Collaborative medication reviews in community pharmacies—Drug-related problems and the process of communicating them with physicians: A retrospective validation study
Abstract
Background and Aims
Cooperation between practicing community pharmacists (PPs) and primary care physicians has traditionally been limited, with scarce communication on therapeutic issues. The aim of this study was to assess how PPs communicate in writing with physicians regarding (1) the clinically relevant problems they have identified in patients' medications and (2) recommendations to solve the problems to identify development needs in the communication process.
Methods
This retrospective validation study assessed medication reviews conducted by PPs in collaboration with home care nurses, practice nurses, and physicians for 46 older (≥65 years) home care clients in the Municipality of Lohja, Finland. The therapeutic and communicative appropriateness of clinically relevant drug-related problems (DRPs) identified by PPs and reported in writing to physicians was blindly evaluated by (1) an accredited pharmacist (AP) and (2) two physicians specialized in geriatric pharmacotherapy. Descriptive statistical analysis was conducted to compare the assessments.
Results
The PPs (n = 13) identified 189 DRPs and made 4.1 recommendations per patient in 46 written reports to physicians. Of the PPs' written recommendations for medication changes, 46% (155/334) were the same as those by the AP. The two specialized physicians evaluated 69% and 67% of PPs' recommendations to be clinically relevant. The way the DRPs and recommendations to solve them were communicated was evaluated as appropriate in 38% and 38%, respectively, of the case reports written by the PPs.
Conclusion
The PPs were able identify DRPs quite well, particularly inappropriate medication use, according to current care guidelines and formularies. It was found that improvement was needed in the communication of DRPs in written reports with physicians. Interprofessional learning by working in care teams would be suitable for strengthening patient care–oriented competencies.
Key points
-
Communication between practicing community pharmacists and physicians has been historically limited.
-
This study evaluates the quality of this communication process in a real-world context, assessing the therapeutic and communicative appropriateness of identified drug-related problems and proposed solutions written by community pharmacists. It was found that while community pharmacists could identify drug-related problems quite well, their written communication with physicians needed improvement.
-
The study emphasizes the need for improved written communication between PPs and physicians in patient care. It also suggests that interprofessional learning through care teams can strengthen patient care–oriented competencies, enhancing the effectiveness of identifying and resolving drug-related problems.
1 INTRODUCTION
Cooperation between community pharmacists and physicians has typically focused on managing patients' medications in primary care. This has been carried out with limited communication, mainly due to statutory practices for prescribing and dispensing medicines.1, 2 Communication about therapeutic issues has been scarce.1 The situation is changing, with community pharmacists increasingly involved in reviewing the medications of primary care patients.2 In particular, older medicine users have become targets of pharmacists' clinical interventions, since multimorbidity and polypharmacy are common in this age group, posing an elevated risk for medication-related problems.3 A previous systematic review indicated that community pharmacists' contributions to medication reviews have positive outcomes, including increased adherence and decreased medication-related problems.3 The same systematic review concluded that community pharmacists could be more involved in medication review interventions for older adults and that their contributions could be extended from the identification of drug-related problems (DRPs) to having a more comprehensive contribution to medication therapy management.3
Although community pharmacists are increasingly involved in collaborative medication reviews, few studies have focused on their competence in communicating drug-related problems with physicians and recommendations to solve them.4 Quite often, DRPs are reported to physicians in written reports.5 Few studies have assessed the therapeutic and communicative appropriateness of these reports, although such reports may influence physicians' responses and actions on the clinical findings by pharmacists.1 Previous studies have mainly presented the number of DRPs identified by pharmacists and the proportion of them accepted by physicians.6, 7 To identify community pharmacists' needs for developing their therapeutic and communication processes, the aim of this study is to assess how they communicate the following in writing with physicians: (1) the clinically relevant problems they have identified with patients' medications and (2) recommendations to solve the problems.
2 METHODS
This retrospective validation study assessed medication reviews conducted by practicing community pharmacists (PPs) in collaboration with home care nurses, practice nurses, and physicians for older (≥65 years) home care clients in the Municipality of Lohja, Finland.8 The data were derived from an randomized controlled trial (RCT) study aiming to enhance cooperation and coordination between home care and a local community pharmacy in the management of medication use among older home care clients.8, 9 Enhanced coordination was achieved by clarifying the tasks of each care team member and optimizing the use of existing resources in both home care and the community pharmacy.8
The core of the intervention was to identify home care clients with clinically relevant risks and problems with their medications that needed solving. For this purpose, practice nurses were trained to use a DRP risk assessment tool (DRP-RAT)10 to more systematically identify potential medication-related risks during routine home visits and to report their findings to the clinically trained coordinating pharmacist. The DRP-RAT tool is a validated tool designed for practice nurses to identify DRP risks in home care clients aged ≥65 years. The tool has been content validated using a three-round Delphi consensus survey involving 18 geriatric care and pharmacotherapy experts and requiring ≥80% agreement on items.11 The feasibility of the DRP-RAT tool has been evaluated among practice nurses (n = 36) in home care.12 The preliminary DRP-RAT tool exposed to the validation process in the Delphi study was based on two systematic reviews.11 The coordinating pharmacist reviewed the DRP risk findings reported by the practice nurses after the home visits and selected cases that needed a physician's consultation in a triage meeting. At the collaborative triage meeting, the home care physician decided on further medication review actions for clients with clinically relevant DRPs. These medication reviews were conducted by community pharmacists, and the comprehensiveness of the reviews were decided upon each patient's needs.9 These medication reviews by community pharmacists were used as data for the present study. All PPs, physicians, home care staff, and clients were treated pseudonymously throughout the study.
The community pharmacists reported the identified DRPs and recommendations for solving them to the physician in writing on a manual form that included the following three sections: (1) recommendation (identified DPRs; e.g., “Could the dose of metformin be reduced to 2000 mg?”), (2) argumentation (e.g., “The patient's renal function is impaired, and the glomerulus filtration rate (GFR) is now 50 ml/min/1.73 m2”), and (3) the physician's comments. The data documented in Sections 1 and 2 were evaluated. Home care staff, the community pharmacists, and other health care providers involved in the study were aware of the ongoing RCT study, the study protocol, and the procedure for communicating medication reviews.8, 9
2.1 Selection of the study participants
The patient cases for this retrospective validation study consisted of all older (>65 years) home care clients in the Municipality of Lohja who had given consent and met the inclusion criteria to participate in a randomized controlled trial (RCT) conducted during 2015–2017 aiming at enhancing coordination of care between local home care services and community pharmacies to reduce medication risks in older home care clients.8, 9, 13 The study participants for the RCT study were recruited by home care nurses and practice nurses during September to December 2015. All home care clients who fulfilled the following criteria were included in the study: (1) age 65 years and older, residing at home; (2) receiving regular home care from the Municipality of Lohja; (3) using at least one medicine; and (4) participating voluntarily (written informed consent to participate in the study was given by the participant or the closest proxy). Older home-dwelling residents (>65 years) who did not receive regular home care services from the Municipality of Lohja were excluded.
The clients selected for this retrospective validation study were those ones identified at the baseline triage meeting in 2016 needing a more comprehensive review of their medication.8, 9 The more comprehensive medication review after the triage meeting was conducted by a PP working in the local community pharmacy. The PPs were in-house trained to conduct medication reviews as part of their usual work.
2.2 Evaluation of written medication review reports prepared by the community pharmacists for the physicians
PPs' written medication review reports were blindly assessed by a clinically trained pharmacist and two physicians specialized in geriatric pharmacotherapy (Figure 1). The clinically trained pharmacist had an MSc (Pharm) degree, accreditation in comprehensive medication reviews (CMRs),14 and extensive experience in conducting medication reviews in practice (termed AP in this report, Figure 1). At the time of the study, CMR accreditation training in Finland consisted of 35 European Credit Transfer and Accumulation System (ECTS) credits and took 2 years to complete through part-time study alongside work.15
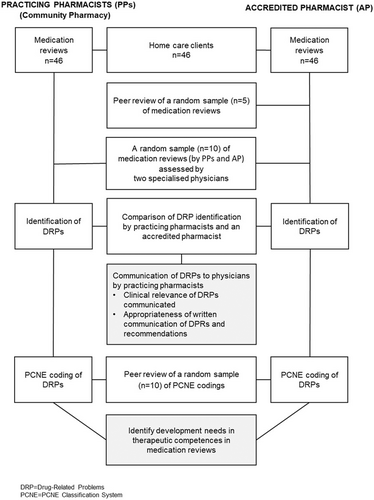
First, the AP independently and blindly reviewed the medications of the same home care clients as did the PPs (Figure 1). When reviewing the medications, no access was available to the patients' medical histories and laboratory test results other than the GFR. Both the PPs and the AP used the same medication review procedure with similar patient information sources and medication-related risk assessment tools (Table 1). To confirm the validity of the medication reviews by the AP, an external AP independently reviewed the medications of a random sample of five study participants (Figure 1). The external AP was a pharmacist with the same kind of training and work experience as the AP (i.e., had a Master of Science in Pharmacy degree, accreditation in CMRs, and extensive experience in conducting medication reviews).
| Medication risk management tool | Description | Content |
|---|---|---|
| DRP-RAT11 | Medication risk assessment tool (manual) for use by practice nurses working with community and home-dwelling older adults | Basic client data, potential risks for DRPs in medication use, characteristics of the client's care and adherence, and recommendations for actions to resolve DRPs |
| Medication list | A manual of electronic list of Rx and OTC drugs and natural products/food supplements in use | Name, strength, dosage form, indication, and time(s) for taking the drug |
| Blood test | Glomerulus filtration rate GFR | GFR estimate based on serum creatinine concentration, age, and gender |
| SALKO16,a | Electronic medication review tool for community pharmacists (The Association of Finnish Pharmacies) | The effect of sensitivity, anticholinergic and serotonergic load of patient's medication, appropriateness for aged patients based on four different criteria, and metabolization via six different CYP enzymes |
| Riskbase17,a | Drug interaction database designed for clinical decision support systems (National Health Portal) | Information about adverse effect profile of drugs: Cumulative scoring of the anticholinergic, bleeding risk, constipation, orthostatic hypotension, prolongation of the QT interval, nephrotoxic effects, sedation, convulsion risk, and serotonergic of the patient's medication |
| Inxbase18,a | Electronic drug interaction database and adverse effects database | Drug–drug interactions and clinically most relevant interactions between drugs and food or natural products |
| Renbase19,a | Electronic database for drug dosing in renal failure | Renal function and appropriateness of doses/medicines used |
- a Routinely available in community pharmacies at the time of the study.
- Abbreviations: DRP-RAT, drug-related problem risk assessment tool11; GFR, glomerular filtration rate; OTC drug, over-the-counter drug; Rx-drug, prescription drug.
The PPs' communication of clinical relevance and the appropriateness of the recommendations to the physician in the written medication review reports were assessed by two independent physicians, namely a clinical geriatrician and a clinical neurologist with expertise in geriatric pharmacotherapy (Figure 1). The physicians were given 10 randomly selected medication review reports written by PPs (including recommendations and argumentations) of the study participants. The cases were chosen using simple random sampling with a random number generator.20 In these reports, recommendations communicated by the PPs and AP were combined and presented in a random order per case. Thus, the physicians did not know which of the communications on DRP findings and recommendations had been written by a PP or the AP in each case. The physicians had the same patient information sources and medication risk management databases (Table 1) available to the PPs and the AP while reviewing the medications.
Both physicians independently assessed the clinical appropriateness of the findings and recommendations and the way they were communicated in writing. For that purpose, the physicians evaluated each individual finding and recommendation in each case report by using the following two questions: (1) Is this recommendation clinically relevant? and (2) Is this recommendation communicated in an appropriate way in this written report? The options yes/no were given for appraisal. The physicians were instructed to evaluate the recommendations based on their subjective views as physicians and how they were used to communicate within an interprofessional team.
2.3 Coding of DRPs according to the PCNE classification system
After the physicians had assessed the clinical relevance and appropriateness of recommendations from medication reviews by the PPs and AP, all recommendations were coded using the PCNE classification of DRPs.21 During coding, the AP identified and documented the possible discrepancies between the reports by PPs and the AP concerning the identified DRPs and recommendations for solving them.
We chose to use the validated PCNE classification of DRPs (V8.02)21 in this study because it is widely used in research to classify the nature, prevalence, and incidence of DRPs. The PCNE classification has primary domains for problems (P-codes, three primary domains), causes (C-codes, eight primary domains), interventions (I-codes, five primary domains), and acceptance of the intervention proposals (A-codes, three primary domains). In this study, only primary domains and subdomains for problems (P-codes) and causes (C-codes) were used by the AP to code the recommendations.
The following aspects were considered in the coding:
(1) In cases where the DRPs and the recommendations to solve them concerned a potentially inappropriate medication for older adults, the American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults22 were used to identify the best standardized results for coding.
(2) If the recommendation contained comments concerning two different drug-related problems, these comments were considered to be two separate recommendations. However, the recommendation was not divided into two separate ones if it concerned the management of possible adverse drug reactions caused by two or more medicines.
(3) The combination of metamizole 500 mg and pitofenone 5 mg (brand name Litalgin®® 500/5 mg) is used in geriatric care in Finland but not found in the Beers Criteria.22 The compound was coded as “inappropriate drug according to guidelines/formulary” (PCNE Classification, code 1.1).21
(4) Two pharmacists' recommendations were coded under the PCNE cause code 8.1 (no or inappropriate outcome monitoring, including therapeutic drug monitoring, TDM) using new subcategories for (1) drug or electrolyte concentration monitoring and (2) blood or orthostatic pressure monitoring.
(5) Two types of recommendations were coded under code 8.2 (other cause; specify) using new subcategories for (1) new drug or product and (2) other notification.
The validity of the DRP categorization by the AP was confirmed by an external AP with experience in PCNE coding. The external AP blindly reviewed the coded DRP recommendations of 10 randomly selected cases. The cases were also chosen using simple random sampling with a random number generator.20
All the medicines included in the identified DRPs and the recommendations to solve them were coded using the ATC classification system.23
2.4 Statistical analysis
Data are presented as means (standard deviations) and numbers (percentages). Inter-rater reliability of the clinical relevance and appropriateness of the written communication of recommendations between the two raters was assessed by kappa coefficients (κ) with a 95% confidence interval (CI). The proportion of agreement (%) with a 95% CI was used to calculate the agreement rate. The χ2 test or Fisher's exact test (two-sided) was used to calculate the difference in the recommendations given by the AP and PPs. p < 0.05 were considered statistically significant. All statistical analyses were carried out using SAS 9.4 System for Windows (SAS Institute Inc.).
2.5 Ethical approval
This retrospective validation study was conducted as part of the randomized controlled study in the Municipality of Lohja under the research protocol approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS), Finland (number 153/13/03/00/15). Informed consent was obtained from each patient and/or their closest proxy before any study procedures were performed. All community pharmacists, physicians, home care staff, and clients were treated pseudonymously throughout the study.
3 RESULTS
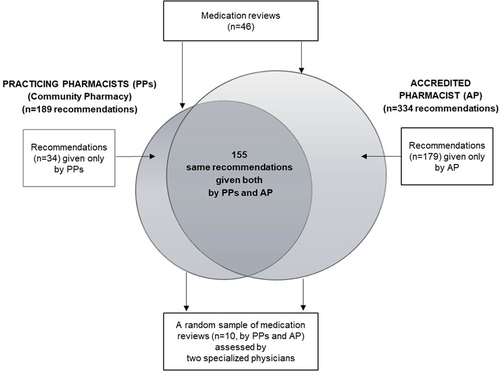
In the triage meeting, decisions on actions needed to optimize medication were made for a total of 99 clients, of which 46 were referred to the community pharmacy for medication review. The medication reviews (n = 46) were conducted by 13 PPs. The mean age of the home care clients included in this study (n = 46) was 83 years, and 69% of them were women. The mean number of prescribed medicines used regularly and as needed was 13 (SD 4.5, range 3–31) per person. The PPs reported altogether 189 recommendations for medication changes, and the most common DRPs were “adverse drug event” (n = 108) and “untreated symptoms or indication” (n = 27) in their medication review reports (n = 46) to physicians (mean 4.1 recommendations per patient) (Figure 2). The AP generated 334 recommendations for medication changes for the same home care clients (mean 7.3 recommendations per patient). Of the recommendations, 155 (42% of all the recommendations given, n = 368) were the same, while 34 (9%) were given by PPs only and 179 (49%) by the AP only (Figure 3).
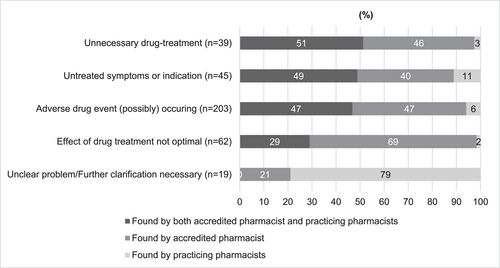
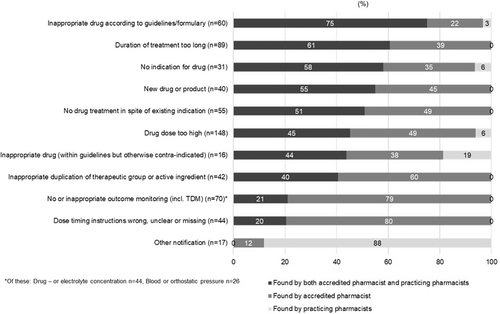
The highest agreement rate between PPs and the AP in identifying DRPs (P-codes, i.e., problems) was found in cases of “unnecessary drug treatment” (51% of the DRPs were identified by both PPs and the AP), “untreated symptoms or indication” (49%), and “potential adverse drug events” (47%) (Figure 2). Correspondingly, the highest proportion of the same causes of DRPs (C-codes) identified by PPs and the AP related to “inappropriate drugs according to current care guidelines/formulary” (75% of the causes identified by both the PPs and AP), “duration of the treatment too long” (61%), and “no indication for drug” (58%) (Figure 4). The lowest proportion of the same causes of DRPs related to “checking blood or orthostatic pressure” (12% of the cases identified by both the PPs and AP), “wrong, unclear, or missing dose timing instructions” (20%), and “checking drug or electrolyte concentration” (27%). These causes of DRPs were mainly identified by the AP.
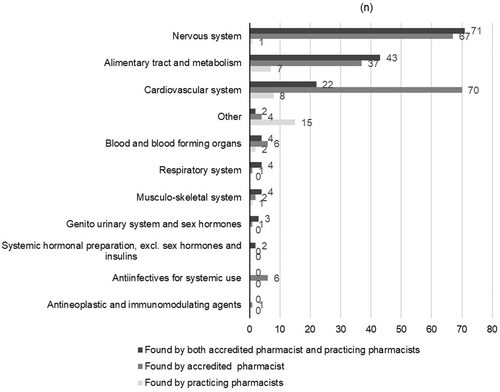
The proportion of the total recommendations by PPs and the AP to solve the identified DRPs varied between therapeutic groups of medicines (Figure 5). The highest proportion of the same recommendations (71/155, 46%) concerned nervous system medications, followed by medicines for alimentary track and metabolism (43/155, 28%) and for cardiovascular systems (22/155, 14%).
The two physicians independently rated 69% of the identified DRPs and 67% of the PPs' suggested recommendations to solve them as clinically relevant, while their ratings were 95% and 83% for the DRPs and the AP's suggested recommendations, respectively (Table 2). The difference between the AP and PPs in providing clinically relevant recommendations to physicians was statistically significant (p < 0.001, p = 0.037, respectively). According to the physicians, the written communication of identified DRPs and recommendations to solve them was appropriate in 38% of the cases reported by PPs (both physicians had the same estimate). The corresponding figures for the appropriateness of written DRP communication by the AP were 97% and 90%, respectively. The difference between the AP and PPs in communicating DRPs appropriately in writing to the physician was statistically significant (p < 0.001 for both).
| The clinical relevance and appropriateness of recommendations communicated in writing | Recommendations given by | |||
|---|---|---|---|---|
| PPs and AP (n = 120) | PPs (n = 42) | AP (n = 78) | p Value | |
| Rater A | ||||
| The recommendation is clinically relevant (%) | 69.1 | 94.9 | <0.001 | |
| The recommendation is appropriately communicated in writing (%) | 38.1 | 97.4 | <0.001 | |
| Rater B | ||||
| The recommendation is clinically relevant (%) | 66.7 | 83.3 | 0.037 | |
| The recommendation is appropriately communicated in writing (%) | 38.1 | 89.7 | <0.001 | |
| Inter-rater reliability (κ) | ||||
| The recommendation is clinically relevant | 0.23 (fair) | 0.18 (slight) | 0.17 (slight) | |
| The recommendation is appropriately communicated in writing | 0.57 (moderate) | 0.39 (fair) | 0.17 (slight) | |
| Proportion of agreement (%) | ||||
| The recommendation is clinically relevant (95% Cl) | 76.7 (69.1–84.2) | 64.3 (49.8–78.8) | 83.3 (75.1–91.6) | |
| The recommendation is appropriately communicated in writing (95% Cl) | 83.3 (75.1–91.6) | 71.4 (57.8–85.1) | 89.7 (89.0–96.5) | |
- Note: The recommendations (n = 120) were assessed by two raters (physicians with geriatric pharmacotherapy expertise) who assessed a random sample of 10 out of 46 medication reviews. Inter-rater reliability (Kappa coefficient κ with 95% confidence interval [CI]) and proportion of agreement (%) were used to calculate the agreement rate. χ2 test or Fisher's exact test (two-sided) was used to calculate statistical significance.
- Abbreviations: AP, accredited pharmacist in comprehensive medication reviews; PPs, practicing community pharmacists.
The results for inter-rater reliability (Kappa = κ, 95% CI, and % agreement) for the two physicians as raters (A and B) are presented in Table 2. Between the physicians, the degree of agreement was slight (κ = 0.18), while the proportion of agreement was 64.3% (95% CI: 49.8–78.8), with 27/42 of the estimates being the same concerning the clinical relevance of the identified DRPs and the suggested recommendations by PPs to solve them. The corresponding degree of agreement on the clinical significance of the identified DRPs and the AP's suggested recommendations to solve them was also slight (κ = 0.17), while the proportion of agreement was 83.3% (95% CI: 75.1–91.6), with 65/78 of the estimates being the same. In addition, the degree of agreement between the physicians' estimates for the appropriateness of the written communication of the cases reported by PPs was fair (κ = 0.39), the proportion of agreement being 71.4% (95% CI: 57.8–85.1), with 30/42 of the estimates being the same. The corresponding degree of agreement on the appropriateness of the written communication of the cases reported by the AP was slight (κ = 0.17), the proportion of agreement being 89.7% (95% CI: 83.0–96.5), with 70/78 of the estimates being the same.
4 DISCUSSION
This study evaluated the practices of community pharmacists in conducting medication reviews, specifically in terms of identifying clinically relevant DRPs and communicating them to a physician in a predetermined written format. We used two pharmacists with accreditation in comprehensive medication reviews and two physicians with expertise in geriatric pharmacotherapy as references. The main finding was that community pharmacists were able to identify DRPs quite well, particularly inappropriate drug use, according to current care guidelines or formularies; excessive treatment duration; and cases where there was no indication for the treatment or no treatment despite the indication being identified. Most commonly, the PPs did not identify DRPs in cases where the DRP identification would have required individual optimization of a complex medication regimen, such as in cases of medication with a high-risk load or the risk of falls, such as induced by drugs adding to orthostatic load.
Our findings indicate that solving the most complex DRP problems requires specific expertise that cannot be expected to be routinely available in community pharmacies. Furthermore, community pharmacists do not traditionally have access to the patient data necessary for comprehensive medication reviews that also consider clinical condition and illnesses/comorbidities.24 In such complex cases, community pharmacists' responsibilities might be limited to identifying patients at risk and knowing the procedure for referring them to a physician or accredited pharmacist. Indeed, the triage procedure developed in the first phase of this intervention study to manage the risks of medications of older home care clients is in line with this suggestion.9 Applying the triage procedure indicated that community pharmacists identified 45% of home care clients having their medications in order and 55% needing further collaborative review of their medications, while 7% of them needed a comprehensive medication review by an accredited pharmacist and home care physician.8
In light of the study reported here, further intensified collaboration between community pharmacists, home care personnel, local physicians, and accredited pharmacists is recommended to refine the practice so that community pharmacists can identify cases where the individual optimization of a complex medication regimen is needed (e.g., in cases of medication with a high-risk load or the risk of falls). Intensified cooperation would also help community pharmacists learn more about geriatric care and pharmacotherapy by working in a care team and communicating with physicians and other team members about patient care and medication-related issues.
Our study identified the need to improve the communication of DRPs in the written reports provided by PPs to physicians. Pharmacists' skills required for effective interprofessional communication and related clinical skills should be mapped and standardized on a larger scale to improve their contribution to patient care. In our study, some physicians were reluctant to make the changes that PPs had suggested to medication regimens.8 A previous study by Snyder et al.25 found the primary prerequisite for establishing trust between physicians and pharmacists to be the provision of high-quality clinical recommendations that improve patient outcomes. Further research is needed to identify and assess such quality factors in written and verbal communication between community pharmacists and physicians while reviewing medications and managing clinically relevant risks and problems that can potentially harm patients.
Our results may still reflect the long history of community pharmacists working separately from other primary care providers and focusing on dispensing. Therefore, essential pedagogics for them to acquire missing patient care–oriented competences would involve learning by doing. Working together as more integral members of a care team would help community pharmacists to learn more about applied pharmacotherapy and optimize the therapy according to each patient's clinical health status. Simultaneously, they could develop their communication skills in care teams, both verbally with physicians and in writing about medication therapies. At the same time, physicians may learn how to cooperate with community pharmacists. The need for interprofessional learning and case-based learning has been recognized in Finland and internationally.26, 27 In our study, the pharmacists worked in a community pharmacy that had regular in-house training and collaboration with home care,8, 9, 28 which may have positively impacted on the results. If the study were conducted in another Finnish community pharmacy without such actions, the results might not have been as encouraging.
Even though pharmacy education in Finland has since the early 1990s systematically focused on fostering medication counseling skills, less attention has been paid to interprofessional communication on patient care–related issues. Therefore, interprofessional case-based learning should be added to undergraduate pharmacy and medical studies in Finland. Likewise, more patient-centered small-group training involving physicians should be included in the continuing education of practicing pharmacists to improve the quality of their written and verbal communication in terms of DRPs and strategies to collaboratively manage them. Locally, health care institutions, including community pharmacies could organize regular joint conferences, workshops and in-house training events for health-care professionals to enhance their communication methods and raise awareness of DRPs and other clinically relevant medication risks to be collaboratively managed in everyday routine practice.
4.1 Study limitations
One of the major limitations of this study concerned the time allocation in the community pharmacy, which did not allow the pharmacists to fully concentrate on reviewing the medications; rather, they conducted the reviews in between other work duties. The study design simulated the current practice in community pharmacies; therefore, the PPs and the AP did not have access to patients' medical records and laboratory results while conducting medication reviews. Like all community pharmacists in Finland, the PPs and AP had access to the same electronic databases for medication risk management as do physicians and other health-care professionals2, 29 (Table 1). In most cases, the PPs and AP used the SALKO electronic medication review tool, which was specially designed for medication reviews in community pharmacies. These study design issues may have affected the comprehensiveness of the medication reviews, and thus the identification of DRPs and the communication of recommendations to physicians in the written reports. The information content and way of communicating DRPs may also have been influenced by the fact that the community pharmacists had been instructed to call the physician immediately after sending the report to discuss the case. However, these communications were often delayed due to busy schedules.
The study results cannot be generalized to all PPs in Finland. The data used in this study were collected in 2016, which may have influenced the results due to curricular changes since then and increased attention on the inappropriate use of medicines in older adults, which can lead to potentially severe harm.30 These concerns were recognized in a government program in 2015,31 leading to the establishment of the Rational Pharmacotherapy Action Plan,32 the implementation of which is still ongoing as part of a major social and health services reform in Finland.33
5 CONCLUSION
The PPs were able to identify DRPs quite well, particularly inappropriate medication use according to current care guidelines and formularies. A need for improvement was found in the communication of DRPs in the written reports for physicians. Interprofessional learning in care teams would be suitable for strengthening patient care–oriented competencies.
AUTHOR CONTRIBUTIONS
Jonna-Carita Kanninen: Conceptualization; data curation; investigation; methodology; project administration; visualization; writing—original draft. Terhi Toivo: Validation; writing—review & editing. Marja Airaksinen: Conceptualization; data curation; investigation; methodology; supervision; writing—review & editing. Anu Holm: Conceptualization; data curation; funding acquisition; investigation; methodology; supervision; writing—review & editing. Eeva Savela: Writing—review & editing. Maarit Dimitrow: Validation; writing—review & editing. Jarkko Tuunanen: Validation; writing—review & editing. Saija Leikola: Writing—review & editing. Juha Puustinen: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; supervision; validation; writing—review & editing.
ACKNOWLEDGMENTS
The Municipality of Lohja and the 1st Pharmacy of Lohja are gratefully thanked for their contributions to this study Biostatistician, Dr, Tero Vahlberg is warmly thanked for his assistance in conducting quantitative analyses of the data. This study was supported by Data Lake Innovation Testbed for Future Hospital, 1/2021 onwards (writing of the report; the decision to submit the report for publication), Satasairaala Central Hospital and Grant State Research Funding (writing of the report). Open-access publishing was funded by the University of Helsinki, Finland. We declare that the supporting sources did not have any involvement in study design; collection, analysis, and interpretation of data; writing of the report; the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Jonna-Carita Kanninen, Jonna-Carita Kanninen affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the Western Uusimaa Wellbeing Services County. However, restrictions apply to the availability of these data, which were used under license for the current study, and therefore are not publicly available. The study was conducted as part of a larger randomized controlled study in the Municipality of Lohja, Finland, which was registered with ClinicalTrials.gov (NCT02545257) before January 1, 2019. A data-sharing plan was not included in the trial's registration. However, all requests for data sharing activities were conducted in compliance with all applicable laws and regulations concerning data privacy and confidentiality.