Effects of a leave-on product on the strength of the dermoepidermal junction: An exploratory, intraindividual, randomized controlled trial in older adults with dry skin
Trial registration: German Clinical Trials Register ID: DRKS00031151.
Abstract
Background and Aims
Skin aging is associated with dry skin and a decrease of the strength of the dermoepidermal adhesion, which increases the risk for lacerations (skin tears). Application of leave-on products improves dry skin and seems to reduce skin tear incidence. The aim of this study was to measure the effects of a humectant containing leave-on product on the strength of the dermoepidermal junction in older adult participants with dry skin.
Methods
A randomized controlled trial using a split body design was conducted. One forearm was randomly selected and treated with a lipophilic leave-on product containing 5% urea for 8 weeks. The other forearm was the control. The parameters stratum corneum hydration (SCH), transepidermal water loss, pH, roughness, epidermal thickness and skin stiffness were measured at the baseline, Weeks 4 and 8. At Week 8, suction blisters were created and time to blistering was measured. Blister roofs and interstitial fluid were analyzed for Interleukin-1α, 6 and 8.
Results
Twelve participants were included. After 8 weeks treatment, SCH was higher (median difference 11.6 AU), and the overall dry skin score (median difference −1) and median roughness (Rz difference −12.2 µm) were lower compared to the control arms. The median group difference for Interleukin-1α was −452 fg/µg total protein (TP) in the blister roofs and −2.2 fg/µg TP in the blister fluids. The median time to blister formation was 7.7 min higher compared to the control arms.
Conclusion
The regular application of humectant containing leave-on products improves dry skin and seems to lower inflammation and contribute to the strengthening of the dermoepidermal adhesion. This partly explains how the use of topical leave-on products helps to prevent skin tears.
1 INTRODUCTION
Aging is associated with physiological and morphological changes of the skin, increasing the susceptibility to many dermatological conditions and skin injuries.1 Dry skin (xerosis cutis) is associated with intrinsic aging and prevalence estimates in older adults range between 41.2 and 99.1%.2-6 The prevalence of skin dryness increases with increasing age.7 In xerosis cutis, decreased stratum corneum hydration (SCH),8 increased pH,9, 10 increased roughness, decreased elasticity,7 possible subclinical inflammation11 and altered molecular markers12 have been reported. Dry skin related pruritus might affect patients' quality of life.13 Scratching can lead to painful wounds.14 There are various visual analogue scales and scoring systems to assess the severity of skin dryness. One widely used system, described by the European Group on Efficacy Measurement of Cosmetics and other Topical Products, is the “overall dry skin score” (ODS) where the severity of dryness is evaluated from “slight” to “extreme” xerosis.15, 16
Ageing related changes also affect the dermoepidermal junction (DEJ). DEJ is an anchoring system formed by interdigitation of epidermal protrusions downward into the dermis and dermal papilla projections upward into the epidermis.17 In older adults, DEJ is gradually disorganized, epidermal protrusions and dermal papillae are reduced,18 which lead to significant thinning and flattening of DEJ and resulting in increased fragility.19-21 Though a direct relationship between a fragile DEJ and skin tears has not been established in clinical research, in-vitro studies show DEJ damage by inflammatory cytokines and subsequent formation of skin tears.22 Interestingly, skin dryness is also one of the strongest predictors of skin tear development23 and the risk factor is considered modifiable.24 Especially in care dependent populations the skin tear prevalence is up to 22%.5, 25, 26 For measuring the strength of DEJ adhesion in clinical research, suction blistering can be used.20, 27 Suction blistering is an artificial and controlled technique28, 29 and is widely used in dermatology, for example, for studying wounds or epidermal grafting.30, 31 A constant negative pressure (suction) is applied on the skin surface, and after time sub-epidermal vesicles arise and eventually coalesce to form a single cavity filled with interstitial fluid, as the complete dermoepidermal separation along the DEJ occurs.32 The parameter “time to blistering” was suggested as a clinically relevant outcome, which reflects the resistance and mechanical integrity of DEJ.20, 27, 33
Basic leave-on products are helpful in decreasing skin dryness, improving skin barrier function, as well as reducing the risk of skin tear development in the older adults.34, 35 Humectants in combination with basic leave-on products are effective in this regard and any effect on the skin is due to the total composition of the product.36 Urea is widely accepted as a potent humectant and is one of the most extensively studied product ingredient for the treatment of dry skin,7 which was found to improve hydration, barrier function, to reduce transepidermal water loss (TEWL), skin pH37, 38 and roughness.39 Urea added to lipophilic leave-on products was associated with stronger hydrating effect.40 Products containing 5% urea are considered tolerable on moderately scaly skin.7 Previously we have shown, that the application of petrolatum in skin healthy older people improved DEJ adhesion.33 Since dry skin is one prognostic factor for skin tear development,23 we hypothesized that the effect might be stronger in dry skin; in terms of increased DEJ adhesion and subsequent reduction of the risk of skin tears. Thus, the main aim of this study was to investigate the effects of a 5% urea containing leave-on product on the adhesion of the DEJ in older adult participants with dry skin.
2 METHODS
2.1 Trial design
An exploratory, within person randomized controlled trial was conducted from January to April 2023 at the Clinical Research Center for Hair and Skin Science (CRC) at Charité - Universitätsmedizin Berlin, Germany (German Clinical Trials Register ID: DRKS00031151, registration date: 30 January 2023).41 Using a split-body design, the volar surface of one forearm of the participants was randomly selected for applying a leave-on product. The other forearm was considered as control arm on which no product was used for the entire trial period. The study was approved by the ethics committee of Charité - Universitätsmedizin Berlin (application number: EA1/228/22, date of approval: December 12, 2022). No changes were made after the commencement of the study.
2.2 Participants
Inclusion criteria were 65−85 years old males or females, having skin phototype I−III according to the Fitzpatrick classification, body mass index between 20 and 30 kg/m2, nonsmoker since at least 1 year and provided written informed consent. Eligibility criteria for the body sites were slight to moderate skin dryness (ODS category 1−2) on the volar surface of the forearms according to the ODS system,15 absence of skin diseases and lesions including atopic dermatitis, urticaria, psoriasis, scars, wounds or tattoos on the investigational skin areas. Major exclusion criteria were severe or extreme dryness (ODS category 3 or 4) on the skin area of interest, diabetes mellitus, unstable chronic condition, current skin malignancy, known defect of healing, use of anti-inflammatory drugs, retinoids, etc on the forearms within the past 4 weeks, hormone replacement therapy within last 3 months and any known allergy to the compounds of the investigational product and band-aids.
2.3 Intervention
The study participants applied a 5% urea containing lipophilic product (Lipophile Harnstoff-Creme 5% NRF 11.129; containing urea, (S)-lactic acid, sodium-(S)-lactate and hydrophobic base cream DAC) which was prepared by the hospital pharmacy. The study personnel demonstrated application of the product and the recommended amount (two-fingertip units, approximately equivalent to 1 g). The participants were instructed to apply the product to the selected intervention forearm twice daily (in the morning and evening) at home for 8 weeks; after washing, showering or before going to sleep. To assess adherence to the intervention, study personnel checked participants' diaries during visits. The product bottles were also weighed at Weeks 4 and 8. The participants were asked not to apply any other leave-on product and not to change their currently used cleansing product. The other forearm remained untreated (control arm), hence use of any leave-on product on the control arm was not allowed. No placebo group was used because we did not intend to measure the effect of urea as an active ingredient, but rather the effect of topical application of a hydrating leave-on product. Furthermore, the participants were requested not to have physical therapies (e.g., massages, laser applications) or strong natural or medical UV-exposure on the forearms. Intake of systemic anti-inflammatory drugs, retinoids, vitamin C, vitamin A derivatives more than five consecutive days was also discouraged while participating in the study.
2.4 Outcomes
Due to the exploratory nature of the study, no distinction was made between primary and secondary outcomes. No change regarding the trial outcomes was made after commencement of the trial. Outcomes for both the treatment and control skin areas were the blistering time, SCH, TEWL, skin surface pH, skin structural parameters (ODS, Rz, epidermal thickness, stiffness) and molecular markers Interleukin-1α (IL-1α), 6 (IL-6), and 8 (IL-8). The occurrence of adverse events was monitored during the study period on participant's reporting and diary entries and was rated based on their intensity and causal relationship to the intervention.
2.4.1 Time to blistering
“Time to blistering” (minutes) was defined as (a) time to first vesicles (from the start of suction pressure until the appearance of first macroscopically visible vesicles), (b) time to full blister (from the start of suction pressure until the development of a full blister). Suction blisters were raised at Week 8 (end of treatment). Room temperature ranged from 20 to 24°C and relative humidity from 40 to 60%. Skin areas were marked on similar locations on the right forearm (A, B, and C) and the left forearm (D, E, and F), and the inter-area distances were recorded. Hairy skin areas were avoided. Participant's forearms were positioned comfortably on arm supports of examination chairs and the skin areas were disinfected. A styrofoam block served as a stable housing for six upside-down positioned syringe barrels, assembled with tubes connected to a vacuum pump (MEDAP BORA UP 2080, FALK MedizinTechnik). Upon starting the vacuum pump, the syringe bases (8 mm in diameter) were simultaneously placed on the skin areas in the same direction, and the initiation time of the blistering process was recorded. Vesicle formation was continuously and closely monitored and duration was recorded. When a blister was fully formed, the corresponding tube was closed to halt negative pressure, and the time was noted. Upon completion, the syringe barrels were removed, and the blister fluids (from three blisters on each side) as well as the blister roofs (two on each side; A and B, D, and E) were collected and stored at −80°C for subsequent laboratory analysis. Vaseline and band-aids were applied on the wounds. Successful wound healing was checked after 2 weeks.
2.4.2 Skin barrier parameters
SCH, TEWL and skin surface pH were measured by using Corneometer CM 825, Tewameter TM 300, and Skin-PH-meter pH 905 (Courage + Khazaka electronic GmbH). SCH was measured in arbitrary units (AU) and ranges from 0 to 120; where higher value indicate higher SCH.42 The measuring probe for TEWL detects the continuous permeation of water through a defined surface of the SC per unit time and is expressed as grams per square meter per hour (g/m2/h).43 Skin surface pH is expressed as the concentration of the hydrogen ion detected by the pH measuring electrode due to the extraction of water soluble constituents from the skin surface.44 The reliability of the above-mentioned measurements was supported in previous studies.45, 46 Measurements were performed in duplicate on the upper part of the volar forearms. SCH and TEWL measurements were conducted at baseline as well as at Weeks 4 and 8, while pH measurement was done at baseline and Week 8. The participants were instructed not to bath, sauna or apply products locally 12 h before the measurements and also not to drink caffeinated beverages 3 h beforehand. Before the measurements, the participants were acclimatized for 30 min in a room temperature adjusted to 22 ± 2°C and a relative humidity to 50% (±10).
2.4.3 Clinical and structural parameters
ODS categories included no skin dryness (category 0), faint scaling, faint roughness and dull appearance (category 1), small scales with few larger ones, along with roughness and whitish appearance (category 2), small and larger scales uniformly distributed with definite roughness with a few superficial cracks and possible slight redness (category 3) and large scales, advanced roughness, redness, eczematous changes and cracks (category 4).15 ODS of the forearms was evaluated by visual examination at baseline, at Weeks 4 and 8 by an investigator who was blinded to the treatment allocation.
Mean roughness was measured as Rz using the Visioscan VC 98 USB (Courage & Khazaka) which assesses the grayscale photograph of the epidermis surface.47, 48 Rz is expressed in µm as arithmetic mean of the maximum peak-to-valley height of five successive sections of the sampling line of the skin surface.
Epidermal thickness (ET) was measured by optical coherence tomography (OCT) using the OCT imaging system from Thorlabs, Germany according to standard operating procedures. Images were analyzed using the ImageJ software.49 ET was expressed in micrometer (µm). Structural skin stiffness was measured with the Cutometer MPA 580 (Courage & Khazaka) following standard operating procedures. The measuring probe (2 mm in diameter) was placed on the skin surface and by means of a defined intake pressure (450 mbar), skin surface was pulled into the probe (suction on, for 2 s) and released again (suction off, for 2 s) for five repetitions, evaluating the maximum extensibility, Uf (in mm).50, 51
2.4.4 Molecular inflammatory markers
IL-1α, IL-6 and IL-8 were analyzed from the epidermal blister roofs and the interstitial fluid samples. Blister roofs were cut into small pieces, incubated with extraction buffer (100 mM Tris, pH 7.4; 150 mM NaCL, 1% Triton-X-100, 1 mM EDTA) and then sonicated in ice-water to extract the analytes. Blister fluid diluted in assay buffer was used for analysis. Total protein (TP) measurement was done in triplicates by colorimetric method using Pierce™ 660 nm Protein assay reagent from Thermo Scientific™, Rockfeld. The ILs were quantified in duplicates using commercial kits for specific enzyme-linked immunosorbent assay (ELISA) (Human IL-1 alpha/IL-1F1 DuoSet ELISA from R&D Systems, Minneapolis, USA; Human IL-6 and IL-8 CytoSet™ from Invitrogen by Thermo Fisher Scientific and Bender Medsystems GmbH) according to the manufacturer's protocols. Absorbance was measured with EnSpireTM multilabel reader (Perkin Eimer Singapore Pte. Ltd., Singapore). TP values were expressed as µg/mL. The concentrations of the inflammatory markers were calculated from the standard curve (pg/mL) and normalized by dividing the values by the concentration of TP of the corresponding sample. The normalized values were expressed as fg/µg TP.
2.5 Sample size
Due to the explorative character of the study, a formal sample size estimation was not performed. Following the recommendation by Julious et al. regarding pilot studies,52 it was planned to include 12 participants.
2.6 Randomization and blinding
There was a concealed random allocation of the treatment arms. A simple computer generated randomization table having 1:1 allocation left versus right was created by a statistician not involved in the study conduct. Sequentially numbered, opaque, sealed envelopes containing the allocation were prepared and opened after confirming eligibility, inclusion and baseline skin measurements. The treatment allocation procedure, product dispensation and instructions for use was performed by a study nurse independently from the investigators. Due to the nature of the intervention, blinding of the participants was not possible. The investigators and outcome assessors were blinded throughout the study. Participants were requested not to reveal any information regarding the allocation of the treatment arm during clinical assessments and skin measurements.
2.7 Statistical analysis
Participant characteristics were described using mean and spread estimates. Comparisons between intervention and treatment arms were done descriptively using parametric (mean, standard deviation) and nonparametric (median, 25%−75% interquartile ranges; IQR) statistics and group differences were presented. Because of the exploratory design of the trial, statistical hypothesis testing was not conducted. However, p values based on Wilcoxon signed-rank tests (related-samples, 2-sided test) between the treatment and control arms were provided, considering all p values to be descriptive. Calculations were performed by using IBM SPSS Statistics for Windows, version 29 (IBM Corp.).
3 RESULTS
3.1 Participant flow
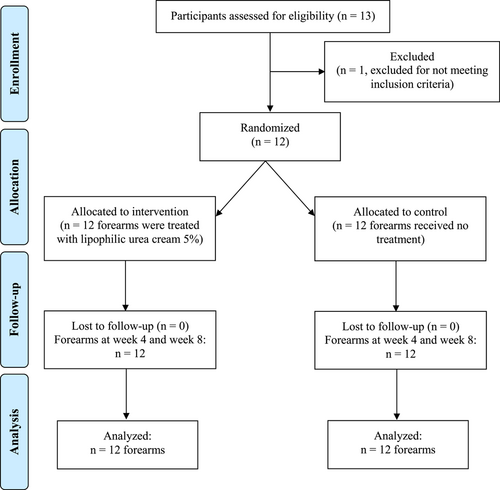
Thirteen participants were screened for eligibility whereas one subject was excluded for not meeting inclusion criteria. Twelve participants were included in the study. For all included participants, one forearm was randomly allocated for intervention while the other forearm was considered as control arm. All participants adhered to the study protocol, wrote regular diary entries and completed all the study visits. A participant flow diagram is shown in Figure 1.
3.2 Recruitment
Recruitment took place between January and February 2023. By April 2023, all participants had completed the final visits.
3.3 Baseline data
Mean age of the participants was 77.9 (SD 5.6) years, with a mean BMI of 24.7 (SD 2.4) kg/m2. Most of them had skin phototype II according to Fitzpatrick scale. Participant characteristics in detail are shown in Supporting Information: Table 1.
3.4 Outcomes and estimation
The results for time to blistering, skin barrier characteristics and clinical and structural parameters are shown in Table 1. “Time to first vesicles” and “time to full blister” for the treatment forearms was longer compared to the control forearms (median difference 2.3 min and 7.7 min, respectively).
| Intervention | Control | Difference | ||
|---|---|---|---|---|
| Time to first vesicles (min) | ||||
| Mean (SD) | 52.8 (26.8) | 50.1 (23.7) | 2.7 (9.4) | |
| Median (IQR) | 47.3 (37.0−58.9) | 46.5 (34.1−54.7) | 2.3 (−5.4 to 9.0), p = 0.27 | |
| Time to full blister (min) | ||||
| Mean (SD) | 88.7 (25.1) | 82.8 (24.0) | 5.9 (11.4) | |
| Median (IQR) | 83.8 (71.2−99.0) | 77.3 (70.8−89.8) | 7.7 (−1.7 to 12.2), p = 0.07 | |
| Stratum corneum hydration (AU) | ||||
| Baseline (Week 0) | Mean (SD) | 35.5 (8.7) | 35.4 (7.7) | 0.1 (5.5) |
| Median (IQR) | 36.3 (26.9−45.5) | 36.3 (29.8−41.3) | 2.5 (−5.4 to 4.7) | |
| Week 4 | Mean (SD) | 49.2 (10.4) | 32.6 (7.1) | 16.6 (9.5) |
| Median (IQR) | 46.7 (42.1−56.9) | 35 (26.4−36.5) | 20.1 (7.1−25.6), p = 0.002 | |
| Week 8 | Mean (SD) | 45.3 (7.1) | 32.6 (4.2) | 12.7 (6.0) |
| Median (IQR) | 45.1 (40.3−48.8) | 33.0 (29.6−34.4) | 11.6 (9.1−15.1), p = 0.002 | |
| Transepidermal water loss (g/m2/h) | ||||
| Baseline (Week 0) | Mean (SD) | 7.7 (2.1) | 7.5 (1.8) | 0.2 (2.0) |
| Median (IQR) | 7.8 (5.7−8.4) | 7.6 (5.8−9.5) | 0.2 (−1.4 to 1.5) | |
| Week 4 | Mean (SD) | 7.4 (5.0) | 7.8 (1.8) | −0.4 (3.8) |
| Median (IQR) | 7.0 (4.3-7.7) | 7.6 (6.7-8.8) | −1.3 (−2.3 to 0.2), p = 0.12 | |
| Week 8 | Mean (SD) | 5.6 (1.1) | 8.3 (1.4) | −2.7 (1.5) |
| Median (IQR) | 6.0 (4.3−6.4) | 8.3 (7.4−8.6) | −2.8 (−3.7 to −1.3), p = 0.002 | |
| Skin surface pH | ||||
| Baseline (Week 0) | Mean (SD) | 5.43 (0.58) | 5.36 (0.64) | 0.07 (0.31) |
| Median (IQR) | 5.55 (4.90−5.97) | 5.47 (4.47−5.97) | −0.01 (−0.12 to 0.27) | |
| Week 8 | Mean (SD) | 5.38 (0.50) | 5.54 (0.60) | −0.15 (0.33) |
| Median (IQR) | 5.41 (5.13−5.79) | 5.71 (5.03−6.01) | −0.14 (−0.22 to 0.04), p = 0.06 | |
| Overall dry skin score | ||||
| Baseline (Week 0) | Mean (SD) | 1.0 (0.4) | 1.1 (0.3) | −0.1 (0.3) |
| Median (IQR) | 1.0 (1.0−1.0) | 1.0 (1.0−1.0) | 0.0 (0.0−0.0) | |
| Week 4 | Mean (SD) | 0.1 (0.3) | 0.8 (0.6) | −0.8 (0.5) |
| Median (IQR) | 0.0 (0.0−0.0) | 1.0 (0.3−1.0) | −1.0 (−1.0 to −0.3), p = 0.003 | |
| Week 8 | Mean (SD) | 0.1 (0.3) | 0.7 (0.5) | −0.6 (0.7) |
| Median (IQR) | 0.0 (0.0−0.0) | 1.0 (0.0−1.0) | −1.0 (−1.0 to 0.0), p = 0.02 | |
| Mean roughness (Rz in µm) | ||||
| Baseline (Week 0) | Mean (SD) | 51.2 (11.0) | 46.7 (9.9) | 4.5 (9.7) |
| Median (IQR) | 49.5 (42.8−57.3) | 43.2 (40.8−53.8) | 7.2 (−0.6 to 9.2) | |
| Week 8 | Mean (SD) | 47.9 (7.0) | 50.0 (8.2) | −7.1 (11.1) |
| Median (IQR) | 47.7 (41.4−53.2) | 54.9 (47.1−59.3) | −12.2 (−15.8 to 4), p = 0.04 | |
| Epidermal Thickness (µm) | ||||
| Baseline (Week 0) | Mean (SD) | 88.2 (11.6) | 97.1 (13.0) | −8.9 (14.9) |
| Median (IQR) | 87.0 (77.7−96.4) | 94.8 (89.3−107.5) | −7.0 (−16.5 to 1.2) | |
| Week 8 | Mean (SD) | 101.6 (15.7) | 92.6 (15.3) | 9.0 (15.7) |
| Median (IQR) | 96.1 (90.4−110.8) | 87.4 (81.4−105.2) | 8.4 (−2.5 to 25.9), p = 0.07 | |
| Skin stiffness (Uf in mm) | ||||
| Baseline (Week 0) | Mean (SD) | 0.273 (0.028) | 0.294 (0.028) | −0.022 (0.027) |
| Median (IQR) | 0.279 (0.256−0.290) | 0.291 (0.256−0.290) | −0.023 (−0.049 to −0.000) | |
| Week 8 | Mean (SD) | 0.295 (0.037) | 0.283 (0.036) | 0.011 (0.030) |
| Median (IQR) | 0.296 (0.275−0.320) | 0.284 (0.250−0.314) | 0.008 (−0.017 to 0.037), p = 0.27 | |
- Abbreviations: AU, arbitrary units; IQR, interquartile ranges.
At baseline, SCH, TEWL and pH values were similar between groups. At Weeks 4 and 8, SCH in the intervention group was higher, with a median difference of 11.6 AU at Week 8. At Weeks 4 and 8 TEWL was lower with a median difference of −2.8 g/m2/h at Week 8. pH values were also lower in the treatment group at Week 8 (median difference −0.14).
Baseline ODS was similar in both groups. At Weeks 4 and 8, the median ODS was one point lower in the intervention group. At Week 8, the median roughness (Rz) was 12.2 µm lower in the intervention group. Median ET and Uf were slightly higher in the intervention group at Week 8.
Table 2 displays the results of the molecular markers analyzed at Week 8. There were small differences in the amount of TP measured in the samples from different participants and no differences were measured between treatment and control arms. Concentration of IL-1α were measured in the blister roofs in pg and in blister fluids in fg range. IL-1α was lower in treatment arms in the blister roofs and fluid samples (median difference −452.4 and −2.2 fg/µg TP, respectively). For IL-6 and 8, lower concentrations were measured which were close to the lower sensitivity limit of the assay. Group differences between IL-6 and IL-8 were minor. The difference in molecular inflammatory markers between men and women from the intervention arm are presented in Supporting Information: Table 2.
| Blister roofs | |||||
|---|---|---|---|---|---|
| Units | Intervention | Control | Difference | ||
| Total protein | µg/mL | Mean (SD) | 166.6 (45.9) | 183.0 (38.6) | −16.4 (46.6) |
| Median (IQR) | 166.3 (126.5−202.4) | 184.2 (144.1−218.3) | −11.5 (−57.7 to 13.6), p = 0.31 | ||
| IL-1α | fg/µg TP | Mean (SD) | 3541.1 (1172.8) | 4374.2 (2034.3) | −833.1 (1113.7) |
| Median (IQR) | 3338.2 (2755.7−4728.9) | 3947.9 (2988.3−5427.9) | −452.4 (−923.3 to −239.4), p = 0.002 | ||
| IL-6 | fg/µg TP | Mean (SD) | 195.8 (79.9) | 149.3 (82.7) | 46.5 (111.4) |
| Median (IQR) | 181.7 (126.7−250.1) | 142.9 (71.8−229.4) | 37.8 (−15.6 to 111.9), p = 0.14 | ||
| IL-8 | fg/µg TP | Mean (SD) | 164.2 (48.7) | 155.0 (68.8) | 9.2 (71.1) |
| Median (IQR) | 161.1 (113.9−208.0) | 137.5 (96.9-205.8) | −5.7 (−32.3 to 63.3), p = 0.81 | ||
| Blister fluid | |||||
| Total protein | µg/mL | Mean (SD) | 21,079.2 (2803.4) | 21,242.4 (2835.9) | −163.2 (2189.1) |
| Median (IQR) | 21,058.3 (19,506.3−22797.9) | 21,183.3 (19,485.4−23,433.3) | 0.0 (−2187.5 to 510.4), p = 0.97 | ||
| IL-1α | fg/µg TP | Mean (SD) | 9.6 (5.4) | 14.3 (11.4) | −4.8 (7.4) |
| Median (IQR) | 8.2 (5.5−12.7) | 9.9 (5.7-22.8) | −2.2 (−4.7 to −0.1), p = 0.01 | ||
| IL-6 | fg/µg TP | Mean (SD) | 2.3 (5.3) | 2.1 (4.7) | 0.2 (0.7) |
| Median (IQR) | 0.8 (0.5−1.1) | 0.7 (0.6−1.1) | 0.0 (−0.1 to 0.1), p = 0.88 | ||
| IL-8 | fg/µg TP | Mean (SD) | 2.1 (2.4) | 1.6 (1.8) | 0.5 (0.9) |
| Median (IQR) | 1.3 (0.8−2.3) | 1.0 (0.6−1.6) | 0.2 (−0.1 to 0.9), p = 0.16 | ||
- Note: Blister roof: Average values of two blister locations (A, B or C, D) on the same forearm; Blister fluid: all fluids from each forearm pooled together.
3.5 Harms
No harms or unintended effects were observed. The wounds created by suction blistering process healed and there was no remarkable difference in wound healing between the intervention and control arm.
4 DISCUSSION
4.1 Interpretation
The overall aim of this study was to investigate the effects of a urea containing lipophilic leave-on product on the strength of the dermoepidermal adhesion in older adults with dry skin. Time to blistering in the treatment arm was longer compared to the control arm. Especially “time to full blister” (median difference 7.7 min) was similar to the results reported by El Genedy-Kalyoncu et. al.33 in a slightly different sample and after a slightly different treatment. This suggests that the application of a topical leave-on product increases the dermoepidermal adhesion in older adults.
Results further indicate that the treatment decreased skin dryness in terms of clinical, functional and structural parameters. Baseline SCH and TEWL values are comparable with results in similar populations.9, 37, 53, 54 Especially the substantially higher SCH in the intervention group indicates the well-known hydrating effects of topical leave-on products containing urea.37, 39, 55, 56 However, how exactly the treatment may influence dermoepidermal adhesion, is not fully understood. Urea regulates epidermal proliferation57 and was found to enhance filaggrin (FLG) expression.37 Previous reports on relative gene expression in the suction blister roof showed that application of urea containing formulation resulted in upregulation of genes like loricrin (LOR) and FLG, which are involved in skin cell differentiation and barrier function.55, 58 LOR was found to be enriched in skin areas where the interdigitation of the epidermis and dermis are more prominent59 which is a characteristic of healthy DEJ. However, because the difference in time to blistering was observed previously by treating with petrolatum only, the overall physiological and structural changes caused by application of leave-on products may also induce changes in the underlying epidermal tissue and the DEJ, hence improving the resistance against mechanical loads.
Values of skin surface pH in our sample are also comparable to previous studies.9, 37, 53, 54 As urea enhance FLG biosynthesis, increased natural moisturizing factors (NMFs) in SC due to catabolic degradation of FLG into NMFs components contributes to the maintenance of skin's acidic pH.60 Beside reducing dryness, topical application of urea containing products exert keratolytic effect, facilitating the removal of top layer of dry skin and improves the dry and rough texture,39 which might also have contributed the reduction of ODS in the treatment arm in our study. Similar to our study, improvement in Rz parameter have also been reported in studies involving leave-on product use.61, 62 ET measurements were also comparable with previously reported results63, 64 and there were no difference after the treatment.33 Previous studies reported improvements in the structural stiffness (Uf) in young participants by using topical formulations.65 Stiffness is mainly influenced by stretching of the collagen and elastic fiber networks.66 This dermal network, which provides mechanical support also for the epidermis67 and therefore, may also influence epidermal stiffness, degenerates with intrinsic aging68 and our results seems to indicate that 8 weeks topical treatment has no effect.
IL-1α, a proinflammatory cytokine capable of inducing neutrophil and macrophage recruitment, is accounted for the vast majority of epidermal-associated IL-1 activity.69 Overexpression of IL-1α is positively correlated with reduced SCH as well as symptom exacerbation in many skin diseases.70, 71 Our result suggest that, the treatment might have reduced possible subclinical inflammation induced by dry skin, as the aged skin may exhibits signs of continuous inflammation.72 Legiawati et al. reported that after 29 days of treating the lower extremities with a leave-on product, IL-1α levels in the control group were not lower than the treatment groups.73 The authors used cyanoacrylate skin surface stripping for analyzing SC extract. IL-1α is expressed by keratinocytes in epidermis and is retained as intracellular stores.74, 75 In our analysis, IL-1α was extracted from the whole epidermis which might have provided analytes also from the lower epidermal cell layers. Another aspect of IL-1α might be relevant in suction blistering process as this produces wounds. Immediately after an incision, cellular recruitment and activation starts within wounds and keratinocytes produce IL-1α.76 However, as blisters were created both on control and treatment arm, blistering should effect the production of IL-1α similarly on both arms. Hence, the lower levels of IL-1α might be due to the treatment. Topical application was reported to normalize serum IL-6 level.77 However, increased serum IL-6 level was significantly correlated with reduced SCH only in the females participants.71 In our study, the value of epidermal IL-6 was not affected by the treatment. This indicates heterogeneity in IL-6 expression depending on gender or analyzed sample material. Schweiger et. al., 2013 reported the amount of IL-8 to be higher in the dry scalp compared to the hydrated scalp after a tonic treatment.11 Our result show that for dry forearm skin (not the scalp region) the marker was not affected by dryness or hydration. Due to very high concentration of TP in the blister fluid, the normalized amount of IL-6 and IL-8 were very low (as low as 0. 2 fg/µg TP). Nevertheless, in our analysis, the amounts of IL-6 and IL-8 in the blister roof extract and the blister fluid were located in the range measurable by the assay. The values of IL-8 and IL-6 were not significantly affected by the treatment and probably they are not proper markers for the endpoint chosen in this study.
4.2 Limitations
We included only Fitzpatrick skin type I-III to reduce heterogeneity. Due to the exploratory nature of the trial, results should be regarded as descriptive and hypotheses generating. Because of the restricted in- and exclusion criteria and the controlled intervention and measurement conditions, results are not generalizable.
5 CONCLUSION
The use of a urea containing leave-on product improves clinical, functional and structural aspects of dry skin and seems to reduce inflammation and to strengthen the dermoepidermal adhesion in older adults. Our result contributes to the understanding of how topical leave-on products help in the prevention of skin tears in older adults.
AUTHOR CONTRIBUTIONS
Ruhul Amin: Data curation; formal analysis; visualization; writing—original draft; writing—review and editing. Fiorenza Rancan: Formal analysis; investigation; validation; visualization; writing—review and editing. Kathrin Hillmann: Conceptualization; data curation; methodology; project administration; writing—review and editing. Ulrike Blume-Peytavi: Conceptualization; funding acquisition; methodology; project administration; resources; software; supervision; writing—review and editing. Annika Vogt: Conceptualization; investigation; methodology; resources; supervision; writing—review and editing. Jan Kottner: Conceptualization; funding acquisition; investigation; methodology; resources; software; supervision; writing—review and editing.
ACKNOWLEDGMENTS
Ruhul Amin's doctoral study is supported by a scholarship from “Bangabandhu Science and Technology Fellowship trust, Ministry of Science and Technology, Bangladesh” and Charité—Universitätsmedizin Berlin. We would like to thank Doris Wilborn and Sarah Maria Richter for contributing to the optical coherence tomography measurements, Annette Andruck and Claudia Blankenstein for helping with data collection and study management, and Sabrina Hadam for her technical assistance during ELISA experiments. Additionally, the authors thank the study volunteers who participated in the study. This investigator-initiated study was fully funded by the Charité—Universitätsmedizin Berlin, Department of Dermatology, Venereology and Allergology, Clinical Research Center for Hair and Skin Science and the Institute of Clinical Nursing Science, Charité Center for Health and Human Sciences, Berlin, Germany. The supporting authorities had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Jan Kottner affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information Material. If requested, anonymized data will be shared by the corresponding author.