Antibodies to specific domains of Plasmodium falciparum erythrocyte membrane protein-1 and its relationship with protection from severe malarial anemia: A prospective study among Ghanaian children
Abstract
Background
Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP-1) is important in malaria pathogenicity as it mediates Pf-infected erythrocytes cytoadherence to host endothelial microvasculature receptors. Naturally acquired antibodies against specific PfEMP-1 antigens may be beneficial in clinical malaria protection. This study determined antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 in children with P. falciparum malaria in Tamale, Ghana.
Methods
Sixty P. falciparum-infected children, and 30 controls, aged 1–12 years were recruited for this case-control study from April to July 2023 in Northern Ghana. Participants with uncomplicated malaria had asexual P. falciparum in peripheral blood and Hb ≥ 5.0 g/dL, and severe malaria was diagnosed when participants had Hb < 5.0 g/dL in addition to asexual P. falciparum in peripheral blood. Blood cell indices were measured using hematology analyzer, and IgG antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 and pro-inflammatory cytokines were detected using enzyme-linked immunosorbent assay. Data were analyzed using SPSS version 26.0.
Results
The prevalence of PfEMP-1 IgG antibodies among P. falciparum-infected children and the uninfected group was 65.0% and 6.7%, respectively. PfEMP-1 IgG antibodies were present in 83.3% of uncomplicated malaria cases, and 46.7% in severe malaria subjects. Plasma levels of PfEMP-1 IgG antibodies were elevated in participants with uncomplicated malaria compared to those with severe malaria (p < 0.001). Hemoglobin, RBC, HCT, and platelet were significantly lower among P. falciparum-infected children without PfEMP-1 IgG antibodies than among those with the antibodies. Prevalence of anemia among children with PfEMP-1 IgG antibodies and those without the antibodies were 74.4% and 100%, respectively.
Conclusion
The high prevalence of PfEMP-1 IgG antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains observed in participants with uncomplicated malaria, and the relationship between PfEMP-1 IgG antibodies and blood cell parameters could indicate that the antibodies may be related to effective erythropoietic response in P. falciparum malaria. Immune antibodies against DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 may suppress the deteriorating effects of PfEMP-1 antigens and provide immune protection against severe malarial anemia in children.
1 INTRODUCTION
The detrimental effects of malaria, especially in sub-Saharan Africa are overwhelming, as one child dies from the disease nearly every 2 min. The World Health Organization (WHO) reported total malaria cases of 249 million with 608,000 related deaths in 2022, of which approximately 80% of its mortality is among African children below 5 years of age.1 In areas of high malaria endemicity such as Ghana, with prevalence ranging from 2.6% to 41.9%,2-6 almost all malaria-infected infants and young children experience mild-to-severe life-threatening complications, including fever, severe malarial anemia (SMA) requiring blood transfusion, cerebral malaria, hypoglycemic, respiratory distress syndrome, and acute kidney injury.7, 8 The severity of P. falciparum malaria is largely mediated by the sequestration of P. falciparum-infected red blood cells in the microvasculature, especially in the brain, bone marrow and placenta, provoking intense inflammation, blood flow impairment, hypoxia, and anemia.9, 10 In the erythrocytic stage of the life cycle of plasmodium parasites, P. falciparum-infected red cells express surface antigens including P. falciparum erythrocyte membrane protein-1 (PfEMP-1) which mediates the adherence of infected red cells to the endothelium by binding to endothelial cell adhesion molecules such as endothelial protein C receptor (EPCR), vascular cell adhesion molecule (VCAM)−1, intercellular adhesion molecule (ICAM)−1, P-selectin or E-selectin.10 PfEMP-1 antigens mediate sequestration of P. falciparum-infected and uninfected erythrocytes to endothelial surfaces and enhance excessive release of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-ɣ), interleukin (IL)−1β, and IL-6, which has negative impacts on erythropoiesis.10-12 PfEMP-1 contributes to the development of severe anemia by promoting intense inflammation that acts directly against the bone marrow, renders iron unavailable for use, and inhibits the expression of erythropoietin, preventing the proliferation and differentiation of erythroid progenitor cells.11, 13-15
Naturally acquired antibodies against variant surface antigens on the surface of infected erythrocytes have been suggested to protect individuals from severe and symptomatic malaria by inhibiting the rosetting and cytoadhesion of infected erythrocytes to host receptors; thereby preventing adverse complications.16, 17 After exposure to P. falciparum, individuals develop antibodies against blood-stage parasite antigens which may help protect against severe malaria by preventing parasite multiplication, decreasing tissue-specific sequestration and inflammation, resulting in reduced parasite density as unbound parasites are eliminated in the spleen.17, 18
An earlier study conducted in a semirural area of Ghana reported that more than half of P. falciparum-infected children residing in the area developed antibodies to PfEMP-1, and the antibodies' levels correlated positively with age.18 The study by Dodoo et al. was limited to children living in the Southern part of Ghana, and may not be applicable to children residing in Northern Ghana with different geographical conditions. In addition, their study did not assess the protective effects of the antibodies against SMA. The ability of immune antibodies against DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 to impede cytoadherence of infected red blood cells to the endothelium, reduce sequestration in tissues including bone marrow, suppress inflammatory response and minimize hemolysis of infected red cells, may protect infected individuals from SMA. To the best of our knowledge, no study has assessed the association between specific immune antibodies to PfEMP-1 variants and severe malaria-induced anemia among P. falciparum-infected children in Northern Ghana.
Hence, the present study determined the presence of antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 specific domains of PfEMP-1 in the plasma of children with P. falciparum malaria, and assessed the relationship between the antibodies and SMA in northern part of Ghana. These findings will be useful in the clinical management and counseling aimed at preventing or controlling malaria.
2 MATERIALS AND METHODS
2.1 Study setting and participants
This case-control study was conducted at Tamale Teaching Hospital (TTH) from April to July, 2023. The TTH is located in Tamale and remains a referral center for the five regions in Northern Ghana where P. falciparum transmission is holoendemic.19 P. falciparum malaria remains a major cause of morbidity and mortality to the inhabitants of the area, especially children and pregnant women. Major malaria transmission occurs in the rainy season (April to June) when approximately 80% of the estimated number of infective female anopheles mosquito bites per year occurs.20 A total of 90 children aged 1–12 years were recruited for the study. The participants comprised 60 treatment-naïve febrile children who presented to the outpatient department and pediatric ward of TTH with P. falciparum malaria, without any other illness. Thirty apparently healthy children without malaria were selected from a Basic School in Tamale as controls. The appropriate sample size for the study was determined using the Kelsey's sample size determination formula. Children on antimalarial drugs, with cerebral malaria, non-falciparum malaria, sickle cell disease, G6PD deficiency, HIV, with history of other comorbidities, and whose parents or caregivers withheld their consents were excluded from the study. Sixty P. falciparum-infected children were split into two categories. The uncomplicated malaria group included children with asexual P. falciparum in peripheral blood, and hemoglobin (Hb) ≥ 5.0 g/dL. Severe malaria was described when a child had positive blood smear for asexual form of P. falciparum, and Hb < 5.0 g/dL.21 About 4 mL of venous blood specimen was aseptically collected in ethylenediaminetetraacetic acid vacutainer tubes at the point of admission, before antimalarial drugs were administered. The blood specimens were used for the measurement of full blood count (FBC), malaria diagnosis, PfEMP-1 IgG antibody detection, and assessment of plasma proinflammatory cytokines (TNF-α, IFN-ɣ, IL-1β, and IL-6). The study was approved by the Institutional Review Board of the University for Development Studies, Ghana (UDS/IRB/15/2023). After obtaining permission from the management of TTH, written informed consent was received from the parents/guardians of the study participants.
2.2 Laboratory analysis
FBCs were measured using five-part automated hematology analyzer (URIT-5250) at TTH Hematology Laboratory. For the detection and quantification of P. falciparum parasite density, 10% Giemsa-stained thick and thin smears were prepared and examined under the microscope by two microscopists. The number of P. falciparum parasites per ≥200 leukocytes was estimated using the equation:
Parasites per µL of blood = 22, 23 Plasma specimens extracted from the whole blood were stored at −20°C until analysis. Antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 recombinant domains of PfEMP-1 and the proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-ɣ) were measured using sandwich enzyme-linked immunosorbent assay (ELISA), with ELISA kits from Biobase, China, and protocols were followed according to manufacturer's instructions. The microplate wells were precoated with the recombinant domains of PfEMP-1 (supplied ready to use) for the detection of the PfEMP-1 antibodies specific to the domains. Using 96-well microplates, 50 µL of the standard was added to the standard well, and 10 µL of participants' plasma was added to the sample wells. Sample diluent (40 µL) was then added to the sample wells, but nothing was added to the blank well. Fifty microliters of HRP-conjugate reagent was added to the various wells, except blank well, and the preparation was sealed with a glue strip and incubated for 30 min at 37°C. The wells were aspirated and washed by loading each well with the wash solution (400 µL) using autowasher (Poweam, WHYM201). Remnants of the solution after the final wash were removed by aspirating and pouring. The plates were turned upside down and blotted against spotless paper towels. About 50 µL each of chromogen solutions A and B were introduced to the individual wells. The preparation was agitated gently and incubated for 10 min at 37°C, away from light. Stop solution (50 µL) was introduced to the wells and there was a color conversion from blue to yellow. The optical densities (OD) and the concentrations of PfEMP-1 IgG antibodies, and proinflammatory cytokines in each well were read at 450 nm within 15 min using automated plate reader (Poweam, WHYM200) at UDS Laboratory. Upon running the standards, the auto-analyzer automatically generated standard graph. Subsequently when the various samples were run the analyzer measured the OD and generated their respective concentrations (ng/L) from the standard graph. The negative cut-off values for PfEMP-1 IgG antibodies were based on the OD obtained for the negative standard provided by the manufacturer (OD ≤ 0.018). Positive control was obtained from a pool of plasma specimens obtained from five clinically immune Ghanaian children who reside in Tamale and have successfully completed the four doses of malaria vaccine.
2.3 Statistical analysis
The Statistical Package for the Social Sciences (SPSS) software, version 26.0 was used for the statistical analysis. Shapiro–Wilk and one-sample Kolmogorov–Smirnov tests were used to test for the distribution of the continuous data. Parametric data were presented as mean ± standard deviation whilst nonparametric data were presented as median (25th–75th percentiles). Categorical data were compared using either Pearson Chi-square or Fisher's exact test. Bivariate continuous data were compared using either independent sample t-test (for parametric data) or Mann–Whitney test (for nonparametric data). Kruskal–Wallis test was used to compare the plasma levels of PfEMP-1 IgG antibodies among multivariate group (uncomplicated malaria, severe malaria and controls). The data were presented in tables and figures, and statistical significance was set at p < 0.05.
3 RESULTS
3.1 Demographic characteristics of the study participants
Of the 90 children recruited, 60 (66.7%) were infected with P. falciparum malaria and 30 (33.3%) had no malaria. The median age of the participants was 3.0 (1.0–7.0) years, with the majority (63.3%) within 1–4 years. There were no significant difference for sex and age among the study participants (Table 1).
| Variables | Total (N = 90) | Study participants | p-Value | |
|---|---|---|---|---|
| With P.f malaria (N = 60) | Without P.f malaria (N = 30) | |||
| Age (Years) | 3.0 (1.0-7.0) | 2.0 (1.0-6.8) | 3.0 (1.0-7.0) | 0.858 |
| Age category | 0.643 | |||
| 1–4 | 57 (63.3) | 37 (64.9) | 20 (35.1) | |
| 5–10 | 33 (36.7) | 23 (69.7) | 10 (30.3) | |
| Sex | 0.545 | |||
| Males | 53 (58.9) | 34 (64.2) | 19 (35.8) | |
| Females | 37 (41.1) | 26 (70.3) | 11 (29.7) | |
- Note: N = Number of participants; P. f = Plasmodium falciparum. Age was presented in median (25th –75th percentiles) and was compared using Mann–Whitney U-Test. Categorical data were presented in frequencies with corresponding percentages in parenthesis. Pearson's Chi-square was used to compare categorical data. p < 0.05 was considered statistically significant.
3.2 Prevalence of PfEMP-1 IgG antibodies among the study participants
The prevalence of PfEMP-1 IgG antibodies among the 60 P. falciparum-infected children was 65.0%, however, only 6.7% (2/30) of the controls had PfEMP-1 IgG antibodies, which was statistically significant (p < 0.001). The prevalence of PfEMP-1 IgG antibodies in the plasma of study participants with uncomplicated P. falciparum malaria was higher than that in the severe malaria group (83.3% [25/30] vs 46.7% [14/30], p = 0.003) (Figure 1).
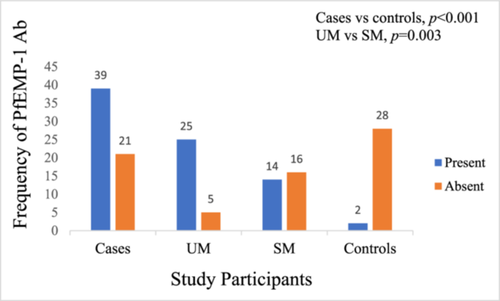
3.3 Age- and sex-specific prevalence of PfEMP-1 IgG antibodies among the study participants
The age- and sex-specific prevalence of plasma PfEMP-1 IgG antibodies among P. falciparum-infected children were 1–4 years: [uncomplicated malaria (84.2%), vs severe malaria (44.4%), vs controls (10%)]; 5–12 years: [uncomplicated malaria (81.8%), vs severe malaria (50.0%), vs controls (0)]; males: [uncomplicated malaria (93.3%), vs severe malaria (52.6%), vs controls (5.3%)]; and females: [uncomplicated malaria (73.3%), vs severe malaria (36.4%), vs controls (9.1)] as shown in Table 2.
| Variables | Participants with uncomplicated malaria | Participants with severe malaria | Controls | ||||
|---|---|---|---|---|---|---|---|
| Categories | Present (%) | Absent (%) | Present (%) | Absent (%) | Present (%) | Absent (%) | |
| Age (years) | 1–4 | 16 (84.2) | 3 (15.8) | 8 (44.4) | 10 (55.6) | 2 (10.0) | 18 (90.0) |
| 5–12 | 9 (81.8) | 2 (18.2) | 6 (50.0) | 6 (50.0) | 0 | 10 (100) | |
| Sex | Males | 14 (93.3) | 1 (6.7) | 10 (52.6) | 9 (47.4) | 1 (5.3) | 18 (94.7) |
| Females | 11 (73.3) | 4 (26.7) | 4 (36.4) | 7 (63.6) | 1 (9.1) | 10 (90.9) | |
- Note: Data were presented in frequencies with corresponding percentages in parentheses. p < 0.05 was considered statistically significant.
3.4 Plasma levels of antibodies against DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 among uncomplicated malaria, severe malaria, and the controls
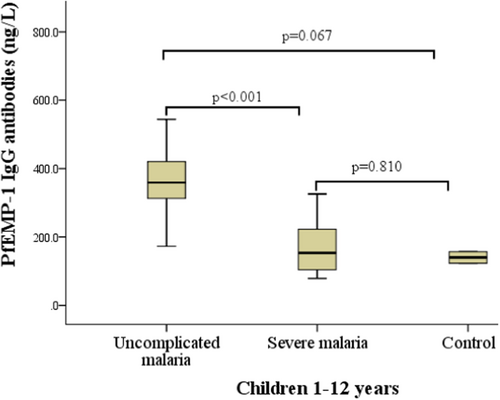
Among the 90 participants, the plasma levels of PfEMP-1 IgG antibodies were higher in P. falciparum-infected children than in the controls. However, within the cases, levels of PfEMP-1 IgG antibodies were significantly elevated in the participants with uncomplicated P. falciparum malaria compared with their counterparts with severe malaria (Figure 2).
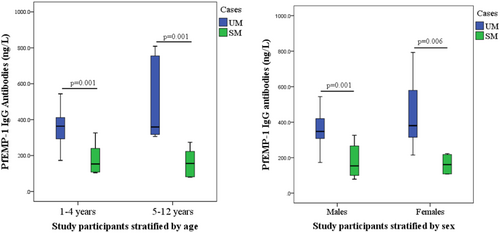
3.5 Age- and sex-specific plasma levels of PfEMP-1 IgG antibodies among P. falciparum-infected participants
When P. falciparum-infected children were stratified by age, the plasma levels of PfEMP-1 IgG antibodies (ng/L) were higher in those with uncomplicated than severe malaria among both children less than 5 years (p = 0.001) and children from 5 to 12 years (p = 0.001) Similarly, PfEMP-1 IgG antibodies were higher in both male (p = 0.001) and female (p = 0.006) participants with uncomplicated than severe malaria (Figure 3).
3.6 Hemogram of the P. falciparum-infected children stratified by the presence or absence of antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1
P. falciparum-infected children without plasma PfEMP-1 IgG antibodies had significantly lower hemoglobin, red blood cell (RBC), hematocrit (HCT), platelet, and plateletcrit (PCT), but higher red cell distribution width-coefficient of variation (RDW-CV), platelet distribution width (PDW), and platelet large cell ratio (P_LCR) than those with the antibodies in the plasma and the controls. Other blood cell indices did not differ between participants with or without PfEMP-1 IgG antibodies and the controls (Table 3).
| Variables | Pf. Infected Children | Controls (N = 30)c | p-Value | Pairwise comparison | |
|---|---|---|---|---|---|
| PfEMP-1 IgG Ab Present (N = 39)a | PfEMP-1 IgG Ab Absent (N = 21)b | ||||
| RBC×/L | 3.6 (3.3–3.9) | 1.7 (1.4–2.8) | 4.0 (3.9–4.5) | <0.001 | a&b, a&c, b&c |
| Hb (g/dL) | 10.5 (9.4–11.0) | 4.8 (4.0–7.8) | 11.9 (11.4–12.6) | <0.001 | a&b, a&c, b&c |
| HCT% | 31.0 (27.9–33.4) | 16.5 (13.4–23.5) | 35.6 (34.6–37.0) | <0.001 | a&b, a&c, b&c |
| MCV (fL) | 76.8 ± 9.1 | 74.4 ± 11.1 | 75.9 ± 11.2 | 0.689 | – |
| MCH (pg) | 27.0 ± 4.5 | 25.2 ± 4.1 | 26.0 ± 4.8 | 0.0.303 | – |
| MCHC (g/dL) | 34.6 ± 3.2 | 33.4 ± 3.5 | 34.0 ± 2.1 | 0.0.309 | – |
| RDW-CV% | 9.4 (8.3-11.2) | 11.5 (10.1-13.9) | 9.0 (8.1-11.7) | 0.005 | a&b |
| TWBC×/L | 8.6 (5.8-11.1) | 9.8 (6.7-14.9) | 9.9 (6.9-14.0) | 0.254 | |
| Neut. #×/L | 4.9 ± 2.2 | 4.9 ± 1.8 | 5.1 ± 3.6 | 0.915 | – |
| Lymph. #×/L | 2.7 (1.4-4.1) | 3.7 (1.5-6.0) | 3.5 (2.4-5.7) | 0.108 | |
| Mon. #×/L | 0.6 (0.4-1.1) | 1.0 (0.4-1.7) | 0.7 (0.4-1.1) | 0.229 | |
| Eos. #×/L | 0.10 (0.05-0.20) | 0.10 (0.08-0.80) | 0.1 (0.1-0.5) | 0.316 | |
| Baso. #×109/L | 0.008 (0.005-0.012) | 0.01 (0.009-0.016) | 0.01 (0.01-0.01) | 0.276 | |
| PLT. #×/L | 235.0 (162.0-351.0) | 133.0 (106.0-206.0) | 358.0 (296.0-388.3) | <0.001 | a&b, a&c, b&c |
| MPV (fL) | 5.9 ± 0.8 | 6.2 ± 0.8 | 5.9 ± 0.9 | 0.318 | – |
| PDW% | 6.7 (6.1-8.0) | 8.6 (7.5-10.3) | 6.6 (6.0-7.5) | <0.001 | a&b, b&c |
| PCT% | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.3 ± 0.1 | <0.001 | a&b, a&c, b&c |
| P-LCR% | 18.4 (14.5-25.0) | 23.5 (19.0-38.7) | 16.6 (11.9-27.8) | 0.008 | a&b, b&c |
- N = Number of participants,
- a = P. falciparum-infected children with PfEMP-1 IgG antibodies.
- b = P. falciparum-infected children without PfEMP-1 IgG antibodies.
- c =Controls, PfEMP-1 IgG Ab, Plasmodium falciparum erythrocyte membrane protein-1 immunoglobulin G antibodies; RBC, Absolute red blood cell count; Hb, Hemoglobin concentration; HCT, Hematocrit, MCV, Mean cell volume; MCH, Mean cell hemoglobin; MCHC, Mean cell hemoglobin concentration; RDW-CV, Red blood cell distribution width-coefficient of variation; TWBC, Total white blood cell count; Neut. # = Absolute neutrophil count, Lymph. # = Absolute lymphocyte count, Mon. # = Absolute monocyte count, Eos. # = Absolute eosinophil count, Baso. # = Absolute basophil count, PLT. # = Platelet count; MPV, Mean platelet volume; PDW, Platelet Distribution width; PCT., Plateletcrit; P-LCR, Platelet large cell ratio. Parametric data presented as mean ± standard deviation were compared using one-way ANOVA test, and nonparametric data presented as median (25th–75th percentiles) were compared using Kruskal–Wallis test. Bold values p < 0.05 was deemed statistically significant.
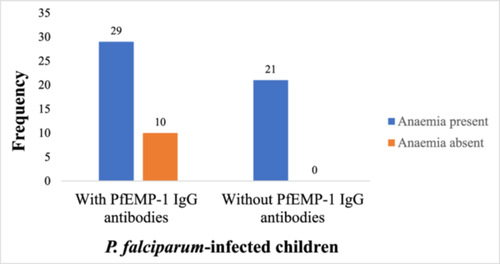
3.7 Prevalence of anemia between children with PfEMP-1 IgG antibodies and those without the antibody
Of the 39 participants with PfEMP-1 IgG antibodies, 29 (74.4%) of them had anemia. However, all the 21 (100%) P. falciparum-infected children who had no PfEMP-1 IgG antibodies in plasma were anemic (Figure 4).
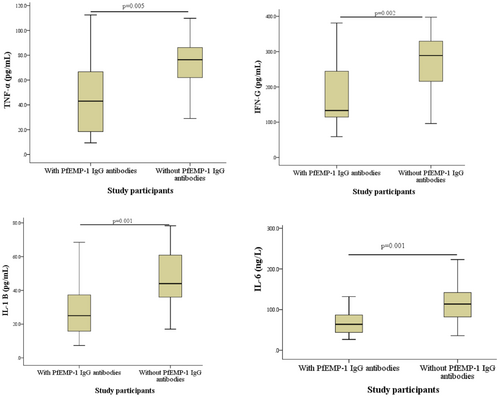
3.8 Relationship between PfEMP-1 IgG antibodies and proinflammatory cytokines among P. falciparum-infected participants
Plasma levels of TNF-α (p = 0.005), IFN-ɣ (p = 0.002), IL-1β (p = 0.001), and IL-6 (p = 0.001) were significantly reduced in P. falciparum-infected children with plasma PfEMP-1 IgG antibodies compared with their counterparts without the antibodies (Figure 5).
4 DISCUSSION
PfEMP-1 contributes to malaria pathogenesis by mediating the adherence of P. falciparum-infected red blood cells to the microvasculature, triggering massive inflammatory response that leads to life-threatening complications.9-11, 14, 15 Naturally occurring antibodies against specific PfEMP-1 variants may play a significant role in the protection against clinical malaria.22 This study determined the presence of antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 in children with P. falciparum malaria, and assessed the relationship between the antibodies and SMA in Northern Ghana.
In the present study, the prevalence of PfEMP-1 IgG antibodies was higher (65.0%) among the P. falciparum-infected children than in the uninfected group (6.7%). When the P. falciparum-infected participants were classified, those with uncomplicated malaria had a higher prevalence (83.3%) of PfEMP-1 IgG antibodies in the plasma compared with their counterparts in the severe malaria group (46.7%). The higher prevalence of PfEMP-1 IgG antibodies identified in this study is similar to findings from a previous study that detected the presence of the PfEMP-1 antibodies in more than half of the different study participants.18 However, contrary to the Dodoo et al. study that reported more than 50% of the protected group with PfEMP-1 antibodies, the present study found the antibodies in only 6.7% of the uninfected group. The differences in the findings may be related to variations in the assay protocols and geographical settings. The study by Dodoo and colleagues used whole infected erythrocytes to detect antibodies against variant antigens which would possibly include other antigens in addition to PfEMP-1. Also, the current study was conducted among children residing in northern Ghana, while the study by Dodoo et al. involved children living in Dodowa, a semirural area in southern Ghana. Following exposure to one or two severe episodes of P. falciparum malaria, an individual may develop naturally occurring antibodies to surface antigens expressed by the parasites. Higher levels of the PfEMP-1 antibodies have been suggested to confer natural immunity or protection to children from severe P. falciparum malaria, and these antibodies could persist for a period before being eliminated.22 Development of the IgG antibodies to PfEMP-1 antigens could be due to previous exposure to P. falciparum malaria.
In the current study, the plasma levels of the PfEMP-1 IgG antibodies were significantly elevated in the group with uncomplicated P. falciparum malaria compared with those with severe malaria. The PfEMP-1 antigens expressed by P. falciparum contribute significantly to severe malaria pathogenesis by binding to endothelial cell adhesion molecules such as EPCR, VCAM-1, and ICAM-1 to facilitate cytoadherence of infected erythrocytes to the endothelial surfaces.9, 10 Eventually, the endothelium becomes hyperactive, with an enhanced inflammatory response that would promote the development of severe malaria. Continuous sequestration of infected and uninfected red cells in the brain, bone marrow and other tissues enables the parasite to escape elimination in the spleen and cause life-threatening complications including cerebral malaria, SMA, and respiratory distress syndrome among others.10 The presence of the antibodies to PfEMP-1 antigens may suppress the destructive properties of the antigens and avert the occurrence of severe malaria. This could explain why children with raised plasma levels of the antibodies to PfEMP-1 antigens experienced uncomplicated malaria. This is in line with the assertion that progression of malaria severe enough to require hospitalization is related to insufficient antibody reactivity to the variant antigens expressed by the plasmodium parasite.18 The plasma levels of the PfEMP-1 IgG antibodies were not associated with age and sex of the participants, and this contradicts earlier findings in Ghana.18 The differences in the findings may be related to variations in the assay protocols, sample size and study participants, as some subjects in the protected group were obtained from Sudan in the Dodoo et al. study.
P. falciparum-infected children without the PfEMP-1 IgG antibodies had significantly reduced hemoglobin, RBC, platelet and PCT than those with the antibodies in the plasma. Also, while anemia was present in 74.4% of children with PfEMP-1 IgG antibodies, all (100%) participants without PfEMP-1 IgG antibodies were anemic. Persistent sequestration of plasmodium-infected red blood cells in the bone marrow and other tissues induced by the PfEMP-1 antigens enhances excessive hemolysis, dyserythropoiesis and promotes intense inflammatory response leading to the associated anemia.11, 12 The bone marrow parenchymal cells have been recognized as niches for the accumulation of parasites during malaria pathogenesis. In this study, participants with PfEMP-1 IgG antibodies in their plasma had reduced levels of proinflammatory cytokines TNF-α, IFN-ɣ, IL-1β, and IL-6 compared with those without the antibodies. The intense inflammatory response experienced by participants with reduced plasma levels of PfEMP-1 IgG antibodies may impair erythropoietic response to SMA. Following the excessive sequestration of P. falciparum-infected red cells in severe malaria, there is increased release of inflammatory cytokines such as TNF-α, IFN-ɣ, IL-1β and IL-6, and these cytokines have negative impacts on erythropoiesis.12 Thus, PfEMP-1 antigens contribute to the development of severe anemia by enhancing intense inflammatory environment that acts directly against the bone marrow, restricts iron to a storage site, and suppresses the release of erythropoietin; inhibiting the proliferation and differentiation of erythroid progenitor cells.11, 13-15 However, the presence of PfEMP-1 IgG antibodies could block PfEMP-1 from binding to their target proteins on endothelial surfaces in the bone marrow, liver, brain, and other tissues. The presence of the PfEMP-1 IgG antibodies may reduce the related microvasculature occlusions and inflammatory response, and eventually enhance bone marrow erythropoietic response to the SMA. This may explain why Ghanaian children with plasma levels of PfEMP-1 IgG antibodies seemed less likely to develop severe malaria anemia than those without the antibodies.
4.1 Strengths and weaknesses of the study
This study identified high prevalence of antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 among Ghanaian children with malaria. The association between PfEMP-1 IgG antibodies and blood cell indices was observed, with relatively higher prevalent of anemia in children without PfEMP-1 IgG antibodies.
This study was limited to children from 1 to 12 years of age and findings may not be inferred to other age groups. Also, the study could not assess the entire PfEMP-1 proteins expressed by the Pf.-infected red cells in the participants.
5 CONCLUSION
The high prevalence of antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 domains of PfEMP-1 in participants with uncomplicated malaria, and the relationship between PfEMP-1 IgG antibodies and blood cell parameters could indicate that the antibodies may be related to effective erythropoietic response in P. falciparum malaria. Antibodies to DBLα2, CIDRα1, DBLβ12, and DBLγ6 PfEMP-1 domains may suppress the deteriorating effects of PfEMP-1 antigens and provide immune protection against SMA in children residing in Northern Ghana. Findings from this study will be useful in the clinical management and counseling aimed at preventing or controlling malaria in children.
AUTHOR CONTRIBUTIONS
Charles Nkansah: Conceptualization; Investigation; Writing—original draft; Methodology; Validation; Visualization; Writing—review and editing; Formal analysis; Supervision; Data curation; Software. Felix Osei-Boakye: Writing—original draft; Writing—review and editing; Formal analysis; Validation. Gabriel Abbam: Writing—original draft; Validation; Writing—review and editing. Samuel K. Appiah: Writing—original draft; Validation; Writing—review and editing. Charles A. Derigubah: Writing—original draft; Validation; Writing—review and editing. Simon B. Bani: Writing—original draft; Validation; Writing—review and editing. Samira Daud: Writing—original draft; Validation; Writing—review and editing. Emmanuel K. Alhassan: Conceptualization; Investigation; Writing—original draft; Methodology; Writing—review and editing; Data curation; Resources; Formal analysis. Isaac Adjei: Investigation; Writing—original draft; Writing—review and editing; Data curation; Formal analysis. Emmanuel Appiah-Kubi: Writing—original draft; Investigation; Writing—review and editing; Formal analysis; Software. Anastasia Koduah: Investigation; Conceptualization; Writing—original draft; Methodology; Writing—review and editing; Data curation; Resources; Formal analysis. Bright Boakye: Investigation; Writing—original draft; Writing—review and editing; Formal analysis. Samsiyatu Abdulai: Investigation; Writing—original draft; Writing—review and editing; Formal analysis. Neena I. Anass: Writing—original draft; Writing—review and editing. Dorcas Serwaa: Writing—original draft; Writing—review and editing. Boniface N. Ukwah: Writing—original draft; Writing—review and editing; Validation. Victor U. Usanga: Writing—original draft; Writing—review and editing; Validation. Ejike F. Chukwurah: Conceptualization; Writing—original draft; Writing—review and editing; Supervision.
ACKNOWLEDGEMENTS
Authors appreciate the efforts of the Senior Members at the Department of Biomedical Laboratory Sciences, School of Allied Health Sciences, University for Development Studies, and the staff of the Department of Haematology, TTH especially MLS Prince Ottah and Dr. Sylvanus Kpankpali for their support. We salute all the children and their parents or guardians who willingly volunteered to be part of this study. This work received no external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Charles Nkansah affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The original data that support the findings of this study are available from the corresponding author upon reasonable request. All relevant data are within the article.