Reduction in FEV1 following spinal anesthesia is associated with intraoperative complications: A prospective study
Abstract
Background and Aims
Although Spinal Anesthesia (SA) remains the technique of choice for many surgeries below the umbilicus, it is associated with multiple intraoperative complications. Sympathetic blockade and Bezold-Jarisch reflex do not fully explain SA-related cardiopulmonary complications. Reduction in FEV1 has been reported as a predictor of sudden cardiac death. This study aimed to determine the association between reduction in FEV1 following SA and adverse intraoperative cardiopulmonary complications.
Materials and Methods
A prospective study of 48 patients of ASA status I and II with no history of primary cardiopulmonary disease scheduled for elective surgery under SA. Spirometry was performed based on ATS/ERS guidelines before induction and 30 min after induction of SA. FEV1% predicted was determined using GLI 2012 equations. Participants were grouped into two (∆FEV1% < 10% and ∆FEV1% ≥ 10%) based on reductions (∆) in FEV1% predicted following SA. Logistic regression analyses were used to examine associations between ∆FEV1% and intraoperative hypoxia, hypotension, bradycardia, and nausea/vomiting, with adjustments for age, gender, and BMI.
Results
The mean FEV1% predicted following SA was lower than the mean FEV1% predicted before SA (83.42 vs. 95.31, p = 0.001). In a fully adjusted model, ∆FEV1% ≥ 10% was associated with an increased risk of hypoxia [AOR 13.55; 95% CI, 1.07–171.24, p = 0.044]. The positive associations between ∆FEV1% ≥ 10% and hypotension [2.02 (0.33–12.46), 0.449], bradycardia [1.10 (0.28–4.25), 0.895] and nausea/vomiting [9.74 (0.52–183.94), 0.129] were not statistically significant.
Conclusion
Reduction in FEV1% predicted following SA was associated with adverse intraoperative outcomes. FEV1 may play an important role in the association between SA and cardiopulmonary complications.
1 BACKGROUND
Spinal Anesthesia (SA) is the preferred anesthetic technique for short surgical procedures below the umbilical region including obstetric, gynecological, genitourinary, and orthopedic procedures.1, 2 Compared with general Anesthesia, SA is a less expensive, simple, reliable, and quick procedure, and gives optimal operating conditions in terms of muscle relaxation. It is also associated with a lower incidence of intra-operative hypertension and tachycardia, reduced postoperative analgesic requirement, and a shorter hospital stay.3-5
Despite its advantages over general Anesthesia, SA is associated with an increased risk of perioperative cardiac-related complications including hypotension, bradycardia, dysrhythmias, nausea and vomiting, and intraoperative cardiorespiratory arrest.2, 6, 7 Mechanistically, sympathetic chain blockage and/or the Bezold-Jarisch reflex mediated by 5-hydroxytryptamine (5-HT3) receptors are known to play important roles in these cardiac-related complications.8, 9 However, these mechanisms do not fully explain the cardiac-related complications following SA.
In the general population, a reduction in forced expiratory volume in 1 s (FEV1) was shown to be a predictor of sudden cardiac death in patients without primary heart or lung disease.10, 11 SA is known to impair pulmonary mechanics including a reduction in FEV1.12, 13 Therefore, it is biologically plausible that a reduction in FEV1 following SA could explain some of the cardiac-related complications of SA. However, this has not been previously investigated. We therefore determined the association between reduction in FEV1 following SA and adverse intraoperative cardiopulmonary complications.
2 METHODS
2.1 Study design and settings
This was a prospective study of Ghanaians scheduled for elective surgery under SA at the Greater Accra Regional Hospital (GARH) in 2019. GARH is a teaching and referral hospital in Ghana's capital, Accra, and attends to about 3500 surgical cases annually including about 800 elective non-obstetric surgical procedures under SA.
2.2 Recruitment
The study involved 50 patients aged 18 to 59 years with American Society of Anesthesiologists (ASA) physical classification I and II with no history of primary cardiopulmonary disease scheduled for orthopedic, gynecological, and general surgical procedures.
All study participants received standard perioperative care, based on guidelines of the GARH. Before surgery, all participants were seen at the preanesthetic clinic and optimized for surgery. Informed consent was obtained from study participants.
2.3 Data collection
A structured questionnaire was used by a trained research assistant to collect participant data. Data collected included demographics, socioeconomic status, smoking status (never smoked, currently smokes and previously smoked) and alcohol (never drink, occasional drinker, and regular drinker), previous anesthetics, and comorbidities including diabetes, hypertension, sickle cell disease (determined by cellulose acetate haemoglobin electrophoresis), and previous history of acute coronary syndrome. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg and/or current use of antihypertensive agents and/or physician-diagnosed based on hospital records. Diabetes was defined by fasting plasma glucose concentration of ≥7.0 mmol/L and/or the use of glucose-lowering agents and/or physician-diagnosed based on hospital records. Weight and height were measured in light clothing without shoes with SECA 22089 scale (gmbh & co. kg, Germany). Body mass index (BMI) in kilogram per square meter (kg/m2) was determined by dividing the weight in (kg) by the square of the height in (m).
2.4 Spirometry
A trained anesthetist performed spirometry for each participant before induction and 30 min after induction of SA in the supine position with a 30-degree head elevation. Spirometry was done using the Morgan FVL spirometer according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guideline.14 The spirometry procedure was first demonstrated to each participant, after which the participant was made to perform spirometry. Each participant was made to inhale rapidly and completely from functional residual capacity, after which a breathing tube was inserted into the participant's mouth, with the lips sealed around the mouthpiece and the tongue not occluding the mouthpiece. The forced vital capacity (FVC) maneuver was then started with minimal hesitation (within 1–2 s after inspiring to total lung capacity). The participant was prompted to ‘blast” air from their lungs and was encouraged to fully exhale. Throughout the FVC maneuver, enthusiastic coaching of each participant was carried out using appropriate body language and phrases, such as “keep blowing” or “keep going”. Measured and calculated spirometric indices from FVC maneuver included FEV1, FVC, and ratio of FEV1 to FVC (FEV1/FVC). The predicted values of FEV1, FVC, and FEV1/FVC ratio were determined for each participant based on their age, gender, height, and ethnic group using the Global Lung Initiative 2012 equations.15 Intraoperative spirometry could not be done for two participants due to a change in position during surgery and were therefore excluded from the analysis.
2.5 Intraoperative measurements
Intraoperative noninvasive measurements of blood pressure, pulse oximetry, electrocardiography, and temperature were done using Carescape Monitor B650 (GE, Finland) and recorded. Using a structured complication monitoring chart, the minimum heart rate, blood pressure, peripheral capillary oxygen saturation (SpO2), and temperature were documented every 3 min after induction of SA. SpO2 and pulse rate were measured with finger probes using the middle finger.16, 17 Hypoxia was defined as SpO2 of <95%.18 The study adapted the hospital's protocol to treat sustained hypoxia with supplemental oxygen and/or manage underlying causes. Blood pressure was measured on the right upper arm using appropriately sized cuffs. Hypotension was determined as systolic blood pressure (SBP) < 90 mmHg or reduction in SBP > 40 mmHg from baseline.19 Heart rate and rhythm were evaluated using 3-lead electrocardiography. Bradycardia was defined as a heart rate of less than 60 beats per minute. Episodes of nausea (self-reported) and vomiting were recorded.
2.6 Spinal anesthesia
Study participants fasted as per ASA fasting guidelines.20 Participants were intravenously infused 1 litre of lactated Ringer solution over 30 min immediately before the institution of SA. SA was induced in the sitting position with 3mls of 0.5% heavy bupivacaine (15 mg) and 0.5 ml (25 mcg) of fentanyl given over 15 s using gauge 26 pencil-point spinal needles at L4/L5 intervertebral space. Participants were positioned supine with head elevated to 30°. Alcohol preparation was used to assess the level of sensory block every minute. Sensory blockade to cold was achieved between T8 and T5 dermatomes. The modified Bromage score13 was used to assess motor blockade at 3 min following SA and recorded. To cater for fluid maintenance and replacement of deficit, intravenous crystalloids were given intraoperatively at 500 mls/h using an electronic infusion pump (DRE AVANTI NXT3, USA). A bolus of 5 ml/kg was administered as a rescue dose in hypotension. We further provided a warmer (Bair Hugger Model 505, USA) to mitigate hypothermia and hypoperfusion. Adverse effects following SA including difficulty in breathing, hypotension, bradycardia, nausea/vomiting, and hypoxia were managed based on the GARH standard guidelines.
2.7 Ethics and confidentiality
The principles of the Declaration of Helsinki and its appendices, as well as national legislation, were followed during the conduct of the study and all participants provided written informed consent. The study received ethical approval from the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana (CHS-Et/M.6-5.17/2018-2019).
2.8 Statistical methods
Data were analyzed using the IBM Statistical Package for Social Sciences version 23 software package. Data with normal distribution were presented as mean (standard deviation); whereas those not normally distributed were presented as median (interquartile range). Categorical data were presented as frequencies (percentages). Participants were grouped into two (∆FEV1% < 10% and ∆FEV1% ≥ 10%) based on reductions (∆) in FEV1% predicted following SA.21 Differences in demographic characteristics and intraoperative complications between ∆FEV1% < 10% and ∆FEV1% ≥ 10% were assessed by chi-square test or Fisher's exact test for categorical variables, and t-test for continuous covariates or the Mann–Whitney U-test for covariates not normally distributed. Logistic regression analyses were used to examine the associations between ∆FEV1% predicted and intraoperative hypoxia, hypotension, bradycardia, and nausea/vomiting, with adjustments for age, gender, and BMI. A power of study 80% at 95% confidence interval was used. The sample size was calculated using paired sample size formula based on previous studies13 and a p < 0.05 was considered as statistically significant.
3 RESULTS
3.1 Baseline characteristics
The study aimed to recruit 50 participants and follow them up during the perioperative period. Two of the 50 study participants were excluded because they were unable to perform technically acceptable spirometry after induction of SA. Therefore, the current analysis included data from 48 study participants. Baseline characteristics are presented in Table 1. The mean age and BMI, and the male-to-female ratio were similar in the ∆FEV1% < 10% and ∆FEV1% ≥ 10% groups. Additionally, the proportion of smokers, individuals who consume alcohol, and hypertensive patients were similar in the two groups. Further, the mean baseline FEV1 & predicted, FVC% predicted, and the FEV1/FVC were similar between the ∆FEV1% < 10% and ∆FEV1% ≥ 10% groups.
| Characteristics | All participants | ∆FEV1 < 10% group | ∆FEV1 ≥ 10% group | p-value |
|---|---|---|---|---|
| N = 48 | N = 31 | N = 17 | ||
| Age | 38.77 (±11.53) | 39.00 (±11.51) | 38.35 (±11.26) | 0.858 |
| Gender | 0.536 | |||
| Male | 30 (62.5%) | 18 (58.1%) | 12 (70.6%) | |
| Female | 18 (37.5%) | 13 (41.9%) | 5 (29.4%) | |
| Alcohol use (%) | 22 (45.8%) | 14 (45.2%) | 8 (47.1%) | 0.756 |
| Never smoked (%) | 46 (95.8%) | 31 (100.0%) | 15 (88.2%) | 0.121 |
| Diabetes (%) | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Hypertension (%) | 3 (6.3%) | 3 (9.7%) | 0 (0.0%) | 0.543 |
| BMI (kg/m2) | 24.93 (±4.69) | 25.79 (±5.16) | 24.40 (±3.79) | 0.349 |
| Baseline FEV1% pp | 93.82 (±28.30) | 89.16 (±20.93) | 103.41 (±37.59) | 0.097 |
| Baseline FVC % pp | 92.79 (±30.40) | 88.37 (±22.54) | 100.71 (±40.45) | 0.184 |
| Baseline FEV1/FVC pp | 102.15 (±10.23) | 102.55 (±10.94) | 103.65 (±7.40) | 0.174 |
| Baseline SpO2 (%) | 98.29 (±1.47) | 99.32 (±1.59) | 98.24 (±1.35) | 0.853 |
| Post SAB SpO2 (%) | 98.31 (±1.55) | 98.54 (±1.57) | 98.00 (±1.46) | 0.262 |
| Change in SpO2(%) | −0.02 (±1.39) | −0.21 (±1.24) | 0.24 (±1.48) | 0.301 |
- Note: ∆ spo2 is the difference between spo2 before spinal anesthesia and spo2 after spinal anesthesia.
- Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; pp, percentage predicted.
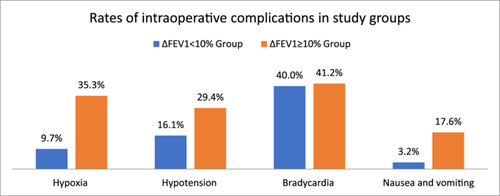
3.2 Rates of intraoperative complications
Figure 1 compares the rates of intraoperative complications in the two groups. The mean SpO2 before induction of SA, the mean SpO2 following SA, and the change in SpO2 following SA did not differ between the two groups. However, the rate of hypoxia was about five times higher in the ∆FEV1% ≥ 10% group compared to the ∆FEV1% < 10% group. The rates of hypotension and nausea/vomiting were approximately twice and five times higher in the ∆FEV1% ≥ 10% group than in the ∆FEV1% < 10% group, although the differences were not statistically significant.
3.3 Change in FEV1 and association with intraoperative complications
Mean FEV1% predicted following SA was lower than mean FEV1% predicted before SA (83.42 vs. 95.31, p = 0.001). The mean change in FEV1% predicted was 11.89 (95% CI 4.89–18.89). In an unadjusted model, ∆FEV1% ≥ 10% was associated with a sevenfold increased risk of intraoperative hypoxia (OR 7.09, 95% CI 1.23–40.75, p = 0.028). After adjustment for age, gender, and BMI, ∆FEV1% ≥ 10% was still associated with an increased risk of hypoxia [AOR 13.55; 95% CI, 1.07–171.24, p = 0.044]. The positive associations between ∆FEV1% ≥ 10% and hypotension [2.02(0.33–12.46),0.449], bradycardia was not statistically significant in the unadjusted model and in models adjusted for age, gender, and BMI (Table 2).
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Lower | Upper | Lower | Upper | |||||
| Hypoxia | 7.09 | 1.23 | 40.75 | 0.028* | 13.55 | 1.07 | 171.24 | 0.044† |
| Hypotension | 2.50 | 0.57 | 11.05 | 0.227 | 2.02 | 0.33 | 12.46 | 0.449 |
| Bradycardia | 1.08 | 0.32 | 3.69 | 0.900 | 1.10 | 0.28 | 4.25 | 0.895 |
| Nausea and vomiting | 5.79 | 0.55 | 60.87 | 0.144 | 9.74 | 0.52 | 183.94 | 0.129 |
- 1 Model 1 – Unadjusted: Model 2 – Adjusted for age, gender, and BMI.
- † p < 0.05.
4 DISCUSSIONS
4.1 Summary of key findings
In the current study, there was a significant decline in FEV1 percentage (%) predicted following SA. A relatively high proportion of individuals (35%) had a change in FEV1 ≥ 10% following SA. The reduction in FEV1% predicted ≥ 10% was associated with nearly 14-fold increased risk of intraoperative hypoxia.
4.2 Discussion of key findings
In a population of Ghanaians undergoing elective surgery under SA without any cardiac and/or pulmonary disease, we demonstrated that there were significant reductions in the percentage predicted FVC and FEV1 30 min following induction of SA. The effect of SA on spirometry indices including FEV1 and FVC has been previously reported in other populations12, 13, 21, 22 but not in sub-Saharan Africans. Considering the role of ethnicity in pulmonary dysfunction23 this study was warranted. Our results confirm previous reports12, 13 and have expanded the evidence to sub-Saharan Africans. However, our findings on the change in FEV1 contrast reports among Austrians and Chinese13, 21 which did not show a significant reduction in FEV1 following SA; in those studies, there was a significant reduction in FVC.
A relatively high proportion of individuals had a change in FEV1% predicted exceeding 10%. Reduction in FEV1% predicted was associated with intraoperative complication. There have been fewer publications on the association of lung function with the risk of developing SA-related complications. Many cohort studies within other populations have shown that reduction in FEV1 is a predictor for cardiovascular diseases such as first-time stroke, heart failure, myocardial diseases, atrial fibrillation, and even sudden cardiac death.10, 11, 24 Several studies have also observed reduction in blood pressure and heart rate after induction of spinal SA.25, 26 For instance, in the 511 participants by Fakherpour et al study, there was significant incidence of hypotension classified as mild, moderate, and severe 20%, 35% and 40% respectively post SA induction. The role of sympathetic blockade in these studies has been highlighted. However, how a reduction in pulmonary function predicts such cardiopulmonary-related complications remains unknown.
We demonstrated for the first time that participants with a reduction in FEV1 ≥ 10% had a sevenfold increased risk in intraoperative hypoxia post-SA. The risk of hypoxia was persistent even after we adjusted for age, gender, and BMI. There were also positive associations between the reduction in FEV1% predicted and the risk of hypotension and bradycardia. These findings indicate that a reduction in FEV1% predicted may be associated with a high risk of intraoperative hypoxia post-SA. The mechanistic basis is unclear. However, it is biologically plausible that the increased reduction in FEV1% predicted may indicate a reduction in pulmonary function reserves. Therefore, individuals whose pulmonary reserves are challenged following SA, evidenced by a drop in FEV1% predicted are likely to have poorer pulmonary gas exchange and decreased arterial oxygenation. This may affect tissue oxygenation, evidenced by intraoperative hypoxia. Our study used reduction in SpO2 as a surrogate marker for hypoxia since it is a simple, reliable, and objective measurement that approximates arterial oxygen saturation (SaO2). The relationship between FEV1 and mortality independent of baseline cardiac function has been previously described in the general population.10 Also in the general population, FEV1% predicted is a known robust predictor of sudden cardiac death independent of cardiac function.11 These findings give credence to our observation of a positive association between a reduction in FEV1% predicted and cardiac dysfunction following SA, although the biological basis remains unclear.
4.3 Limitations
In assessing tissue hypoxia, SpO2 was used. In addition to known deviations from SaO2, other factors affect the accuracy of SpO2 measurements; these include motion, low perfusion, hypothermia, and excessive ambient light.27-29 As precautionary measures, we ensured adequate hydration, normothermia, and limited movement of the probe site. The wide 95% CI for the odds of intraoperative hypoxia warrants the need for a larger sample-sized study testing the association between change in FEV1 and intraoperative hypoxia.
5 CONCLUSION
In conclusion, SA results in reduction in FEV1% predicted. Reduction in FEV1% predicted following SA is associated with adverse intraoperative outcomes including hypoxia. FEV1 may play important roles in the association between SA and cardiopulmonary complications. Our findings provide opportunities for future research assessing the mechanism of intraoperative complications following SA. Oxygen therapy might be beneficial in patients with increased risk of reduced FEV1 following SA.
AUTHOR CONTRIBUTIONS
Melody Kwatemah Agyei-Fedieley: Investigation; writing—original draft; writing—review and editing; resources; conceptualization; methodology; formal analysis; visualization; validation; funding acquisition; data curation; software; project administration. Ebenezer Owusu Darkwa: Conceptualization; methodology; validation; supervision; writing—review and editing; writing—original draft; resources; data curation. Charles F. Hayfron-Benjamin: Conceptualization; data curation; formal analysis; methodology; software; validation; writing—original draft. Adeyemi Olufolabi: Software; conceptualization; supervision; validation; funding acquisition; resources; writing—original draft; writing—review and editing. Evans Atito-Narh: Data curation; conceptualization; writing—original draft; resources; investigation; methodology. Jerry Agudogo: Conceptualization; data curation; investigation; writing—original draft; resources; writing—review and editing. Bartholomew Dzudzor: Conceptualization; methodology; investigation; validation; formal analysis; supervision; funding acquisition; visualization; project administration; resources; writing—review and editing; writing—original draft; data curation.
ACKNOWLEDGMENTS
The authors are very grateful to the Head of Surgery, Greater Accra Regional Hospital (GARH), Dr. Reuben Ngissah, the Department of Anaesthesia and Critical Care, GARH for their immense support in patient recruitment. George Aryee and Raymond Essuman of the Anaesthesia Department, University of Ghana medical school assisted with data analysis. We thank Richard Fedieley and all participants for their dedicated contribution of time and effort.
TRANSPARENCY STATEMENT
The lead author Bartholomew Dzudzor affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data set used and/or analyzed during the current study are available at \.\Downloads\Reduction in FEV1 data.xls.