Depression is associated with discoordination between heart rate variability and physical acceleration in older women
Abstract
Background and Aims
It is well known that depression is closely associated with the autonomic nervous system and physical acceleration (PA), which may cause functional time-deviance between these two parameters. Exploring this relationship is important in sustaining the mental and physical health of older adults in daily life. However, few studies have assessed the relationship between depression and the coordination of parasympathetic nervous activity (PSNA) and PA. The present study was designed to investigate whether the coordination between PSNA and PA is associated with the mental state of healthy volunteers in normal daily lives and the underlying mechanism.
Methods
In total, 95 adult women were divided into non-older and older groups comprising 50 (aged 20–59 years) and 45 (aged 60–85 years) women, respectively. PSNA and PA data were simultaneously obtained every minute for 24 h during the free-moving day using the ActiveTracer accelerometer. Lag time was determined as the time difference between PSNA and PA, and it was introduced as a parameter of %lag0, which is the percent ratio of the lag = 0 min between PSNA and PA in 1 h. The General Health Questionnaire 28 (GHQ28) was used to evaluate the effects of psychological distress, including depression.
Results
In the hour before sleep, %lag0 was significantly lower in older women (38.7 ± 6.4) who had higher GHQ28 values (subscale D = 0, n = 12) compared with that in older women (19.4 ± 10.5) with lower values (subscale D ≧ 1, n = 33) (p < 0.05).
Conclusion
Impairments in coordination between PSNA and PA are significantly associated with depression in older women, particularly in the hour before sleep on free-moving days.
Key points
-
Impairments in coordination between parasympathetic nervous activity and physical acceleration are associated with depression in older women, particularly in the hour before a night's sleep.
-
The lag is determined by the cross-correlation analysis between the time-series of heart rate variability and physical acceleration.
-
The coordination between parasympathetic nervous activity and physical acceleration can be performed noninvasively and easily during the free-moving day.
1 INTRODUCTION
Depression is one of the most prevalent mental illnesses globally and is associated with an increased risk of morbidity and suicide.1 It is associated with physical and cognitive decline, compromised social life, and greater self-neglect, thereby also increasing mortality.2 According to a survey conducted by the World Health Organization, in 2017, the prevalence of depression was highest in Ukraine (6.3%) followed by the United States, Greece, and Portugal. Moreover, according to the survey, the prevalence of depression is 4.2% in Japan. Moreover, the prevalence of depressive symptoms in patients aged >60 years may reach up to 40%.3
Nevertheless, psychological distress is widely used as an indicator of public mental health. Psychological distress implies an undifferentiated combination of symptoms ranging from depression and general anxiety symptoms to personality traits, functional disabilities, and behavioral problems.3 Reportedly, the incidence of psychological distress in women is approximately twice that in men, although the frequency increases in both men and women with age.4
The General Health Questionnaire 28 (GHQ28) has often been utilized for the evaluation of psychological distress from subscales of somatic symptoms, anxiety and insomnia, social impairments, and severe depression.5, 6 Furthermore, the GHQ28 is one of the most widely used and validated questionnaires to screen for emotional distress and depression.7
Moreover, physical acceleration (PA) has been utilized to evaluate physical activity in participants with severe mental illnesses8 and in multiple clinical settings.9 The health benefits of PA have also been widely studied and were demonstrated as an effective treatment for depression in older patients.2 Plausible mechanisms to explain the association of PA with depression include PA-induced changes in physiological/neurological and psychological parameters.10 Overall, PA may prevent depression by increasing the functional activity of monoamines.11
Although habitual activity and heart rate variability (HRV) have been reported to differ between men and women,12, 13 during exercise with an increasing PA, an increase in the heart rate and decrease in vagal discharge have been detected.14 Moreover, heart rate increases immediately after initiating exercise due to a withdrawal of parasympathetic nervous activity (PSNA) and increased sympathetic nervous activity.15
HRV is a noninvasive tool for assessing variations in beat-to-beat intervals and autonomic nervous system activity. The HRV spectrum involves the power of high-frequency (HF) and low-frequency (LF) ranges.16, 17 Normalized spectral indices, defined as HFnu = HF/(LF + HF), are regarded as markers of PSNA.18
Cross-correlation analysis generates a series of correlation coefficients between two time-series by overlaying and temporally shifting the two series over a range of successive time lags.19 To investigate the association of HRV with a physiological signal, cross-correlation analysis is widely used.20 Based on a cross-correlation analysis between the time-series of HRV and PA, we previously reported an increase in lag with age in the daily lives of free-moving adults.21 Furthermore, we reported that this increase was closely associated with depression, although the sex-specific effect remained unclear owing to the small sample size.21 Moreover, we recently reported that fatigability in adult women is closely associated with a decline in coordination between PSNA and PA.22
In the present study, we aimed to investigate whether psychological distress is associated with impairments in coordination between PSNA and PA in daily free-moving life in adult women by combining %lag0 and the GHQ28 and the underlying mechanism. To our knowledge, this is the first study to evaluate the relationship between depression and the coordination between PSNA and PA in free-moving adult women.
2 MATERIALS AND METHODS
2.1 Participants
A total of 95 healthy women participants aged 22–85 years old were screened from 106 volunteers based on their responses to medical interviews regarding previous and current illnesses, physical findings, blood test results, and electrocardiogram results. Five participants who had consumed alcohol on the day of the experiment, six participants with severe arrhythmias, two participants taking beta-blockers, and three participants with excessive electrical noise in the devices were excluded. These excluded numbers were overlapped. First, the participants were divided into non-older (n = 50) and older age groups (n = 45) based on a cutoff of 22–59 years in the younger group and 60–85 years in the older group (Table 1). Second, participants in each age group were divided into low and high GHQ groups (Table 1 and Figures).
| Group | Non-older | Older | p Value (d value) |
|---|---|---|---|
| Age (years) | <60 | ≥60 | |
| N | 50 | 45 | |
| Age (years) | 42.6 ± 1.7 | 70.5 ± 0.8* | <0.001 (2.60) |
| Body mass index | 22.0 ± 0.4 | 22.8 ± 0.55 | 0.197 (0.27) |
| Sleeping hours | 6.8 ± 0.2 | 7.5 ± 0.2* | 0.038 (0.43) |
| Frequency of nocturnal awakening | 0.48 ± 0.10 | 1.04 ± 0.13* | 0.001 (0.67) |
| GHQ A: Somatic symptoms | 1.9 ± 0.3 | 2.2 ± 0.3 | 0.506 (0.14) |
| GHQ B: Anxiety and insomnia | 2.3 ± 0.3 | 3.0 ± 0.4 | 0.113 (0.37) |
| GHQ C: Social impairment | 0.7 ± 0.2 | 1.5 ± 0.3* | 0.044 (0.54) |
| GHQ D: Severe depression | 0.5 ± 0.2 | 1.2 ± 0.3* | 0.035 (0.46) |
| PA (mG) | |||
| Evening | 39.0 ± 2.0 | 40.5 ± 1.9 | 0.586 (0.11) |
| Morning | 50.2 ± 2.6 | 51.1 ± 3.2 | 0.837 (0.05) |
| HFnu | |||
| Evening | 0.24 ± 0.01 | 0.30 ± 0.02* | 0.023 (0.42) |
| Sleep | 0.47 ± 0.02 | 0.44 ± 0.02 | 0.403 (0.17) |
| Morning | 0.24 ± 0.01 | 0.30 ± 0.02* | <0.001 (0.69) |
- Note: Evening, from the start of the monitoring to sleep at night; Morning, after wake-up to the end of the monitoring; sleep, subjects in bed.
- Abbreviations: HFnu, HF/(LF + HF); HF, high-frequency; LF, low-frequency; PA, physical acceleration.
- * p < 0.05 Non-older versus older.
2.2 Protocols
Participants completed the GHQ28 before or on the day of the assessments before visiting our laboratory at approximately 13:00 and underwent a physical examination and 12-lead electrocardiogram. Thereafter, participants wore a portable monitor (Active Tracer AC301; GMS Inc.) to record their PA and R–R intervals for 24 h. The sampling frequency of the AC301 was 1 kHz. During monitoring, participants were instructed to continue with their usual lives but to avoid bathing. After the 24-h monitoring period, participants returned to the laboratory. The experimental protocols have been described in detail in our previous studies.21-23
2.3 Questionnaires
We used the Japanese version of the GHQ28 to evaluate psychological distress (Nihon Bunka Kagakusya Co., Ltd.).24 The GHQ28 is a self-report instrument frequently used to indicate psychological well-being and the psychological dimensions of quality of life.5 It has four subscales: (A) somatic symptoms, (B) anxiety and insomnia, (C) social impairment, and (D) severe depression.5 Each of these subscales includes seven items, scored using 2-point scores of 0-0-1-1. The sum of scores indicated the severity of mental or psychological distress.5 The GHQ scores were defined as low (total < 8, A and B < 2, C and D = 0) and high (total ≥ 8, A and B ≥ 2, C and D ≥ 1).24
2.4 PA
To measure PA, an ActiveTracer equipped with a triaxial accelerometer (72 g) was used.25 The body of the accelerometer was positioned on the frontal midline of the waist above the navel to avoid disturbing sleep or free movement. The resolution of acceleration was 2 mG, and the sensitivity ranged between 0 and 4.0 G. The absolute values of the resultant vectors, calculated from the signals of triaxial acceleration, were averaged for every minute. The times at which participants fell asleep and woke up were estimated based on records they maintained and changes in body positions were evaluated from the acceleration vectors. We defined three periods: evening, from the start of the monitoring to sleep at night; sleep, time on the bed; and morning, from wake-up to the end of the monitoring.
2.5 HRV analysis
The HRV was analyzed at 1-min intervals using the MEMCalc System software (Suwa Trust Co., Ltd.). One-min HRV analysis was performed in mobile settings described in a previous study.26 The method was based on a maximal entropy combined with the least square method and was useful for frequency analyses using small sample size data.27 LF (0.04–0.15 Hz) and HF power (0.15–0.40 Hz) were analyzed as HRV parameters.16 In this study, PSNA was defined as HFnu = HF/(LF + HF).16
2.6 Definition of %lag
The definition of %lag is shown in previous literature.21, 22 In brief, we determined lag time by the maximum correlation coefficient obtained from the cross-correlation analysis between PSNA and PA. Each cross-correlation coefficient was calculated over 10-min time-windows during 60-min consecutive periods. Our preliminary cross-correlation analysis confirmed that similar results were obtained regardless of whether the implemented time window was 10 or 20 min for evaluating the lag between PSNA and PA (data not shown). When the correlation coefficient of HFnu and PA showed maximum correlation at lag = 0 min, the HFnu and PA were synchronized. If this synchronization continued for 1 h, then the %lag0 = 100%. Thus, low levels of %lag0 indicated an impairment in the coordination between PSNA and PA.
2.7 Statistical analyses
Data are expressed as mean ± standard error of the mean. Statistical analysis was performed using the Student's t-test, Mann–Whitney U-test, and Bonferroni procedure for multiple comparison correction using Excel and SPSS 21.0, as appropriate. The significance of the cross-correlation coefficient at match position was evaluated based on Pearson correlation coefficients. For significant differences, we calculated Cohen's d effect size. p < 0.05 was considered statistically significant.
3 RESULTS
As shown in Table 1, older participants had longer sleep duration and experienced more nocturnal awakenings than younger participants. There were no significant differences between groups in the subscale scores of GHQ28 A: somatic symptoms and B: anxiety and insomnia; however, the older group had significantly higher scores in the GHQ28 subscale C: social impairment and D: depression. The older group had significantly higher HFnu in the evening and morning than the non-older group but not during sleep.
To understand %lag0 better, an example is shown in Figure 1. Figure 1 illustrates a participant's HFnu and PA in 1 h. The top panel shows lag = 0 min, and the next panel shows 5 min PA precedence (lag = 5). The bottom panel shows that the correlation coefficient was calculated every minute from HFnu precedence 10 min to PA precedence 10 min. This case indicated that the time-series between them were synchronized because their correlation coefficient indicated maximal correlation at lag = 0 min. If this synchronization continues for 1 h, the %lag0 = 100%, as defined in Section 2.
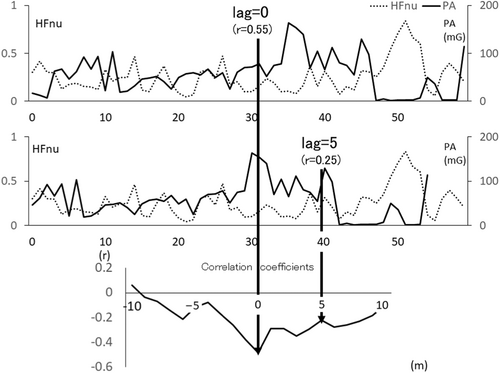
Figure 2 illustrates the %lag0 between PSNA and PA 1 h before sleep. At 1 h before sleep, the older group with higher total GHQ and GHQ A–D scores had significantly lower %lag0 than the non-older group (#, p < 0.05). Remarkably, the older group with high GHQ D scores (lowest Figure 2) showed significantly lower %lag0 between the time-series of PA and HFnu than the older group with low GHQ D scores 1 h before sleep (*, p < 0.05, Cohen's d = 0.55). Nonetheless, in the non-older group, no significant differences were found in the %lag0 between those with low and high GHQ total and A–D scores.
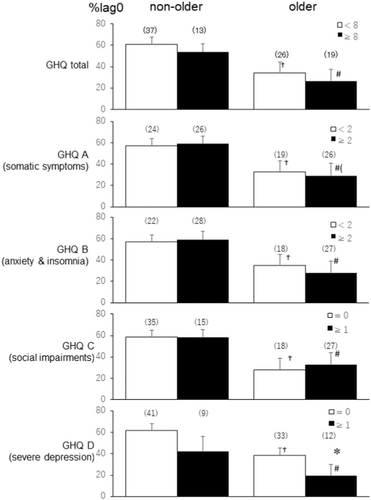
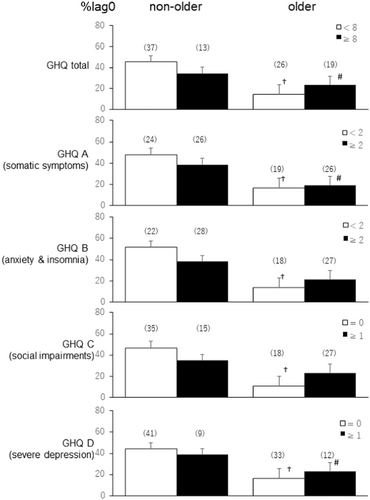
Figure 3 illustrates the %lag0 between PSNA and PA 1 h after sleep. At 1 h after wake-up, the older group with high GHQ A–D scores had significantly lower %lag0 than the non-older group (#, p < 0.05), except in %lag0 of PA and HFnu in GHQ B (p > 0.05, Cohen's d = 0.41) and C (p > 0.05, Cohen's d = 0.43), although they indicated the same tendency. However, no significant difference was observed between the older group with high GHQ D scores and the older group with low GHQ D scores.
4 DISCUSSION
In our previous report, we demonstrated that %lag0 after wake-up is significantly reduced with aging even in healthy adult women.21 In this study, older women with depression (high GHQ D > 1) had significantly lower %lag0 1 h before sleep. These results indicate that depression is closely associated with impairments in the coordination between PSNA and PA before sleep in older women.
In this study, we divided the participants into non-older and older age groups based on a cutoff of 22–59 years in the younger group and 60–85 years in the older group. In our previous study, participants were divided into three groups, young (20–39 years), middle-aged (40–59 years), and older (≥60 years).21 Significant differences were observed in the %lag0 between the middle and older groups in 1 h before sleep; however, o significant differences were observed between the young and middle-aged groups. Therefore, we divided the participants into non-older (20–59 years) and older (≥60 years) in this study.
We defined lag = 0 as the time difference within −30 to 30 s indicated by the minimum p-value obtained from an analysis of the cross-correlation between the HFnu and PA. Active and passive changes in fundamentally different cardiovascular effects for approximately 20 s (within 30 s) involve the central command, muscle receptors, and high- and low-pressure receptors.28 Therefore, the definition of lag was based on the hypothesis that PA causes an immediate PSNA response under healthy conditions. We determined lag = 0 (−30 to 30 s) as an indicator of the coordination between PSNA and PA.
In this study, no significant differences in %lag0 were detected between participants with irregular sleep cycles or without incidents of nocturnal awakening on the experimental day, based on the questionnaires (data for reviewers). Patients with depression have also been reported to have sleep disorders and low sleep efficiency,29, 30 and participants with sleep difficulties are threefold to fourfold more likely to be depressed. PA and PSNA discoordination before sleep, especially 1 h before sleep, may be associated with reduced sleep efficiency and quality.
The total GHQ28 reflects the level of psychological stress31; this is demonstrated in our results showing depressive tendencies in older and non-older individuals based on their GHQ28 D subscale scores and lower %lag0 between HRV and PA. However, the decrease in %lag0 was not significant in non-older individuals. These results indicate that depression is only significantly associated with the discoordination between HRV and PA in older women, particularly 1 h before sleep.
The menopausal years in Japanese women reportedly begin at approximately 52 years of age.32 We divided participants into non-older (≤59 years) and older (≥60 years) groups. Therefore, the older group might have comprised postmenopausal women alone. Conversely, some participants in the non-older group might also have been postmenopausal, as they were in their late 40s and early 50s. Estrogen reportedly reduces cardiomyocyte contractile function and sympathovagal nervous activity, whereas androgen enhances them.32 Estrogen acts on the central nervous system to reduce sympathovagal activity.33 In postmenopausal women, sympathovagal nervous activity is elevated due to changes in the hormonal balance.34 Further, menopause or the menstruation cycle can also affect depression.35 In the present study, we could not establish whether menopause affected the %lag0 in non-older and older women. Therefore, further studies are needed to investigate the effects of the menstruation cycle on the coordination of HRV and PA.
This study has some limitations. First, the analytical method used to evaluate the coordination between the PSNA and PA has limitations. The reason for the significant reduction of %lag0 in older people with depression remains unclear. Second, we could not explain why menopause affected the %lag0 in non-older and older women. Third, this study did not control for the comorbidity score or physical condition of participants. All participants had no or mild diseases. Fourth, in this study, we defined the times at which the participants fell asleep and woke up based on the records of changes in body position evaluated from the acceleration vectors. Although the times obtained by this method may differ from the actual time, they did not significantly affect the %lag0, even when the exact time of falling asleep was off by approximately 10 min. Fifth, menopause may be an important factor that could have affected our results. However, as described above, our previous experiments showed no significant difference between the 20–39 years and 40–59 years groups, whereas a significant difference was observed between the 40–59 years and ≥60 years groups. Lastly, as the number of participants was limited, the effects of diseases on %lag0 remained unclear. Taking the above limitations into consideration, further controlled studies are required in the future.
5 CONCLUSIONS
Using the “%lag0” index, determined by using the time-series correlation analysis between PSNA and PA, this study suggests that impairments in the coordination between PSNA and PA are closely associated with depression in older women, particularly 1 h before sleep on free-moving days. Although further studies are required in the future, this parameter may be useful as a noninvasive index to detect depression in older women in clinical or even home settings.
AUTHOR CONTRIBUTIONS
Kentaro Taniguchi: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; software; validation; visualization; writing—original draft. Naoya Jinno: Data curation; investigation; methodology; visualization; writing—review & editing. Akitoshi Seiyama: Conceptualization; methodology; project administration; supervision; writing—review & editing. Akito Shimouchi: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; supervision; validation; visualization; writing—review & editing.
ACKNOWLEDGMENTS
We thank all the volunteers who participated in this study. We also would like to express special thanks to Ms. Noriko Inui, Mariko Mori, Azusa Kawamura, Shoko Nagahiro, Mariko Komatsu, Nobue Nishi, Hiroko Hayashi, and Yoshiko Kokusho for their technical assistance. We would like to thank Editage (www.editage.com) for English language editing. This study was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Science, and Culture (Grants 17659207 to Akito Shimouchi, and 15J08579 and 19K21435 to Kentaro Taniguchi); the Center of Innovation, Science and Technology-based Radical Innovation and Entrepreneurship Program, Japan, to Akito Shimouchi; the Japan Agency for Medical Research and Development to Akito Shimouchi; and the Intramural Research Fund of the National Cerebral and Cardiovascular Research Center (25-2-1) to Akito Shimouchi. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee at the National Cerebral and Cardiovascular Research Center (M18-19-2, M26-158), Chubu University (280031), and Nagahama Institute of Bio-Science and Technology (006). All participants provided written informed consent before participation in this study.
TRANSPARENCY STATEMENT
The lead author Kentaro Taniguchi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.