Advanced Imaging Techniques (PET, fMRI, DTI) in Early Detection of Neurodegenerative Diseases: A Systematic Review
ABSTRACT
Background
Neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and vascular and frontotemporal dementia (FTD), are characterized by progressive cognitive and motor decline. So, timely detection, especially early in the disease process, is crucial. Positron Emission Tomography (PET), Functional Magnetic Resonance Imaging (fMRI), and Diffusion Tensor Imaging (DTI) are advanced neuroimaging techniques that have shown promise for early diagnosis.
Objective
This review evaluates the diagnostic accuracy and clinical utility of PET, fMRI, and DTI in the early detection of neurodegenerative diseases.
Methods
A systematic search was conducted using PubMed, Google Scholar, and Cochrane Library for studies published between 2014 and 2024. Inclusion criteria focused on phase 2 and 3 clinical trials involving adult patients with AD, PD, and FTD. Studies were assessed for diagnostic accuracy, sensitivity, specificity, and identification of early biomarkers using PET, fMRI, and DTI. Data were extracted and analyzed from 14 selected studies.
Results
PET imaging with tracers like 18F-flortaucipir provided visualization of amyloid and tau aggregates in AD and dopaminergic changes in PD. PET showed a strong association with amyloid and tau pathology in AD, with up to 95% diagnostic performance. Another useful technique in identifying early changes in the brain networks was resting-state fMRI (rs-fMRI), with a diagnostic accuracy of 80%–95%. DTI offered essential data on white matter connectivity and showed microstructural alterations that pointed to early neurodegenerative processes. Integrating these neuroimaging modalities with machine learning models further enhanced diagnostic accuracy.
Conclusion
PET, fMRI, and DTI are valuable tools for the early diagnosis of neurodegenerative diseases. These techniques can identify structural and functional changes in the brain before the onset of clinical signs. Integrating these imaging techniques with machine learning improves diagnostic outcomes. Further large-scale studies with standardized methodologies are needed to validate these findings and implement these techniques in clinical practice.
1 Introduction
Alzheimer's disease (AD) [1, 2], Parkinson's disease (PD), Vascular dementia (VD), and frontotemporal dementia (FTD) are regarded as some of the most significant diseases of the present time because of their progressive course and the involvement of cognitive-motor functions [3]. These disorders, especially Alzheimer's disease and Parkinson's, can be associated with autonomic nervous system impairment, which can even increase the risk of arrhythmias like atrial fibrillation [4]. These disorders should be diagnosed at an early stage so that appropriate measures can be taken, which may slow down the progression of the disease and enhance the life of the patients [3, 5]. The present approaches to diagnosing these conditions are clinical examination and neuropsychological tests, which are not very helpful in early diagnosis of the conditions when intervention is most useful [6]. New technology in imaging has brought a big change in imaging the brain and has been useful in early detection as well as the tracking of neurodegenerative illnesses. Among the mentioned techniques, Positron Emission Tomography (PET), Functional Magnetic Resonance Imaging (fMRI), and Diffusion Tensor Imaging (DTI) can be regarded as the most advanced [7]. All of these techniques provide information on the structural and functional organization of the brain and help in the detection of early changes that occur before the manifestation of the disease. New tracers enable the visualization of pathological processes in vivo using PET, for instance, amyloid and tau protein deposits in AD and alterations in the dopaminergic system in PD [8]. Such molecular imaging capacities have located PET at the epicenter of the identification of the first molecular alterations that define neurodegeneration. fMRI is a noninvasive technique that measures the brain activity based on the changes in blood flow. It is especially helpful when investigating the cohesiveness of connections and networks that are engaged in the initial stages of neurodegenerative diseases. Rs-fMRI has been applied in the examination of functional alterations in which the patient does not perform any tasks applicable to various clinical populations. DTI is an advanced MRI technique that relies on the assessment of water molecules' diffusion in the white matter tracts [9]. This technique provides information on the health of the microstructure of white matter that is equally impacted in neurodegenerative diseases. FA and MD obtained from DTI can be helpful in the early identification of white matter degeneration and, thus, neurodegenerative diseases [10].
1.1 Rationale
AD, PD, and FTD are neurodegenerative diseases that cause gradual neuronal loss, resulting in severe cognitive and motor dysfunction [11, 12]. Indeed, the conventional methods of assessment that rely on clinical observations and cognitive tests do not help in the early diagnosis of these diseases when interventions are most helpful. This is important so that the recommended treatments that may help to control the progress of the disease and, consequently, the fate of the patients can be initiated. PET, fMRI, and DTI appear to have the possibility of identifying early pathological changes in the brain before clinical manifestations. As such, these modalities can help to define early biomarkers and improve diagnostic accuracy by offering specific information about the structural and functional integrity of the brain.
1.2 Objectives
This systematic review aimed at assessing the diagnostic performance of PET, fMRI, and DTI in detecting neurodegenerative diseases such as AD, PD, and FTD at their preliminary stages. The purpose of this review is to evaluate the diagnostic accuracy of these imaging techniques in depicting early pathological changes of these diseases. Also, the review aims to identify how these imaging techniques can be integrated with machine learning models for enhanced diagnosis. This systematic review, therefore, seeks to summarize the recent findings on the clinical applicability of PET, fMRI, and DTI and to highlight areas that require further investigation in future research.
2 Methodology
2.1 Inclusion Criteria
This systematic review was based on the following inclusion criteria: Studies that gave particular information on how such techniques as PET, fMRI, and DTI can be used in the early diagnosis of neurodegenerative diseases were considered. In all reviewed research, only patients over the age of 18 with neurodegenerative diseases took part; the study aimed at targeting the population of adult patients. The review was mainly done on phase 2 and 3 trials, and the information given on clinical trial methods gave an insight into the efficacy and safety of these imaging procedures. These trials may have been of an open-label design, DBPC, or RW design. Regarding the evaluation of the outcomes, the authors identified descriptors of outcomes as sensitivity, specificity, diagnostic accuracy, and identification of early biomarkers. Regarding the article selection, the inclusion criteria required the articles to be only those published in peer-reviewed journals during 2014–2024 and in the English language to exclude the use of low-quality, non-English data. In this review, “early pathological changes” were defined as biomarker or imaging-based abnormalities detectable before the onset of overt clinical symptoms or during prodromal phases such as Mild Cognitive Impairment (MCI). This included amyloid and tau deposition, dopaminergic decline, functional disconnection, and white matter microstructural alterations.
2.2 Exclusion Criteria
The criteria for exclusion were intended to prevent studies that would be irrelevant to the context of the review from being included. Such populations as children or animals were not included. In addition, articles that used PET, fMRI, and DTI in a secondary manner were also excluded to filter out the studies where these techniques were not the main focus. Other excluded study designs were observational studies, case reports, reviews, editorials, and nonclinical comparative studies to limit the review to clinical trial-level evidence.
2.3 Database Search
The database search for this systematic review employed PubMed, Google Scholar, and Cochrane Library to obtain papers on PET, fMRI, and DTI in relation to the efficiency and safety of the early detection of neurodegenerative diseases. The search terms were composed of different synonyms and connected words for neurodegenerative diseases, early diagnosis, and each imaging method. A detailed search strategy can be found in the Supporting Information S1: Table S1.
2.4 Screening of Studies
The systematic review involved a comprehensive literature search across three major databases: The sources used in the current literature search included PubMed, Google Scholar, and the Cochrane Library. Searching through PubMed, using the keywords “neurodegenerative diseases”, “early diagnosis”, “PET”, “fMRI”, and “DTI”, and limiting the results to randomized controlled trials from the years 2014 to 2024, 151 results were obtained. Google Scholar was searched using the following terms: Neurodegenerative diseases AND Parkinson's disease AND dementia AND Alzheimer's disease early detection AND PET AND fMRI AND DTI, and the search returned 3100 hits. The search made in Cochrane Library using the keywords “neurodegenerative diseases”, “fMRI”, “PET”, and “DTI” yielded 621 papers.
2.5 Outcome Measures
The main outcome of this systematic review was the diagnostic performance of PET, fMRI, and DTI in identifying early AD alterations. The secondary objectives of the study were to determine early markers, sensitivity, specificity, and the possibility of tracking disease progression. Other assessed parameters included the shift in cognitive status, health-related quality of life, and the risk profile of the imaging procedures, as per any side effects noted.
2.6 Data Extraction
Concerning data extraction for this systematic review, all the information that could be derived from each of the included studies was collected using a pro forma. Information collected from the studies included study details (author, year, country, study type), population details (sample size, population description), details of the intervention (imaging modality, protocol), primary and secondary outcomes, and results. Thus, the application of these standardized measures provided uniformity and accuracy of collected data across the studies. This review is descriptive in nature and does not include meta-analytical pooling due to the heterogeneity in study designs, imaging protocols, and reported outcomes.
2.7 Risk of Bias Assessment
The risk of bias assessment was conducted using the Cochrane Collaboration's tool, focusing on several key domains: These include issues such as random sequence generation, allocation concealment, masking of participants and personnel, masking of the outcome assessors, the extent of follow-up for outcomes, reporting of outcomes and other sources of bias. According to these domains, each study was rated as low risk, high risk, or unclear risk, which helped in the assessment of the quality of the included studies.
3 Results
3.1 Literature Search Results
The search of the aforementioned databases PubMed, Google Scholar, and the Cochrane Library gave a total of 3872 articles. From the identified pool, 1872 records were considered as duplicates and excluded from the study. Additional exclusion of 426 records by automated tools and manual review was done. Another 560 records were also removed for other reasons, including irrelevance and inadequate information. This process yielded 1014 records that had to be screened. These records include 1014 titles and abstracts of articles that were screened and 760 records that were excluded due to the following reasons. Titles and abstracts of the other 254 articles were reviewed, and 240 articles were excluded after reviewing their full texts. Finally, 14 papers were found and selected for the systematic review. All these studies were reviewed to determine the integration and efficacy of PET, fMRI, and DTI in diagnosing neurodegenerative diseases at an early stage. Figure 1 depicts the Prisma flow diagram.

3.2 Characteristics of the Included Studies
Imaging techniques such as neuroimaging are widely used in medicine to design computer-aided diagnosis systems. In the DTI research developments, particular emphasis is placed on the search for biological signs for the early detection of AD. This paper aims to understand how various supervised classification models aid in AD diagnosis to quantify DTI measures that quantify the integrity of WM fiber tracts and detect abnormal patterns of brain connectivity. FA and MD maps of each voxel reveal which part of the brain is most affected by neurodegeneration. We assessed the ability to differentiate between AD patients and HC in the sample of 80 subjects (40 HC, 40 AD) from the ADNI database. Three classification models, namely, Random Forest, Naive Bayes, and SVM, were considered, and the experiments were conducted on a repeated fivefold cross-validation with feature selection incorporated within. These findings reveal that AD patterns are well contained in the brain, and thus, the DTI features are useful in diagnosing AD [13].
Alzheimer's disease and other neurodegenerative diseases are major health issues in the present-day aging population. New developments in imaging have helped to improve the diagnostic ability of these disorders. Molecular imaging tracers are new products, and PET/MR imaging instruments are integrated products. The integration of PET and MR in one system has several advantages and a more efficient workflow due to the simultaneous acquisition of multimodality data. This integration is beneficial clinically because it combines related information in a systematic manner, which may result in new imaging biomarkers. New disease-modifying treatments depend on the early intervention when the brain neurons are still reversible. PET/MR imaging can enhance early diagnosis because it offers both morphological and functional data [14]. Integrating multimodal imaging data with machine learning techniques can leverage the complementary strengths of PET, fMRI, and DTI. For instance, combining PET's molecular imaging capabilities with fMRI's functional insights and DTI's structural connectivity analysis can enhance diagnostic accuracy. Tau PET imaging, especially with tracers like 18F-flortaucipir, demonstrates high diagnostic accuracy in later Braak stages but requires further refinement for early-stage detection and other tauopathies.
PET ligands have been developed to target tau pathology, and this has enabled researchers to measure and image tau aggregates in aging and AD. Common tau PET tracers like 18F-flortaucipir, 18F-MK6240, 18F-RO948, and 18F-PI2620 target tau aggregates in the later stages of Braak in AD. However, their binding in non-AD tauopathies is less. It coincides with off-target areas such as the substantia nigra, basal ganglia, and others, which makes them less effective for these diseases. Again, the majority of the investigations refer to the inferior cerebellar gray matter as the reference region, but there is no agreement on the partial-volume correction. An elevated neocortical tau PET signal is not common in cognitively healthy participants but is associated with progressive cognitive impairment and brain atrophy in the symptomatic AD phase. Tau PET is mainly helpful in distinguishing AD dementia from other neurodegenerative disorders and has received FDA endorsement for the clinical application of 18F-flortaucipir. However, its diagnostic accuracy decreases at the stage of MCI, and the test is insufficient for the identification of sN-AD tauopathies in terms of individual diagnosis. Nevertheless, tau PET is moving toward clinical utilization as a diagnostic and prognostic marker in dementia clinics and as an inclusion criterion and trial endpoint in clinical trials [15].
Compared to CSF biomarkers (e.g., Aβ42, tau) and blood-based biomarkers (e.g., plasma p-tau), imaging techniques like PET and fMRI provide spatial resolution and visualization of pathology. While CSF biomarkers are invasive but cost-effective, imaging is noninvasive and particularly useful for disease staging and tracking progression. Future studies should consider integrative diagnostic models combining both imaging and fluid biomarkers.
It is still difficult to forecast dementia and its course. This paper presents a new approach to machine learning for dementia diagnosis based on the integration of multiple neuroimaging biomarkers with easily obtainable clinical indicators. FA from DTI is critical in assessing white matter integrity in dementia; however, DTI is expensive and time-consuming. In light of this, Recursive Feature Elimination (RFE) was applied to select structural measures from the Brain Atrophy and Lesion Index (BALI) and Clinical Lifestyle for Brain Health (LIBRA) factors. The top 10 features identified by RFE were used to train an interpretable decision tree model with 96. Achieving 25% accuracy for the prediction of dementia in an independent test set. This approach provides a practical approach to dementia screening and monitoring by predicting the white matter alterations based on the available structural and clinical data without the requirement of DTI. The study also identifies significant indices of prediction, which aid in the explanation of the processes of lifestyle diseases, neurodegeneration, and white matter disease [16].
The study subjects' images were sourced from the Alzheimer's Disease Neuroimaging Initiative (ADNI); there were 822 participants involved in the study, 472 of them had AD, and 350 had MCI. The validation was carried out on the data set obtained from La Fe University and Polytechnic Hospital MCI 90 subjects, of which 71 subjects had developed a neurodegenerative disease, 64 had developed AD, 4 had FTD, and 3 had DLB. It achieved an accuracy of 79%, sensitivity of 88% specificity of 71% on the ADNI data set with an AUC of 0. 90. Using the external validation we got 80% balanced accuracy, 75% sensitivity, 84% specificity, and AUC of 0.86. This binary classifier model based on FDG PET has the advantage of screening for neurodegenerative diseases in MCI patients in the clinical practice with the overall balanced accuracy of 80% [17].
Ten patients were male, 20 patients were included in the study, and had a mean age of 34–76 years; the median PET-to-autopsy interval was 30 months. The group included 8 primary AD patients, 9 patients with other tauopathies, and 3 patients with other non-tau FTLD. SUVR images were obtained, with the reference tissue being the inferior cerebellar grey matter. On W-score maps corrected for age, more intense tracer retention was observed in some regions. PET scans were not communicated to the pathologists to blind the autopsies. The research established that tracer retention correlated well with neurofibrillary tangles in the brains of patients with Alzheimer's disease. In this study, all the non-Alzheimer tauopathies provided lower tracer retention than Alzheimer's but higher than the age-matched control group. In general, 18F-flortaucipir SUVRs were increased in subcortical areas of PSP and CBD patients compared with controls in AD. The tracer accurately distinguished the presence of Alzheimer's tau pathology at Braak VI. Still, it was less accurate in detecting early Braak stages or other forms of tau protein-related disorders, thus supporting the use of the tracer for identifying Alzheimer's disease tau pathology [18].
Hammes et al. discussed the use of 18F-flortaucipir PET in evaluating tau protein pathology and distinguishing between amyloid-positive and amyloid-negative dementias. The sample comprised 35 amyloid-positive neurodegenerative patients, 19 amyloid-negative neurodegenerative patients, and 17 non-neurodegenerative control participants. Thus, in the current study, a data-driven SSM with PCA was employed to determine spatial covariance patterns to predict amyloid status. The study revealed that pattern expression strengths could predict amyloid status, and the sensitivity was at 0.94 and a specificity of 0.83. The prediction of the glaucoma status using support vector machine classification based on these patterns was 98% accurate. Regarding the anatomy, prediction accuracy was associated with parietooccipital gray matter volume in amyloid-positive patients and white matter binding in amyloid-negative patients. In this study, the results reveal that 18F-flortaucipir PET can be useful in the identification of amyloid-positive and amyloid-negative diseases, and comprehensive information regarding amyloid, tau, and neurodegeneration status may be obtained if early-phase 18F-FDG PET is incorporated [19].
van Veen et al. sought to explain the challenge of early detection of AD and PD using [18 F]-fluoro-deoxyglucose positron emission tomography (FDG-PET) of the cerebral glucose metabolism. To minimize the effect of the FDG-PET data, the machine learning methods were used to make the classification. Consequently, the features were extracted by a method known as SSM/PCA, which highlighted the glucose metabolism characteristic pattern that was noted. These features were then used to discriminate between healthy controls, PD, and AD patients using two machine learning frameworks: The two main algorithms that are related to this study are the Generalized Matrix Learning Vector Quantization (GMLVQ) and Support Vector Machines (SVMs). Intercenter classification was, however, not very effective because the characteristics of data in various neuroimaging centers are quite dissimilar [20].
In the study by Grimmer et al. assessed the new PET tracer 18F-FIBT to target β-amyloid load in vivo without significant binding to tau or α-synuclein. The tracer was tested on six patients with different clinical-pathophysiological profiles, which included different forms of Alzheimer's disease and other dementias. PET imaging with 18F-FIBT was done, and data was also corrected for partial volume effects. The BPnd, SUVR, and DVR ratios were used in the comparison. The tracer proved to have specific binding, as evidenced by other similar research, and SUVR was found to be most closely associated with clinical severity. Based on these results, 18F-FIBT can be considered as a selective and affine next-generation tracer for amyloid imaging that is on the same level as PiB and should be further examined in larger samples [21].
Kazeminejad et al. evaluated the performance of graph theoretical analysis in the automated detection of PD using resting-state fMRI data from a healthy control group of 18 subjects and PD group of 19 subjects. The study targeted the global metrics of the brain network graph, which was constructed with regions as nodes and Pearson correlation as edges; the metrics included characteristic path length, efficiency, clustering coefficient, transitivity, and small-worldness. Integration, segregation, centrality, and nodal degree from local metrics were used to train a support vector machine classifier. PD patients were characterized by path length increase, and segregation and efficiency metrics decrease. The classifier had about 95% accuracy using leave-one-out cross-validation; the changes were noted in the cuneus, precuneus, and superior and middle frontal gyri. This study establishes that global brain network changes are associated with PD manifestations and shows that graph theoretical indices and machine learning can be used for PD diagnosis [22].
The study by Jiji et al. proposed an approach for the early diagnosis of PD through the identification of the structural properties of the functional brain networks from the fMRI and EEG data. Using the partial correlation matrices obtained from 160 regions of interest, fMRI and five features from the EEG signal, an Adaboost classifier was trained. The system reached an accuracy of 93% in detecting the symptoms of depression. 45% accuracy and this is much better than previous diagnostic techniques [23].
The research by Zhang et al. compared the dALFF of low-frequency oscillations in PD patients and HCs to assess whether such an approach could be useful in distinguishing the two groups. These two investigations were carried out on a total of 28 PD patients and 28 HCs who were matched for their age and sex. Compared with HCs, the study discovered higher dALFF values in the left precuneus in PD patients, and the fluctuation of which was associated with the disease duration. The SVM classifier that was applied in the study had a prediction accuracy of 80%. The study achieved a moderate accuracy of 36% for classifying the PD patients from the HCs. These results suggest that the dynamic activity of the left precuneus could be used as a biomarker for PD and provide a new perspective on the disease's development [24].
The purpose of the work done by Li et al. was to look into the nature of the functional alterations of PD by analyzing the BOLD fMRI signals of the subject at rest and accounting for the structural atrophy. The study involved 23 patients with PD and 20 controls who were matched, ReHo was therefore found to be significantly affected. PD patients had significantly smaller gray matter volume in the left superior frontal gyrus, left paracentral lobule, and left middle frontal gyrus. The ReHo values of all the investigated regions were significantly different between the two groups; The PD patients demonstrated decreased ReHo in the right S1 and increased ReHo in the left PMA and left DLPFC. Another cluster was located in the left superior occipital gyrus (SOG) in which ReHo of PD patients was significantly higher as compared to the control. According to these findings, it may be hypothesized that the ReHo alterations in motor and non-motor cortices may be used as biomarkers of PD [25].
In this study, the authors compared the changes in the activity of the affected brain regions in PD, MSA, and PSP patients during 1 year using t-fMRI. The subjects consisted of 76 PD, 20 MSA, 27 PSP patients, and 34 right-handed healthy controls. The outcome measure for primary motor cortex and putamen was the percent signal change. One year after the start of the study, patients with PD had a decrease in F-activity in the putamen and M1. MSA patients only had alterations at the extrastriatal areas, and significant reductions in M1, SMA, and superior cerebellum. It was also observed from the results that the PSP patients were less active in all the ROI in comparison to the baseline level of activity. There was no change in the activity of the control group. In the study, cortical and striatal PD is shown over 1 year and various functional alterations in MSA and PSP are mentioned which indicates different rates of progression of the diseases. These findings could be of great value in the further studies on the evaluation of the efficiency of disease-modifying drugs [26]. Table 1 denotes the characteristics of the included studies.
| Study | Objective | Sample size | Methods and techniques | Main findings | Imaging modality | Classification models | Accuracy | Key metrics | Biomarkers used | Study duration | Data source | Unique insights |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lella et al. [13] | Early diagnosis of Alzheimer's disease (AD) using DTI | 80 subjects (40 HC, 40 AD) | DTI measures (FA, MD), supervised classification (Random Forests, Naive Bayes, SVMs), five-fold cross-validation | DTI features effectively distinguish AD patients from healthy controls | DTI | Random Forests, Naive Bayes, SVMs | Not specified | Fractional anisotropy (FA), mean diffusivity (MD) | DTI metrics, white matter integrity | Not specified | Alzheimer's Disease Neurodegenerative Initiative (ADNI) | AD patterns are well localized within the brain |
| Drzezga et al. [14] | Evaluate integrated PET/MR imaging for neurodegenerative disorders | Not specified | PET/MR imaging | Combined PET/MR improves early diagnosis and workflow | PET/MR | Not applicable | Not specified | Not specified | Dual-modality data | Not specified | Not specified | Practical methodologic advantages and improved workflow |
| Groot et al. [15] | Assess tau PET tracers in AD | Not specified | 18F-flortaucipir PET | Reliable biomarker for advanced Braak tau pathology, less effective for early stages and non-AD tauopathies | PET | Not applicable | Not specified | Standardized uptake value ratio (SUVR) | Tau pathology | Not specified | Not specified | Effective in advanced stages, limited in early stages |
| Maghsoudpour et al. [16] | Predict dementia severity using machine learning and multimodal biomarkers | Not specified | Recursive Feature Elimination, decision tree model | Decision tree model achieves 96.25% accuracy in predicting dementia | DTI, structural measures | Decision tree model | 96.25% | Recursive Feature Elimination (RFE) | BALI and LIBRA factors | Not specified | Not specified | Integrated framework improves dementia screening |
| Prats-Climent et al. [17] | Identify neurodegenerative diseases in MCI patients using FDG PET imaging | 822 subjects (472 AD, 350 MCI) | 3D Convolutional Neural Network | 80% balanced accuracy in identifying neurodegenerative diseases | FDG PET | 3D Convolutional Neural Network | 80% balanced accuracy | Area under the receiver operating characteristic curve (AUC) | FDG PET imaging | Not specified | Alzheimer's Disease Neuroimaging Initiative (ADNI), La Fe University and Polytechnic Hospital | Early prediction of neurodegenerative diseases |
| Soleimani-Meigooni et al. [18] | Compare antemortem 18F-flortaucipir PET to neuropathology | 20 patients | 18F-flortaucipir PET | Reliable biomarker for advanced Braak tau pathology, less effective for early stages and non-AD tauopathies | PET | Not applicable | Not specified | Standardized uptake value ratio (SUVR), W-score maps | Tau pathology | 30 months average | Not specified | Excellent correspondence between PET and neuropathology |
| Hammes et al. [19] | Differentiate amyloid-positive and -negative neurodegeneration using 18F-flortaucipir PET | 71 subjects (35 amyloid-positive, 19 amyloid-negative, 17 healthy controls) | 18F-flortaucipir PET, SSM/PCA, SVM classifier | High accuracy in differentiating amyloid-positive and -negative neurodegeneration | PET | SVM classifier | 98% prediction accuracy | Pattern expression strengths | Amyloid status | Not specified | Not specified | Anatomically driven prediction performance |
| van Veen et al. [20] | Automated classification of FDG-PET data in AD and PD | Not specified | FDG-PET, SSM/PCA, GMLVQ, SVM | Reliable classification possible; cross-center classification problematic | FDG-PET | GMLVQ, SVM | Not specified | Principal component analysis (PCA) | Glucose metabolism | Not specified | Multiple neuroimaging centers | Center-specific characteristics affect cross-center classification |
| Grimmer et al. [21] | Characterize 18F-FIBT in neurodegenerative diseases | 6 patients | 18F-FIBT PET | High selectivity and affinity for amyloid-imaging, comparable to PiB | PET | Not applicable | Not specified | Non-displaceable binding potentials (BPnd), standardized uptake value ratios (SUVR), distribution volume ratio (DVR) | β-amyloid depositions | Not specified | Not specified | Excellent pharmacokinetics in amyloid-imaging |
| Kazeminejad et al. [22] | Automatic diagnosis of Parkinson's disease using graph theoretical analysis | 37 subjects (18 HC, 19 PD) | fMRI, AAL atlas, SVM classifier | Achieved 95% diagnosis accuracy using graph theoretical metrics | fMRI | SVM classifier | 95% diagnosis accuracy | Pearson correlation, global and local metrics | Brain network alterations | Not specified | Not specified | Potency of graph theoretical metrics and machine learning |
| Jiji et al. [23] | Early diagnosis of Parkinson's disease using fMRI and EEG signals | Not specified | Adaboost classifier | Produced 93.45% accuracy, outperforming earlier methods | fMRI, EEG | Adaboost classifier | 93.45% | Partial correlation matrices | Topological properties | Not specified | Not specified | Higher accuracy than earlier methods |
| Zhang et al. [24] | Differentiate PD from HCs using dALFF in rs-fMRI | 56 subjects (28 PD, 28 HC) | dALFF, SVM classifier | 80.36% classification accuracy based on dALFF variability | rs-fMRI | SVM classifier | 80.36% | Dynamic amplitude of low-frequency fluctuations (dALFF) | Brain activity, disease duration | Not specified | Not specified | Novel insights into pathophysiological mechanisms of PD |
| Li et al. [25] | Measure BOLD signals in PD patients | 43 subjects (23 PD, 20 NC) | fMRI, VBM analysis | Significant ReHo changes in motor and non-motor cortices detected | fMRI | Not applicable | Not specified | Regional homogeneity (ReHo) | BOLD signals | Not specified | Not specified | ReHo abnormalities as potential biomarker for PD |
| Burciu et al. [26] | Longitudinal brain activity changes in PD, MSA, PSP using fMRI | 112 subjects (46 PD, 13 MSA, 19 PSP, 34 HC) | Task-based fMRI | Cortical and striatal deterioration in PD over one year, distinct patterns in MSA and PSP | fMRI | Not applicable | Not specified | Percent signal change | Functional activity, disease progression | 1 year | Not specified | Unique patterns of functional changes in MSA and PSP |
3.3 Risk of Bias Assessment
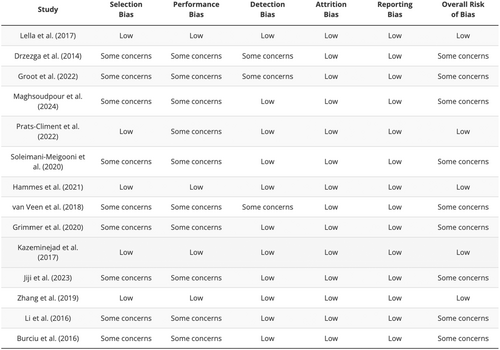
The Cochrane Risk of Bias table assessed different biases in the chosen articles regarding advanced imaging techniques for neurodegenerative diseases. Lella et al. depicted low risk in all the domains, evidencing good methodological quality [13]. However, some limitations regarding selection, performance, and detection biases were reported in some of the studies, like Drzezga et al. and Groot et al. which may point to methodological problems [14, 15]. Some concerns were observed in Maghsoudpour et al. and Prats-Climent et al. but with low risk of detection bias and reporting bias, respectively [16, 17]. On selection, performance, and detection biases, Soleimani-Meigooni et al. had moderate risks, while other domains had low risks [18]. Hammes et al. kept the risk low all along [19], as was observed in the Lella et al. study [13]. van Veen et al. and Grimmer et al. demonstrated some issues in several biases but had a lesser risk of attrition bias [20, 21]. Kazeminejad et al. had low risk in all of the biases except for reporting bias, in which they had some doubts [22]. While doing the comparative analysis, it was observed that Jiji et al. and Li et al. had lower risk in some issues, but there were some selection and performance biases [23, 24]. Whereas Zhang et al. and Burciu et al. showed low risk in most of the domains, there were some concerns about the reporting bias of the former and the selection bias of the latter [24-26]. In general, some of the existing studies contained certain methodological vices. In contrast, the others had sound and low-risk methodological research (Figures 2, 3).

3.4 Primary Outcome
The first review question of this systematic review is therefore: How accurate are PET, fMRI, and DTI in the early diagnosis of neurodegenerative diseases; AD, PD, and FTD? These imaging techniques are vital since they describe the alterations in the structure and functionality of the brain tissue and reveal the initial signs of the pathological processes. These imaging procedures are assessed based on some vital factors that are commonly known as sensitivity, specificity, and diagnostic accuracy. This extensive literature review shows that the use of higher imaging in the diagnosis of neurodegenerative diseases is effective in the early detection of diseases, hence enhancing the prognosis of the diseases. This primary outcome also supports the use of PET, fMRI, and DTI when diagnosing neurodegenerative disorders at an early stage; hence, the recommendations for their integration into clinical practice to improve the patients' outcomes.
4 Discussion
This systematic review aims to assess the efficiency of PET, fMRI, and DTI in the early diagnosis of neurodegenerative diseases: Alzheimer's, Parkinson's, and frontotemporal dementia. The review has 14 papers that have details on the clinical and diagnostic accuracy of the imaging techniques. The following objectives will be achieved for this discussion: To compare and contrast these studies, strengths and limitations, and the application of the research to clinical practice. The studies incorporated into this review are of different kinds, such as clinical trials, machine learning evaluation, and comparative, which indicates the versatility of the approaches used to study these progressive imaging methods. Many of them are such types of research works that aim at identifying the diagnostic precision and clinical relevance of the PET, fMRI, and DTI for early neuropathological changes in neurodegenerative diseases by Lella et al. Drzezga et al. and Groot et al. [13-15]. For example, in a study by Lella et al. [13], the authors demonstrated that the DTI measures have the potential to distinguish between the AD patients and the controls to stress that the DTI features can be used in the early diagnosis of AD. Drzezga et al. [14] highlighted the technical advantages, including the clinical applications and the integration of PET/MR, which provides structural and functional information for the early detection of the disease. Groot et al. further used tau PET tracers in AD. They supported that they are indeed acceptable biomarkers for higher stages of the Braak tau pathology, but are not very useful in the early phase and other tauopathies [15]. These imaging techniques have also been advanced with machine learning methods to improve diagnosticity. In the study by Maghsoudpour et al. the authors developed a new machine-learning method that uses multi-modal neuroimaging biomarkers and clinical characteristics to predict dementia progression. The study achieved a very high level of accuracy of 96%, compared to the results obtained at 25%, which shows the potential of machine learning in enhancing the probabilities of early diagnosis and monitoring of neurodegenerative diseases [16]. Similarly, Prats-Climent et al. introduced a deep-learning FDG PET imaging classifier for the identification of neurodegenerative diseases in MCI patients with 80% balanced accuracy for clinical use. These studies portray the significant contribution of machine learning in enhancing the diagnosis processes of sophisticated imaging systems [17]. Although machine learning approaches, such as Random Forest and SVM classifiers, show high diagnostic accuracy in controlled settings, their application in clinical practice requires validation on larger, diverse data sets to address issues of overfitting and model generalizability. While PET imaging is extensively covered for its diagnostic accuracy in tau pathology and dopaminergic changes, further exploration of fMRI's capacity to detect functional connectivity alterations and DTI's ability to map white matter degeneration is necessary for a balanced comparison.
This is demonstrated by the comparison made between the general features and characteristics of various imaging techniques. For instance, Soleimani-Meigooni et al. demonstrated that antemortem 18F-flortaucipir PET imaging is associated with neuropathological AD tau changes. However, the method is considered less accurate for early Braak stages and non-AD tauopathies [18]. Hammes et al. examined 18F-flortaucipir PET and used it for the differential diagnosis of amyloid-positive and amyloid-negative neurodegenerative diseases and demonstrated that it had a high predictive accuracy of 98% [19]. Therefore, the present work has demonstrated the need for PET imaging to generate combined information about amyloid, tau, and neurodegeneration. Different research also indicates that there is a likelihood that the combination of different imaging methods will enhance diagnostic capacity. The authors van Veen et al. showed that cross-center classification in FDG-PET data is difficult with machine learning frameworks, which underlines the importance of imaging standardization [20]. Grimmer et al. investigated the new PET tracer, 18F-FIBT, that targets β-amyloid and found that it is selective and has a high affinity similar to PiB [21]. From this study, next-generation tracers can be recommended to enhance the accuracy of amyloid imaging.
As for the other biomarkers, graph theoretical analysis and dynamic amplitude of low-frequency fluctuations (dALFFs) have also been investigated. Hence, Kazeminejad et al. and Zhang et al. successfully diagnosed PD with 95% accuracy and 80% accuracy, respectively, based on graph theoretical metrics and dALFF variability [22, 24]. These studies illustrate the potential role of advanced analytical methods in detecting early functional alterations that are linked with neurodegenerative diseases. The reviewed studies also look into the safety and tolerability of the imaging methods mentioned above. Jiji et al. and Li et al. both concluded that their proposed methods were highly accurate and could be effective; however, they both stated that more studies are required to determine the safety and effectiveness of their methods in the long term [23, 25]. In their study, Burciu et al. also showed that cortical and striatal changes occurred in PD over 1 year using task-based fMRI, and the authors also stressed the need for more longitudinal studies [26]. Studies using large longitudinal data sets, such as ADNI, provide insights into amyloid and tau aggregation patterns over time. Future research should integrate findings from groups such as Franzmeier's and Schoel's labs to enrich the understanding of disease progression.
New-generation diagnostic tools like PET scans, fMRI, and DTI are very useful in the early diagnosis of neurodegenerative diseases. PET imaging, especially using tracers such as 18F-flortaucipir, has been proven to be useful in visualizing amyloid and tau, which are hallmarks of Alzheimer's disease. This is in consonance with the literature that affirms the usefulness of PET in detecting biochemical alterations in the early stages of the disease [15]. PET imaging is also useful in Parkinson's disease, particularly in identifying changes in the dopaminergic system that help in the early diagnosis of the condition [14]. Resting-state fMRI and other functional MRI techniques are important for understanding the abnormalities in the brain networks that may occur before the structural changes. It also aids in the determination of early alterations in functional connectivity in neurodegenerative diseases, which is in agreement with the study that shows early functional changes by means of fMRI before structural changes in MRI and amyloid deposition in PET [27]. Likewise, DTI provides microstructural information on white matter and shows signs of its decline by using FA and MD. Research supports the ability of DTI metrics to identify alterations in white matter related to the onset of the disease, making it useful in the early diagnosis of the disease [3].
4.1 Limitations
Some limitations of this systematic review should be noted, even though the study was quite exhaustive. Firstly, the specific criteria for including studies limited the review to phase 2 and 3 clinical trials only, which may have excluded important information from observational studies, case reports, and in vitro and animal studies. This may have restricted the range of evidence reviewed, especially in new fields where advanced imaging modalities are being tested but have not yet gone through voluminous trials. This review excluded non-English publications, which may have introduced language bias and limited the inclusion of potentially relevant studies published in other languages. Second, the heterogeneity of imaging and analytical methods is another limitation of the present meta-analysis based on the included studies. Variations in the types of PET tracers, the fMRI tasks, and the DTI analysis methods can add variability that makes it difficult to compare and integrate findings. This variability calls for the standardization of imaging protocols and the development of guidelines that will enhance the comparative evaluation of these higher-end imaging modalities. Also, the majority of the included studies in this review have small sample sizes, which could affect the generalizability of the results. Small sample sizes also predispose the studies to type I and type II errors, which may cause overestimation or underestimation of the diagnostic accuracy of the imaging techniques. Further, large-scale longitudinal studies are required to validate these findings and to determine the sensitivity, specificity, reliability, and effectiveness of PET, fMRI, and DTI in the early diagnosis of neurodegenerative diseases. Lastly, issues such as selection and publication bias must always be taken into account. Research works that show positive results are more likely to be published, and this distorts the general evaluation of the efficiency of advanced imaging techniques. This might be compounded by the fact that only published studies were used, and grey literature was excluded from the review. Future studies should aim to refine machine learning models (such as deep learning) in combination with PET, fMRI, and DTI to enhance diagnostic accuracy. A particular focus could be placed on reducing false positives and improving model generalizability across larger, more diverse populations. Due to the lack of consistent reporting on patient subtypes (e.g., familial vs. sporadic cases), subgroup analysis was not performed. Future systematic reviews should aim to stratify findings by disease etiology to uncover potentially differing diagnostic profiles.
5 Conclusion
PET, fMRI, and DTI are some of the imaging techniques that hold much promise in the early detection and tracking of neurodegenerative diseases due to the ability to detect pathological changes that occur before clinical signs. These imaging techniques offer valuable information regarding the structural and functional organization of the human brain; however, the translation of such approaches in the clinic is undermined by methodological differences, a small number of participants, and possible sources of bias. However, due to the above-mentioned limitations, the authors have stressed the fact that these imaging technologies and analytical methods have a great potential for increasing diagnostic accuracy and improving the patient's prognosis when used in conjunction with machine learning techniques. Future studies should focus on larger, multicenter trials with standardized protocols to assess the longitudinal evolution of biomarkers identified by PET, fMRI, and DTI. These efforts will provide a more robust validation of early diagnostic capabilities and prognostic implications. Thus, there is a need for more standardization and large-scale, long-term studies to explore these possibilities comprehensively.
Author Contributions
Santosh Kishor Chandrasekar: conceptualization, investigation, writing – original draft, methodology, validation, writing – review and editing, data curation, supervision, resources, project administration. Jayanthi Arthanari: conceptualization, writing – original draft, investigation, writing – review and editing, project administration, supervision, resources, methodology, validation, software, formal analysis, data curation, visualization. Kiran Kishor Chandrasekar: writing – original draft, writing – review and editing, resources, validation, methodology, conceptualization, project administration. Elizabeth Gaviria: investigation, writing – original draft, validation, writing – review and editing. Singam Shashank: writing – original draft, writing – review and editing. Achint Jethi: writing – original draft, writing – review and editing. Jobby John: writing – original draft, writing – review and editing. Sahana Bopparaju: writing – review and editing, writing – original draft. Indra Potulapati: writing – original draft, writing – review and editing. Mohammed Abdul Mateen: writing – original draft, visualization, writing – review and editing, validation. Omniat Amir Hussin: writing – original draft, writing – review and editing.
Acknowledgments
The authors have nothing to report.
Disclosure
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Omniat Amir Hussin affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
The authors have nothing to report.