Can Marburg virus be sexually transmitted?
Abstract
Background and Aim
Marburg virus (MARV) is a highly virulent virus of animal origin and the cause of a lethal infection (known as Marburg virus disease [MVD]) with a case-fatality ratio ranging from 24% to 90%. While the potential nonzoonotic routes of virus spread are plausible, the risk is not yet fully determined. Here, we described the ways by which MARV spreads within the human population focusing mainly on the potential of sexual transmission. In addition, we addressed some measures that should be taken to minimize the risk of sexual spread of the virus and proposed a future research agenda on the risk of sexual transmission.
Methods
For this perspective, we searched four electronic databases (i.e., PubMed, Scopus, Web of Science, and Google Scholar) and included the most relevant studies published since the first identification of the virus in 1967. We used “Marburg virus,” “Marburg virus disease,” “Seminal fluid,” “Sexually-transmitted virus,” “Sexual transmission,” and “Emerging infectious disease” as keywords.
Results
MARV is transmitted to humans via both direct and indirect contact with infected animals (most importantly bats) and individuals who have recently been diagnosed with or died of the disease. The virus transmission through sexual contact has been previously suspected (exclusively from men to their sexual partners). Studies suggest that this virus persists predominantly in testicular Sertoli cells within seminiferous tubules over a relatively long period and is released through seminal fluid (in some reports >200 days post onset of infection) both could potentially threaten sexual health. In addition to men, women could theoretically, although less probably contribute to the sexual transmission of the disease.
Conclusion
MVD, however, rarely, could be passed through sex, and men appear to be the main carriers in this regard. Taking preventive countermeasures and practicing safe sex are recommended to reduce the risk of interhuman transmission.
Key points
-
Marburg virus disease (MVD) is a severe and often lethal viral infection with epidemic potential recorded over the past few decades.
-
Marburg virus (MARV) spreads to humans via animal-to-human and human-to-human transmission.
-
MARV infects the male and likely female reproductive system with testes being the main site of virus persistence.
-
MARV sheds into genital fluids, particularly in the seminal fluid of male survivors, and probably could be passed, however rarely, through sex, even after the disease has resolved.
-
Sexual transmission of MVD is preventable by early detection, taking precautions, and practicing safer sex.
1 INTRODUCTION
In recent years, several outbreaks of an extremely virulent virus with a high case fatality ratio (CFR) [24%–90%] commonly referred to as Marburg virus (MARV) have made headlines exclusively in sub-Saharan Africa amidst the concurrent coronavirus disease 2019 pandemic.1, 2 The basic reproduction number of Marburg virus disease [MVD] is low and varies across outbreaks, ranging from 0.5 [95% confidence interval [CI]: 0.05–1.8] to 1.2 [95% CI: 1.0–1.9], so the frequency of large-scale outbreaks is low, particularly in areas with robust health systems.3, 4 As summarized in Table 1, MARV outbreaks rarely occur (16 recorded outbreaks since 1967, most of which have occurred in Africa [Kenya, Democratic Republic of Congo [DRC], Angola, Uganda, Guinea, Tanzania, and Equatorial Guinea]) with a relatively small proportion of people affected in general, however, given the high lethality, these outbreaks demand both national and international attention as could pose negative consequences on human health.
| Year(s) of outbreak | Location | Number of reported cases | Number of reported deaths |
|---|---|---|---|
| 1967 | Germany | 31 | 7 |
| Yugoslavia (Serbia) | |||
| 1975 | South Africa | 3 | 1 |
| 1980 | Kenya | 2 | 1 |
| 1987 | Kenya | 1 | 1 |
| 1988–1995 | Russia | 4 | 2 |
| 1998–2000 | DRC | 154 | 128 |
| 2004–2005 | Angola | 252 | 227 |
| 2007 | Uganda | 4 | 1 |
| 2008 | US | 1 | 0 |
| 2008 | Netherland | 1 | 1 |
| 2012 | Uganda | 15 | 4 |
| 2014 | Uganda | 1 | 1 |
| 2017 | Uganda | 4 | 3 |
| 2021 | Guinea | 1 | 1 |
| 2022 | Ghana | 3 | 2 |
| 2023 | Tanzania | 9 | 6 |
| 2023 | Equatorial Guinea | 40 | 35 |
- Abbreviations: DRC, Democratic Republic of Congo; US, United States.
MARV is an enveloped single-stranded RNA virus, similar to the Ebola virus (EBOV) (Figure 1), and the first filovirus that was discovered in humans after two simultaneous outbreaks outside of Africa in the 1960s (Table 1).5, 6 This virus is associated with a severe hemorrhagic fever syndrome (known as MVD) that is clinically diagnosed with a sudden onset of flu-like symptoms (i.e., fever, severe headache, muscle pain [myalgia], and sore throat) after a varying incubation period of 2–21 days.7 Patients with severe infection die from the disease (commonly due to severe hemorrhage and shock) between Days 8 and 9 of symptomatic illness.8 In nature, as a result of spillover events, MARV spreads to humans from infected animals (most importantly, bats); however, human-to-human transmission also occurs and makes outbreaks more complex to contain.8 Understanding the routes through which MARV is interpersonally transmitted is crucial for the innovation of impactful life-saving interventions in epidemic-prone regions in future outbreak containment. Here, we will underscore the routes of MARV transmission with a focus on the risk of viral spread to humans via sexual contact followed by infection diagnostics and recommendations for sexually active men and women to minimize the risk of interhuman spread of the disease. This article also discusses several open questions that need to be addressed.
2 HOW CAN MARBURG VIRUS BE TRANSMITED?
As the cause of a lethal zoonotic infection, MARV is transmitted to humans primarily from animal reservoirs, most often bats, either directly or via an intermediate host.5 Indeed, bats (in particular, fruit bats [Rousettus aegyptiacus]) serve as the main natural reservoir that contributes to the virus spread to other bats (horizontally and vertically) and other mammals including humans (mainly those who visit or work in caves or mines inhabited by infected bat colonies).5, 9 In addition to bats, MARV is known to infect a relatively significant number of nonhuman vertebrates which may contribute to its maintenance in nature.5, 10
For MVD, interhuman transmission with many possible modes reported is a matter of concern, particularly in regions with poor health care systems; however, the main route is unknown.4 There are several factors that could increase the risk of MARV transmission and subsequently virus infection in humans. These factors might include the age, sex, and occupation of patients, having a history of traveling to high-risk regions, and sharing meals and objects with infected persons.11 It is believed that humans are most likely to become infected when they come into direct contact (injured skin [wound and scrape] or ruptured mucous membranes [eyes, nose, or mouth]) with the virus-laden biofluids (i.e., urine, saliva, breast milk, amniotic fluid, and semen) of an infected individual usually during the care of patients (by family members and health workers) or via close contact with those who have died of the disease through handling of corpses during burial proceeding.10, 12, 13 Given the risk of virus spread through contaminated material (with stability for 4–5 days on surfaces), the disease may appear in humans through indirect contact.1, 14 Considering the similarities between MARV and EBOV in terms of many anecdotal routes of transmission, that is, infection through biofluids, the question arises as to whether MARV could also be sexually transmissible through genital fluids during sexual intercourse.
3 IS MARV A SEXUALLY TRANSMITTED VIRUS?
For MVD, sexual transmission represents a possible route of virus spread, with the first patient reported to be a woman who probably became infected following sexual contact with a man long after his recovery.15, 16 Factors affecting the sexual transmission of viruses may include the age of patients, the stage of infection, the status of the immune system, and the role of microbial coinfections.17-20 As reviewed in the recent literature, the male genital tract serves as an anatomical site of infection for at least 30 DNA and RNA viruses including human immunodeficiency virus (HIV), herpes simplex virus-2 (HSV-2), human papilloma virus (HPV), and hepatitis B and C viruses (HBV and HCV), that are often released in semen and threaten sexual health.21 As there is no evidence of female-to-male and female-to-female sexual transmission, men are more likely to be the main carriers of MARV, with the testes being a major site of viral localization.22-24 In addition to testicular tissue, the other possible sources of virus within the male reproductive tract have yet to be identified.
How MARV behaves within the human reproductive system remains to be fully understood and data derived from animal models are scarce in this regard. Experiments with nonhuman primates (NHPs) have concluded that the testis is regarded as an “immune-privileged” site (also called sanctuary site), where the virus can reside for a long period with no detrimental activation of the immune system.23 In acutely infected NHPs, MARV was found in the interstitium and endothelial cells of the testis, as well as in smooth muscle cells of the epididymis and prostate stroma.24 Although it is still unclear, the hematogenous pathway appears to be the likely route by which MARV enters the male genital tract and establishes infection.25 From EBOV studies, it seems that infected tissue macrophages carry the virus from stromal replication sites in reproductive tissues to the seminal fluid.26 The involvement of testes may clinically be diagnosed through the signs and symptoms of discomfort, enlargement, and inflammation (referred to as orchitis) weeks after infection.27 Females with MVD may exhibit signs of gynecologic hemorrhage.28 Like EBOV, it is theoretically possible for MARV to be sexually transmitted from females to males, despite being less probable.29 Through the study by Perry et al. who investigated the localization of EBOV in the reproductive tract of macaques that succumbed to the Ebola virus disease (EVD), the authors showed the susceptibility of both male (i.e., the testis, seminal vesicle, epididymis, and prostate gland) and female reproductive tissues (i.e., the ovary and uterus) to the virus infection.26 Through in situ hybridization, MARV's RNA has been found in the uterine endometrial stroma of virus-infected NHPs, which indicates that the female reproductive system might also be a source of viral infection similar to the male reproductive system.24 It was further discovered that the ovary and oviduct are the other sites of viral infection with necrotizing lesions through histological investigations.24
Unlike the liver, lymph nodes, and spleen, which are cleared of viral remnants shortly after an infection, somatic cells (also known as testicular Sertoli cells [TSC]) that form the structure of the blood–testis barrier (BTB) reside in seminiferous tubules, a unique immunological environment that shields the sperm cells from being targeted by the immune system, found to serve as the main cellular reservoir for MARV to persist for an extended period. This may lead to the breakdown of the BTB likely with no adverse effects on overall reproductive function (Figure 2).23 In addition to TSC, further studies revealed that interstitial Leydig cells (LC), peritubular myoid cells, blood vessels, germ cells, and infiltrated macrophages/monocytes to the seminiferous tubules could also act as the additional targets for viral infection (Figure 2).23 Similar to MARV, EBOV infects both TSC and LC with LC being likely more permissive to virus infection.30 In testes where persistent infection has been established, the interstitial tissue contains infiltrated immune cells (i.e., lymphocytes, macrophages, and neutrophils).23 The activation of the immune system in persistently infected seminiferous tubules may cause degeneration of tubule walls, breaking tight junctions, and recruitment of immune cells to the site of infection.23 Whether MVD-associated infertility will be observed in humans remains to be demonstrated.
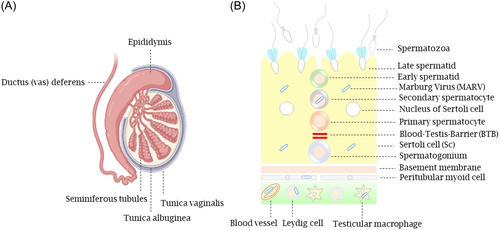
Less is known about the mechanisms involved in viral infection and maintenance in the testes. In light of the recent studies, once the infection of MARV is established, localized immunosuppression elicited by focally infiltrated lymphocytes (with a specific role for the regulatory T cells [Treg]) in the interstitium and seminiferous tubules of the testes likely contributes to a delay in the clearance of virus from infected testes and results in a sustained infection.23 A decrease in the number of these cells is detectable in autoimmune disorders, while their abundance favors the inhibition of immunological responses.23 Therefore, targeting testicular immunosuppression worth further studies, especially the research that seeks to understand the persistence mechanism of MARV in the male reproductive system.
The viral clearance from seminal fluid takes an extended duration as infectivity has been noted in survivors for weeks after recovery.15 For EBOV, the virus has been detected in an individual who remains infected for over 900 days following resolution of acute viral infection raising concerns regarding the potential for long-term sexual transmission.17 Furthermore, it has been observed that male survivors can transmit the infection to their female partners through sexual contact about 470 days after the onset of the disease.31 Recently, seminal amyloid fibrils reported to play a possible role in enhancing EBOV infection by protecting the virus against potential environmental stresses and inducing alternations in physical properties of the virus that are critical for virus transmissibility, however, for MARV the role of these proteins is yet to be determined.32
For MARV, while the specific ways by which the virus is sexually transmitted are still being studied, it is crucial to take the sexual route of transmission into account when evaluating the likelihood of outbreaks. To our knowledge, no cases of MARV transmission from women to men (for instance, through interacting with or touching genital fluid)12 or between homosexual men or women have been reported so far. The first evidence of heterosexual transmission of MARV dates back to the 1967 outbreak in Belgrade, Yugoslavia (Serbia) when a woman contracted the disease, most likely through sexual contact, 2 weeks before being admitted to a medical center (on Day 3 of her illness) with a symptomatic infection and more than 6 weeks after her husband, who was infected through exposure to an animal (most likely grivets) recovered from the associated illness. After the husband's semen was analyzed, the antigen of MARV was detected, and the viable virus was subsequently recovered.15, 16 Indeed, the case was not confirmed as being caused by sexual transmission, though it appears probable, however other routes of transmission (e.g., direct contact with infected blood) are also plausible.16 Years later, in 1980, viral isolation was evidenced in the semen of a survivor from Kenya 2 months after disease recovery.33 According to another document, semen may still contain the infectious virus at least 3 months after infection.34 In a small subset of cases that have recovered from MVD (even if there are no longer acute symptoms of the disease), semen can still be tested positive more than 200 days after the onset of infection.35 Thus, it seems reasonable to assume that sexual fluids transmit the infection over a relatively long time. Here, we underestimate the potential need for extended monitoring of infected individuals and their sexual contacts beyond current practices, considering the extended survival of the virus in the reproductive organs/fluid.
4 HOW CAN MARBURG VIRUS BE DIAGNOSED?
MARV can be diagnosed initially by clinical assessment and specifically by laboratory testing using multiple modalities including (I) cell culture (e.g., using Vero cells), (II) Electron microscopy (EM), (III) molecular analysis, (IV) serological assays (antigen- or antibody capture detection test), (V) Indirect fluorescent antibody test (IFA), and (VI) immunohistochemistry analysis, to detect viral particles, proteins or ribonucleic acid.36, 37 The most commonly used methods in the diagnosis of MVD include reverse transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA).37 For most diagnostics, blood with a minimum volume of 5 mL collected into the EDTA tube (stored at room temperature for a day, refrigerated for a week, or frozen for more than a week to prevent RNA degradation) is the proper biological sample that can be used.38, 39 However, in the lack of the possibility to collect blood, other specimens (e.g., saliva, urine, and breast milk) can alternatively be used for diagnostics.40 The sample-containing tubes should be sent on dry ice (with the highest level of biosafety precautions), regardless of the type of test, to national and/or international reference laboratories to maintain the cold chain throughout transportation.41, 42 Viral inactivation by gamma irradiation, heat treatment, and Sodium hypochlorite has allowed for safe handling and testing of collected specimens.6 All laboratory workers are advised to work in a safe work environment and use personal protective equipment (PPE) while testing infectious material due to high biohazard risk.42 Importantly, one sample with a negative test result probably does not guarantee loss of positivity, thus, testing samples with at least two reliable methods described herein, rather than a single test much preferred as it can minimize any misdiagnosis. In addition to the above methods, low platelet and white blood cell count, high levels of liver enzymes, and proteinuria on urinalysis are valuable diagnostic parameters during the course of illness.37 Despite no fully validated assays for specifically detecting MARV in the semen, the assay of quantitative RT-PCR targeting four most common regions including viral nucleoprotein (NP), glycoprotein (GP), viral protein 40 (VP40), and RNA-dependent RNA polymerase (L) which has already been utilized to test EBOV in the semen specimens could considerably be the preferred diagnostic method for MARV, as well.43-45 However, the lack of or insufficient access to diagnostics especially in resource-poor settings, similarities between the symptoms of MVD and hemorrhagic fevers and other infectious diseases (i.e., malaria, shigellosis, meningitis, typhoid fever) especially in early infections, the need for highly skilled and trained staffs who are experienced enough to work with highly virulent viruses, and the need for the establishment of high-containment Biosafety Level 4 (BSL-4) laboratories with necessary safety protocols, all can cause a delay in the identification of MARV and subsequently increase the risk of virus spread.2, 8 For timely response to the outbreaks of MVD achieved by early detection, isolation, and treatment, there is a need for diagnostic approaches particularly rapid testing tools to be developed, validated, and accessed to remote areas at risk of future outbreak.46
5 RECOMMENDATIONS
The recent outbreak of the rarely reported monkeypox (Mpox), a smallpox-like illness caused by the monkeypox virus (MPXV), underscored the need to take the sexual spread of viruses that have not previously been sexually transmitted, into serious consideration.47 This virus has an incubation period of 6–13 during which it can be transmitted to humans via close contact with virus laden biofluids from an infected individual. The spread of the virus can also occur through sexual intercourse which likely is a new mode of transmission for this virus infection.48, 49 Importantly, among those who were affected by the recent outbreak and disclosed their sexual orientation, 95.8% were homosexual men.50 The analysis of semen yielded positive results for the genetic material of the virus in the majority of infected individuals.48 Skin lesions observed in the anogenital area were reported to be a common feature (>90%) in this outbreak which could indicate that the virus is likely transmissible via skin-to-skin or mucosal contact at the time of sexual intercourse.51
Although sexual transmission of MARV is not much discussed and rarely occurs, the possibility for future outbreaks should not be underestimated as the world witnessed a similar scenario for MPXV. Therefore, raising awareness for the general public and individuals having close contact with MVD cases is essential as insufficient knowledge regarding the sexual spread of infectious diseases such as MVD can lead to misunderstandings, stigma, and discrimination, as well as the further spread of such diseases that makes virus containment more complex.
To minimize the risk of MARV spread, preventive measures are crucial before any exposure to the potential source of infection. As one of the best strategies, developing vaccines with desirable efficacy, tolerability, and safety is essential to provide long-lasting protection against severe disease and death. In this regard, efforts have been made to develop a reliable vaccine with no adverse reactions following immunization. While several candidates have been developed and assessed (i.e., whole-inactivated or subunit vaccines, adenovirus vectored vaccines [e.g., chimpanzee adenovirus type 3-vectored Marburg virus (cAd3-Marburg)], DNA vaccines [e.g., MARV Musoke encoding viral glycoprotein (GP) gene], Recombinant vesicular stomatitis virus (rVSV) vaccine [e.g., recombinant vesicular stomatitis virus (rVSV) expressing the MARV-Musoke glycoprotein], virus-like particles (VLP) [e.g., Marburg Musoke VLPs (mVLPs)], and mRNA-based vaccine [e.g., mRNA-1,360], there is no approved vaccine readily available to contain MVD outbreaks.10, 52, 53 Therefore, standard precautions should seriously be taken to reduce the risk of viral transmission before vaccine administration. In this regard, nonpenetrative sex or using physical barriers (i.e., condoms) properly and consistently during any sexual intercourse (vaginal, anal, and oral sex) for the whole duration of the disease (even at least a full year after that) and safely handled and disposed of barriers after any sexual engagements to avoid any contact with the seminal fluid is highly recommended.22, 45
It is essential for all sexually active males (and possibly females) who have been diagnosed with MVD or have had close contact with an infected person to abstain from engaging in unprotected sexual activity for a relatively long period (approximately 12 months) until they have fully recovered from the disease by having two negative results of virus testing.10 These individuals should also be provided mainly by public health authorities and organizations with detailed information about the risk of sexual transmission throughout and following the duration of the disease. Given the persistence of MARV in the reproductive system, it would be beneficial to monitor all previously diagnosed patients even beyond a year after recovery, since for EBOV the length of time for virus clearance from seminal fluid extends beyond 2 years, as discussed earlier.17 In addition, other preventive strategies such as contact tracing, early identification, the quarantine of cases, and strict application of other approaches are crucial, especially when vaccines cannot be offered. In addition to considering preventive strategies, developing antiviral agents and definite therapeutics equally needs to be prioritized. Indeed, in the absence of effective treatment, the symptoms determine how infected men and women should be treated and the disease managed. Treatment mainly relies on supportive measures including hydration with oral or intravenous fluids and electrolyte replacement, pain, oxygen, and fever management, platelet transfusion, and treatment of secondary infections.54 However, antivirals such as Galidesivir, Favipiravir, Remdesivir, and Polyclonal concentrated immunoglobulin G might be used to treat MVD.39 As discussed for the EVD, there is a need for male survivors to be encouraged to enroll in semen testing and counseling programs to be informed of the risks they can likely pose to their partners during sexual intercourse.55 In addition to men, access to validated testing of genital fluids should also be offered to women, as they could theoretically, although less probably (based on current knowledge), be a source of viral infection.56
Notably, the strategies mentioned are just a fraction of what we can consider for better response to future risks. For future outbreaks, establishing and improving surveillance systems in tracking new cases, expanding research on vaccines and therapeutics development, improving diagnostics, increasing accessibility to health care, and raising the public knowledge about the nature of the threat and dangers of being underestimated needs to be prioritized. There is also a need for intensive global collaboration to be undertaken to ensure responsiveness to highly infectious diseases such as MVD, that could be a threat to sexual health.
6 FUTURE DIRECTION
For MARV, it is essential to address almost all the challenges, knowledge gaps, and misconceptions regarding its possibility for sexual spread to better understand the transmissibility of the virus and better respond to future threats. Given the low possibility of MARV being transmitted sexually, it can be challenging to ascertain the origin of the infection. On the research front, we should address several critical questions to provide a better insight into the potential of MARV for sexual transmission to humans and the impact of MVD on the male, and likely the female, reproductive system and function. These questions include (I) How does MARV enter the male and likely female reproductive system? (II) Can sexually active women be a potential source of MVD? (III) At what concentration MARV can be sexually transmitted? (IV) Where does MARV hide in the human reproductive system in addition to previously identified sites of infection? (V) Does MVD affect male and likely female reproductive function in human survivors? (VI) Are individuals who are immunosuppressed more likely to become chronic carriers? (VII) Does sexually transmitted risk for MVD vary by symptom status? (VIII) Considering the role of tissue-resident immune cells in particular macrophages in the development of EBOV persistence,26 what is the underlying mechanism and role of the immune system in MARV persistence in the human reproductive system? (IX) Is coexisting sexually transmitted infections (STIs) playing an important role in the context of sexual transmission of MARV? (X) Which form of sexual behavior (anal, vaginal, and oral sex) is more likely to result in the transmission of the virus than the others? (XI) Given the length of time that MARV appears to be able to survive in the reproductive organs and or sexual fluids can be on the scale of months, should monitoring of infected individuals and their sexual contacts extend beyond what is currently practiced? (XII) And last but not least, what is the duration of time within which the virus can persist in the reproductive tract of both men and likely women? Is the 200 days observed in men indeed the longest duration, or could it persist in semen even longer?
The answer to these questions may have significant importance regarding public health decisions as it will enable public health officials to recommend safe sex behaviors and effective interventions that might be urgently needed at the time of the virus outbreaks. It also provides better insight into the threats linked to sexual contact and the mechanisms by which MARV establishes a sustained infection in the human reproductive system.
7 CONCLUSION
The interpersonal spread of MARV is a matter of concern as it can potentially result in fatal outbreaks. The contribution of sexual transmission to the spread of the virus remains largely unknown. However, the transmission of MARV via sexual contact should always be considered as a possible secondary mode that threatens sexual health. Considering EBOV, and a few reports on the potential of MARV to infect the reproductive system and shed into genital fluids, the present perspective highlights the necessity of taking preventive countermeasures into serious consideration to minimize, if any, the risk of possible sexual transmission of filoviruses with a focus on MARV to a feasible extent.
AUTHOR CONTRIBUTIONS
Hassan Karami: Conceptualization; supervision; visualization; writing—original draft. Arash Letafati: Investigation; data curation. Somayeh Sadat Hosseini Fakhr: Writing—review and editing.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to the authors of the articles their referenced. Also, the authors thank three anonymous reviewers for their helpful and constructive feedback on the earlier version of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
It is an analysis of online available aggregate data. No ethical approval was needed.
TRANSPARENCY STATEMENT
The lead author Hassan Karami affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.