastrointestinal tract is closely related to the prognosis of sepsis [4, 23]. Imbalances in intestinal motility and destruction of the intestinal barrier are prone to cause translocation of bacteria and toxins in the intestine, aggravating intestinal infections and promoting the pathophysiological process of MODS, which leads to a vicious cycle of explosive infections and inflammatory factor storms [3, 24]. Researchers have gradually discovered that the gastrointestinal tract is not only a target organ for exacerbation of sepsis but also a triggering organ for further damage [23, 25]. Modern medical treatment mainly focuses on anti-infection, stomach protection, and intestinal microbiota, but it cannot fundamentally improve the gastrointestinal function of patients. Moreover, antibiotic resistance, the use of mechanical ventilation, and the deployment of artificial heart, lung, and kidney replacement technologies can potentially cause further damage to gastrointestinal function [26, 27].
EA reduces intestinal inflammation and improves the outcome of S-AGI. The underlying mechanism by which EA alleviates S-AGI intestinal inflammation is unclear. In an experiment involving EA treatment of S-AGI, EA at ST36 was shown to increase the expression of intestinal tight junction protein, reduce serum d-lactic acid level, and improve the permeability of the intestinal mucosal barrier [13]. EA at ST36 performs anti-inflammatory and antioxidant functions, accelerates the recovery of gastrointestinal function in patients with sepsis, and protects the intestinal mucosal barrier function [12]. Recent studies have revealed the intrinsic relationship between ferroptosis and acute intestinal injury. Ferroptosis can regulate the inflammatory cascade at multiple levels and stages, and is closely related to organ function damage in sepsis [28]. Ferroptosis is excessively activated in AGI, which is evidenced by a decline in expression of superoxide dismutase and glutathione peroxidase 4, and a decrease in the level of glutathione. Iron overload, reactive oxygen species, and malondialdehyde production contribute to this increase [29, 30]. These aspects should be verified in future basic experiments.
4.3 Strengths of Our Study
Our study had three main strengths. First, we employed a self-constructed database, ensuring the reliability and comprehensiveness of our data. Our study also used well-defined diagnostic criteria for S-AGI, enhancing the accuracy and validity of our findings. Different from previous studies that may have overlooked comorbidities and treatments, our study included infection source, diabetes mellitus, chronic kidney disease, cerebral vascular disease, cardiovascular disease, chronic respiratory disease, malignant tumor, ventilation and continuous renal replacement therapy in the models, which allows for a more comprehensive analysis of the relationship between EA use and mortality in individuals with S-AGI. Second, no prior research has specifically investigated the effects of EA use on mortality risk in S-AGI patients. Our study shows an association suggesting that the use of EA may be related to a decrease in the 28-day mortality among individuals with S-AGI. Third, we used multiple regression analysis to establish the robustness and reliability of our outcomes. This rigorous analytical approach bolsters the credibility and internal validity of our results.
4.4 Limitations of Our Study
Our study also had several limitations. A major limitation of this single-institute study is the relatively small sample size. Specifically, the treatment group consists of only 50 patients. The small sample size may not be representative of the broader population of S-AGI patients, and thus, the results may not be applicable to other clinical settings or patient cohorts. Another limitations of this study is its single-center nature. The research was carried out in a single traditional Chinese medicine hospital, which may lead to the results not being generalizable to other hospitals with different patient demographics, medical cultures, and treatment protocols. Future multi-center studies involving a more diverse range of hospitals, including both traditional Chinese medicine and Western-medicine hospitals, are needed to validate and expand our findings. In addition, this retrospective study had a potential selection bias. Therefore, more prospective studies with a larger cohort of subjects are needed to support the present findings. Another limitation was that there is no specific mechanism to explore, and further animal experiments are required to explore potential mechanisms.
5 Conclusions
Our study shows an association suggesting that performing EA at ST36-RN4 acupoints may be related to an enhanced 28-day survival rate of patients diagnosed with S-AGI. This discovery highlights the significance of using EA at ST36-RN4 acupoints as a nonpharmacological therapeutic approach for ameliorating S-AGI. Our research provides valuable scientific evidence and offers new perspectives for TCM treatment strategies aimed at addressing S-AGI. However, it is important to note that this study was limited to a single-center retrospective analysis, and further, extensive, multicenter clinical investigations and fundamental research are imperative to elucidate the underlying mechanisms involved.
Author Contributions
Yi Yu: writing – original draft, software, project administration, funding acquisition. Bojun Zheng: methodology, writing – original draft. Jian Xu: investigation, writing – original draft. Jian Li: writing – review and editing, visualization, validation, resources, supervision.
Acknowledgments
We would like to thank the native-English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This study was supported by the National Natural Science Foundation of China (grant number 82104989).
Ethics Statement
The study was approved by the ethical committee of the Guangdong Provincial Hospital of Chinese Medicine (approval no. AF/04-07.0/10) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Jian Li affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
The data utilized in this study are available upon reasonable request from the corresponding author and are subject to Institutional Review Board guidelines.