Fragmented QRS, a strong predictor of mortality and major arrhythmic events in patients with nonischemic cardiomyopathy: A systematic review and meta-analysis
Moein Zangiabadian and Mohammad Sharifian Ardestani contributed equally as first authors.
Abstract
Background and Aims
Fragmented QRS (fQRS), which is associated with rhythm disturbances, can predispose the heart to fatal ventricular arrhythmias. Recently, accumulating studies indicates that fQRS is associated with poor prognosis in various types of cardiomyopathies. Therefore, we assessed the association between fQRS with all-cause mortality and major arrhythmic events (MAEs) in patients with nonischemic cardiomyopathy, in this systematic review and meta-analysis study.
Methods
We performed a comprehensive search in databases of PubMed/Medline, EMBASE, and Web of Science from the beginning to December 31, 2022. Published observational studies (cohorts, case–control, or analytical cross-sectional studies) were included that report the prognostic value of fQRS in patients with different types of nonischemic cardiomyopathies for MAEs (sudden cardiac death, sudden cardiac arrest, sustained ventricular tachycardia [VT], ventricular fibrillation [VF], and appropriate shock) and all-cause mortality. We pooled risk ratios (RRs) through raw data and adjusted hazard ratios (aHRs) using “Comprehensive Meta-Analysis” software, Version 2.0.
Results
Nineteen cohort and three analytical cross-sectional studies were included in this meta-analysis involving a total of 4318 subjects with nonischemic cardiomyopathy (1279 with fQRS and 3039 without fQRS). FQRS was significantly associated with an increased risk of all-cause mortality in patients with nonischemic cardiomyopathy (pooled RR: 1.920; 95% confidence interval [CI]: 1.388–2.656, p < 0.0001/pooled HR: 1.729; 95% CI: 1.327–2.251, p < 0.0001). Also, the risk of developing MAEs in the presence of fQRS was significantly increased (pooled RR: 2.041; 95% CI: 1.644–2.533, p < 0.0001/pooled HR: 3.626; 95% CI: 2.119–6.204, p < 0.0001). In the subgroup analysis, the strongest association between fQRS presence and increased MAEs was observed in patients with hypertrophic cardiomyopathy (HCM) (pooled RR: 3.44; 95% CI: 2.07–5.71, p < 0.0001/pooled HR: 3.21; 95% CI: 2.04–5.06, p < 0.0001).
Conclusion
Fragmented QRS could be a prognostic marker for all-cause mortality and MAEs in patients with various types of nonischemic cardiomyopathies, particularly HCM.
1 INTRODUCTION
Cardiomyopathy is a heterogeneous pathologic cardiac condition that is characterized by myocardial and electrical dysfunction.1 It has remained an important public health issue with increasing prevalence, morbidity, and mortality over the past decades.2 It has been reported that approximately 50% of patients who experience sudden death or undergo cardiac transplantation suffer from cardiomyopathies.3 There are two main categories of cardiomyopathies, namely, ischemic (ICM) and nonischemic cardiomyopathies (NICM). Among various types of nonischemic cardiomyopathies, there are four major types; hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), and arrhythmogenic right ventricular cardiomyopathy (ARVC). Nonischemic cardiomyopathies can be result from various conditions, including genetic abnormalities, idiopathic, cardiovascular diseases, and other systemic disorders.4 It has been shown that patients with ICM have higher overall mortality and worse outcomes compared to those with NICM. The latter group, however, has a lower left ventricular ejection fraction.5, 6 Besides, NICM was found to be associated with higher cumulative incidence for the ventricular arrhythmia recurrence, in comparison with ICM, during the long period.7 However, there was a limited data available on the incidence of NICM and its impacts on patients, compared to ICM. Pathologically, chronic cardiomyopathies are characterized by myocardial scarring and fibrosis, which might be a predisposing factor for major arrhythmic events (MAEs) such as sudden cardiac death and ventricular dysrhythmias, as well as ventricular dysfunction.8, 9
Twelve-lead electrocardiography (ECG) is an important noninvasive method for evaluating patients with cardiovascular disorders, and association between ECG markers with the prognosis of cardiomyopathies.10-12 Fragmented QRS (fQRS), defined as various RSR' patterns, has been identified as an ECG marker of myocardial scarring and fibrosis,13 which could have a prognostic value for poor cardiovascular outcomes and mortality in various cardiac disorders, including coronary artery disease,14 Brugada syndrome15 and heart failure (HF).16
During the past decade, accumulating studies have suggested that the presence of fQRS on ECG may be a predictive factor of higher risk of MAEs and mortality in various types of cardiomyopathies.17-21 However, no systematic review and meta-analysis study is available to address the association between fQRS with MAEs and all-cause mortality in various groups of nonischemic cardiomyopathies. Herein, we aimed to assess the prognostic value of fQRS for mortality and major arrhythmic events in patients with nonischemic cardiomyopathy.
2 METHODS
In this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement and the MOOSE Checklist (Tables S1 and S2).22, 23 Grades of recommendations, assessments, development, and evaluation (GRADE) frameworks were used to assess outcomes.24 PROSPERO (reference number CRD42022371432) was the database where the study was registered.
2.1 Search strategy
We searched PubMed/Medline, EMBASE, and Web of Science for studies reporting the prognostic value of fragmented QRS in patients with different types of cardiomyopathies published up to December 31, 2022. Cohort and analytical cross-sectional studies written in English were selected. We used the following MeSH terms: “‘Cardiomyopathies’, ‘Cardiomyopathy, Restrictive’, ‘Cardiomyopathy, Hypertrophic’, ‘Cardiomyopathy, Dilated’, ‘Takotsubo Cardiomyopathy’, ‘Cardiomyopathy, Hypertrophic, Familial’, ‘Arrhythmogenic Right Ventricular Dysplasia’”. Keyword searches were done with combinations of the terms “nonischemic cardiomyopathy,” “congestive cardiomyopathy,” “non-compaction cardiomyopathy,” “left ventricular non-compaction,” “fragmented qrs,” “fqrs” and “notched qrs” (Supporting Information S2: Tables 3–5). Backward and forward citation searching was performed and gray literatures like conference abstracts were searched.
2.2 Study selection
EndNote X8 (Thomson Reuters) was used to merge and remove duplicate records found through database searching. To exclude unrelated records, two reviewers (M. Z., M. S. A.) separately screened the records by title/abstract and full text. It was the lead investigator (M. A. A.) who resolved any disagreements. Included studies met the following criteria: (i) adult patients (>18 years) were diagnosed with different types of nonischemic cardiomyopathy based on the European Society of Cardiology statement (see below)25; (ii) patients were divided into fQRS+ and fQRS− groups; and (iii) outcomes (major arrhythmic events [sudden cardiac death/sudden cardiac arrest/sustained ventricular tachycardia/ventricular fibrillation and appropriate shock] and all-cause mortality) were reported between the 2 groups (fQRS+ and fQRS−). According to European Society of Cardiology statement25; nonischemic Cardiomyopathy was defined as: “A myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality,” that has five subtypes, including: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), and unclassified cardiomyopathies (left ventricular noncompaction [LVNC] and Takotsubo cardiomyopathy). The definitions of these cardiomyopathies were established by referring to primary sources that mostly utilized a shared set of standards. However, Arrhythmogenic left ventricular cardiomyopathy (ALVC) and myocarditis were not included in European Society of Cardiology statement, we considered them as independent categories of nonischemic cardiomyopathy for our study inclusion. Fragmented QRS was defined as: “Various RSR' patterns such as an additional R prime (R'), notching of the R wave, notching of the S wave, or the presence of multiple (2) R waves, in the absence of a wide QRS (wQRS),” on at least two contiguous leads, with or without Q wave.26 Moreover, only studies with standard ECG filter setting including magnification and sampling frequency, were included. Editorials, reviews, study protocols, and studies focusing on ischemic cardiomyopathies or discussing about the prevalence of fQRS in patients with cardiomyopathy were excluded.
2.3 Data extraction
Two reviewers (M. Z., M. S. A.) designed a data extraction form. These reviewers collected data from all relevant studies, and disagreements were settled by consensus. The following data were extracted: first author name; year of publication; study design and duration; countries where the research was conducted; demographics (i.e., age, sex); Follow-up time; the definition of case and control; the total participants; number of controls and cases and definition of outcomes (all-cause mortality and major arrhythmic events). The corresponding authors of each included study were contacted via email if raw data was missing.
2.4 Quality assessment
Based on the JBI's critical appraisal tools for cohort and cross-sectional analyses, two reviewers (M. Z., M. S. A.) evaluated the quality of the studies.27 Discrepancies were resolved by consulting a third reviewer. A variety of variables were evaluated, including the study population, the measure of exposures, confounding factors, the extent of outcomes, and the statistical analysis.
2.5 Statistical analysis
We analyzed the pooled risk ratios (RRs) for raw data and the adjusted hazard ratios (aHRs) using fixed or random effect models. In cases where the heterogeneity between studies was low, the fixed effect model was used, and in cases where the heterogeneity of the true effect sizes was high, the random effect model was used. To assess between-study heterogeneity, Cochran's Q was used, as well as the I2 statistic. It was considered high heterogeneity if the I2 value was greater than 50%.28 Subgroup analysis was performed to compare the prognostic value of fQRS in different cardiomyopathy subtypes. Moreover, sensitivity analysis with one out remove method was done to assess the effect of each study (especially conference abstracts) on final result.29 To determine publication bias statistically, Egger's and Begg's tests were used. In addition, funnel plots were constructed (p < 0.05 indicates statistically significant publication bias, and funnel plot asymmetry indicates bias).30 All analyses were conducted using “Comprehensive Meta-Analysis” software, Version 2.0 (Biostat).
3 RESULTS
The including process of articles is shown in the PRISMA flow-chart (Figure 1). In this process, 19 cohort and 3 analytical cross-sectional studies were included and classified into the following: six studies that had information to calculate risk ratio and also reported hazard ratio,20, 31-35 nine articles only had raw data but did not report hazard ratio17, 18, 36-42 and seven studies only reported hazard ratio without providing raw data.26, 43-48 According to GRADE framework all studies had moderate certainty except two studies with low certainty.37, 39

Eight studies examined hypertrophic cardiomyopathy (HCM),20, 34, 35, 39, 40, 43-45 while seven articles focused on patients with dilated cardiomyopathy (DCM).17, 26, 36, 37, 41, 42, 46 Two articles investigated arrhythmogenic right ventricular cardiomyopathy (ARVC),32, 47 while two others explored LVNC.31, 33 Additionally, two articles specifically investigated RCM, resulting from sarcoidosis38 and amyloidosis48 respectively. Only one study18 was conducted on patients with nonischemic cardiomyopathy, without any differentiation into subtypes.
Fifteen studies were conducted in Korea,18, 20, 36, 37, 43 Japan,17, 35, 38, 45, 46 and China33, 41, 42, 44, 46 (five studies in each country). Other article's origins were Turkey with three studies,31, 32, 40 the USA with two26, 39 and both Italy48 and Portugal34 with one study. Other study characteristics like publication year, study design, and duration and follow-up time are summarized in Table 1.
| References | Publication year | country | Study design | Study duration | Follow-up time | Type of cardiomyopathy |
|---|---|---|---|---|---|---|
| Das et al.26 | 2010 | USA | Cohort, retrospective, single center | January 1, 2002 to December 31, 2005 | Mean: 16.6 ± 10.2 months | DCM |
| Sha et al.41 | 2011 | China | Cohort, retrospective, single center | January 1, 2009 to December 31, 2009 | Mean: 14 ± 5 months | DCM |
| Kang et al.20 | 2012 | Korea | Cohort, retrospective, single center | January 2000 to December 2005 | Mean: 7.1 ± 2.2 years | HCM |
| Ning et al.33 | 2012 | China | Cohort, retrospective, single center | July 2001 to June 2009 | Mean: 32 ± 24 months | LVNC |
| Pei et al.46 | 2012 | China | Analytical cross-sectional, prospective, single center | July 2005 to December 2009 | Median: 36 months (0.40–65) | DCM |
| Omery et al.39 | 2013 | USA | Cohort, retrospective, single center | January 1, 2000 and December 15, 2011 | NR | HCM |
| Canpolat et al.32 | 2013 | Turkey | cohort, retrospective, single center | NR | Mean: 38 ± 14 months | ARVC |
| Perlini et al.48 | 2013 | Italy | Cohort, retrospective, multicenter | 2008–2010 | Median: 18.7 months | RCM (due to amyloidosis) |
| Ahn et al.36 | 2013 | Korea | cohort, retrospective, single center | October 2002 to June 2008 | Mean: 36.9 ± 24.6 months | DCM |
| Kang et al.43 | 2014 | Korea | cohort, retrospective, single center | February 2001 and April 2007 | Mean: 6.3 years | HCM |
| Nomura et al.45 | 2015 | Japan | cohort, retrospective, multicenter | September 2008 to March 2010 | Median: 4.6 years (4.1–4.8) | HCM |
| Zhao et al.42 | 2015 | China | Cohort, prospective, single center | NR | Mean: 2 years | DCM |
| Cetin et al.31 | 2016 | Turkey | cohort, retrospective, single center | April 2004 to April 2015 | Median: 42.4 months (1.1–120.3) | LVNC |
| Ozyilmaz et al.40 | 2017 | Turkey | Cohort, prospective, multicenter | December 2012 to March 2016 | Mean: 31.7 ± 12.7 months | HCM |
| Kimura et al.47 | 2017 | Japan | Analytical cross-sectional, retrospective, single center | 2007–2013 | Median: 42.5 months (22.0–69.7) | ARVC |
| Lee et al.37 | 2017 | Korea | Analytical cross-sectional, prospective, single center | NR | Mean: 43.6 months | DCM: NR |
| Lu et al.44 | 2017 | China | cohort, prospective, single center | From 2000 to 2012 | Mean: 5.3 ± 2.4 years | HCM |
| Ruivo et al.34 | 2019 | Portugal | cohort, retrospective-prospective, multicenter | April 2013 to April 2015 | Median: 5 years | HCM |
| Ogura et al.35 | 2020 | Japan | cohort, retrospective, single center | April 2004 to April 2017 | Mean: 4.6 ± 4.3 years | HCM |
| Marume et al.17 | 2021 | Japan | cohort, prospective, single center | January 2007 to December 2015 | Median: 3.8 years (1.8–6.2) | DCM |
| Cho et al.18 | 2021 | Korea | cohort, retrospective, single center | February 2004 to June 2014 | Mean: 43.6 ± 37.3 months | NICM: NR |
| Ogura et al.38 | 2021 | Japan | cohort, retrospective, single center | NR | Mean: 5 years | RCM (due to sarcoidosis) |
- Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; fQRS, fragmented QRS; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular noncompaction; NICM, nonischemic cardiomyopathy; NR, not reported; RCM, restrictive cardiomyopathy.
3.1 Quality of the included studies
The JBI's critical appraisal tools,27 indicated that the included studies had a low risk of bias except Omery et al.39 and Lee et al.37 studies. The study conducted by Omery is deemed to be at a significant risk of bias, particularly in cases involving confounding factors and follow-up procedures. Similarly, the study conducted by Lee is at a high risk of bias owing to the handling of study setting and measurement criteria (Supporting Information S1: Tables S6 and S7).
3.2 Patient characteristics
There were 1279 fQRS+ and 3039 fQRS− participants in included studies with a total population of 4318. According to 17 studies,17, 20, 31-36, 40-48 the age range was 31.2–65 years (the mean of total patients was 54-year-old). Males were predominant in 14 out of 17 studies (68% of patients were male).17, 20, 31-36, 39-47 According to 11 studies, the percentage of male participants was 72% in case groups (fQRS+) and 65.3% in control groups (fQRS−).17, 31-33, 35, 36, 40-42, 44, 45 The definition and number of case and control groups and outcomes are shown in Table 2.
| References | Age (mean ± SD of years) | Gender (M/F %) | Total population | Case definition (number of participants) | Control definition (number of participants) | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| Das et al.26 | 63.3 ± 11.4 | NR | 116 | DCM and fQRS+ (31) | DCM and fQRS− (85) | Major arrhythmic event | ||
| Sha et al.41 | 54 ± 14 | 67.5/32.5 | 60 | DCM and fQRS+ (51) | DCM and fQRS− (29) | All-cause mortality | ||
| 71/29 | 62/38 | |||||||
| Kang et al.20 | 62 ± 11 | 71.1/28.9 | 135 | HCM and fQRS+ (51) | HCM and fQRS− (84) | Major arrhythmic event | ||
| Ning et al.33 | 44 ± 18 | 33.3/66.7 | 45 | LVNC and fQRS+ (24) | LVNC and fQRS− (21) | All-cause mortality | ||
| 33/67 | 33/67 | |||||||
| Pei et al.46 | 56.6 ± 14.4 | 77.9/22.1 | 572 | DCM and fQRS+ (116) | DCM and fQRS− (456) | All-cause mortality. Major arrhythmic event. |
||
| Omery et al.39 | NR | 44.1/55.9 | 34 | HCM and fQRS+ (15) | HCM and fQRS− (19) | All-cause mortality | ||
| Canpolat et al.32 | 31.2 ± 11.5 | 65.3/34.7 | 78 | ARVC and fQRS+ (46) | ARVC and fQRS− (32) | Major arrhythmic event | ||
| 67/33 | 62/28 | |||||||
| Perlini et al.48 | 65 ± 17 | NR | 264 | AML with cardiac involvement (RCM) and fQRS+ (75) | AML with cardiac involvement (RCM) and fQRS− (189) | All-cause mortality | ||
| Ahn et al.36 | 55.7 ± 12.5 | 61.6/38.4 | 86 | DCM and fQRS+ (53) | DCM and fQRS− (33) | All-cause mortality. Major arrhythmic event. |
||
| 64/36 | 58/42 | |||||||
| Kang et al.43 | 55 | 36.5/63.5 | 167 | HCM and fQRS+ (67) | HCM and fQRS− (100) | Major arrhythmic event | ||
| Nomura et al.45 | 58 ± 17 | 59.5/40.5 | 94 | HCM and fQRS+ (31) | HCM and fQRS− (63) | Major arrhythmic event | ||
| 65/35 | 57/43 | |||||||
| Zhao et al.42 | 53.2 + 5.4 | 59.1/40.9 | 49 | DCM and fQRS+ (20) | DCM and fQRS− (29) | Major arrhythmic event | ||
| 65/35 | 55/45 | |||||||
| Cetin et al.31 | 38.6 ± 17.7 | 64.7/35.3 | 88 | LVNC and fQRS+ (47) | LVNC and fQRS− (41) | All-cause mortality. Major arrhythmic event. |
||
| 64/36 | 66/34 | |||||||
| Ozyilmaz et al.40 | 46.5 ± 15.3 | 58.2/41.8 | 115 | HCM and fQRS+ (65) | HCM and fQRS− (50) | Major arrhythmic event | ||
| 66/34 | 48/52 | |||||||
| Kimura et al.47 | 52 ± 15 | 77.5/22.5 | 40 | Patients with ILA ≥ 50 and ARVC and fQRS+ (15) | Patients with ILA < 50 and ARVC and fQRS− (25) | Major arrhythmic event | ||
| Lee et al.37 | NR | NR | 307 | DCM and fQRS+ (43) | DCM and fQRS− (264) | Major arrhythmic event | ||
| Lu et al.44 | 51.8 ± 13.5 | 72/28 | 326 | HCM and fQRS+ (105) | HCM and fQRS− (221) | All-cause mortality | ||
| 70/30 | 73/27 | |||||||
| Ruivo et al.34 | 53.2 ± 16.4 | 66.3/33.4 | 911 | HCM and fQRS+ (89) | HCM and fQRS− (822) | Major arrhythmic event | ||
| Ogura et al.35 | 60 ± 16 | 64.3/35.7 | 146 | HCM and fQRS− (46) | HCM and fQRS− (100) | All-cause mortality. Major arrhythmic event. |
||
| 67/33 | 63/37 | |||||||
| Marume et al.17 | 52 ± 15 | 80/20 | 290 | DCM and fQRS+ (213) | DCM and fQRS− (77) | All-cause mortality. Major arrhythmic event. |
||
| 82/18 | 74/26 | |||||||
| Cho et al.18 | NR | NR | 307 | NICM and fQRS+ (99) | NICM and fQRS− (208) | All-cause mortality. Major arrhythmic event. |
||
| Ogura et al.38 | 61 ± 11 | NR | 68 | RCM (sarcoidosis) and fQRS+ (30) | RCM (sarcoidosis) and fQRS− (38) | All-cause mortality. Major arrhythmic event. |
||
- Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; fQRS, fragmented QRS; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; ILA, isolated late activation; LVNC, left ventricular noncompaction; NICM, nonischemic cardiomyopathy; NR, not reported; RCM, restrictive cardiomyopathy; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
3.3 Prognostic value of fQRS in patients with nonischemic cardiomyopathy
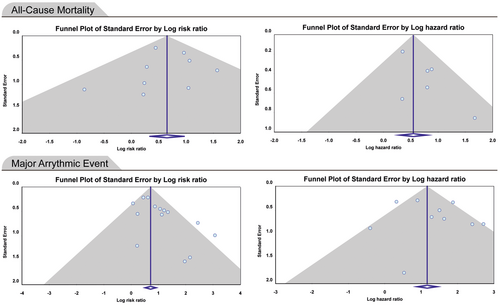
The meta-analysis of included studies showed that all-cause mortality in patients with nonischemic cardiomyopathy and fQRS was about two times more than nonischemic cardiomyopathy patients without fQRS (RR: 1.920; 95% CI: 1.388–2.656, p < 0.0001). The pooled HR of all-cause mortality in patients with nonischemic cardiomyopathy and fQRS was 1.729 (95% CI: 1.327–2.251, p < 0.0001) (Figure 2). There was no evidence of publication bias in reporting RR and HR for all-cause mortality (p > 0.05) (Figure 3). Thus, the presence of fQRS was significantly associated with increased all-cause mortality in patients with nonischemic cardiomyopathy. Also, the risk of developing major arrhythmic events (MAEs) in case groups was twice that of controls (RR: 2.041; 95% CI: 1.644–2.533, p < 0.0001). The pooled HR of MAEs in patients with nonischemic cardiomyopathy and fQRS was 3.626 (95% CI: 2.119–6.204, p < 0.0001) (Figure 2). There was evidence of publication bias in the reporting of risk ratios (Begg's test: 0.048, Egger's test: 0.014)., but not for hazard ratios (Begg's test: 1.000, Egger's test: 0.520) (Figure 3). Therefore, fQRS in patients with nonischemic cardiomyopathy significantly increased the risk of MAEs. Sensitivity analysis showed that removing any of studies could not change the final results significantly except removal of the Pei and colleagues study46 that only reported fQRS on the inferior lead and reported 1.4-fold increased HR for all-cause mortality, and 1.2-fold for MAEs (Supporting Information S3: Figures 1–4). Details of analysis are summarized in Table 3.
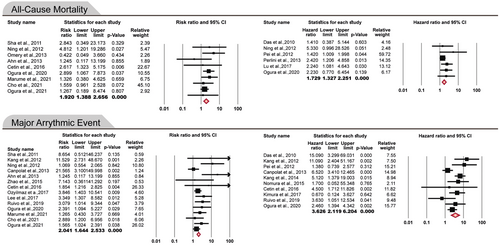
| Outcomes | No. of study | No. of patients | (95% CI)/(p Value) | Heterogeneity I2 (%)/p Value | Begg/Egger test p Value |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| RR: 1.920 | 9 studies | 1144 | (1.388–2.656)/<0.0001 | 0.00/0.580 | 0.75/0.88 |
| HR: 1.729 | 6 studies | 1469 | (1.327–2.251)/<0.0001 | 0.00/0.450 | 0.70/0.08 |
| Major arrhythmic events | |||||
| RR: 2.04 | 14 studies | 2705 | (1.644–2.533)/<0.0001 | 43.7/0.040 | 0.048/0.014 |
| HR: 3.626 | 10 studies | 2347 | (2.119–6.204)/<0.0001 | 61.5/0.005 | 1.00/0.52 |
- Abbreviations: CI, confidence interval; HR, hazard ratio; NO, number; RR, risk ratio.
3.4 Subgroup analysis
The comparison of prognostic value of fQRS in different nonischemic cardiomyopathy subtypes is shown in Tables 4 and 5. The strongest association between fQRS presence and increased MAEs was in HCM patients (RR: 3.44; 95% CI: 2.07–5.71, p < 0.0001/HR: 3.21; 95% CI: 2.07–5.71, p < 0.0001). Also, the HR of all-cause mortality in HCM patients was significant (HR: 2.23; 95% CI: 1.22–4.08, p = 0.009). fQRS significantly increased the risk of all-cause mortality and MAEs in patients with LVNC (RR for all-cause mortality: 2.94; 95% CI: 1.59–5.43, p = 0.001/RR for MAEs: 1.58; 95% CI: 1.10–2.53, p = 0.012) but the risk of MAEs in patients with ARVC and fQRS was not significant (HR:2.40; 95% CI: 0.26–21.9, p = 0.438). For DCM patients, RR in MAEs and HR in all-cause mortality showed a significant association (RR for MAEs: 2.39; 95% CI: 1.25–4.56, p = 0.008/HR for all-cause mortality: 1.41; 95% CI: 1.02–1.97, p = 0.038).
| Subgroups | No. of study | No. of patients | Risk ratio (95% CI)/p Value | Heterogeneity I2 (%)/p Value | Begg/Egger test p Value |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| DCM | 3 studies | 84 | 1.54 (0.58–4.11)/0.380 | 0.00/0.810 | 1.00/0.69 |
| HCM | 2 studies | 235 | 1.41 (0.22–8.78)/0.700 | 60.2/0.110 | NA |
| LVNC | 2 studies | 149 | 2.94 (1.59–5.43)/0.001 | 0.00/0.440 | NA |
| Major arrhythmic events | |||||
| DCM | 5 studies | 84 | 2.39 (1.25–4.56)/0.008 | 0.00/0.480 | 1.00/0.58 |
| HCM | 4 studies | 235 | 3.44 (2.07–5.71)/<0.0001 | 17.24/0.300 | 0.30/0.08 |
| LVNC | 2 studies | 149 | 1.58 (1.10–2.53)/0.012 | 47.4/0.160 | NA |
- Abbreviations: CI, confidence interval; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular noncompaction; NA, not applicable; NO, number.
| Subgroups | No. of study | No. of patients | Hazard ratio (95% CI)/p Value | Heterogeneity I2 (%)/p Value | Begg/Egger test p Value |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| DCM | 2 studies | 84 | 1.41 (1.02–1.97)/0.038 | 0.00/0.990 | NA |
| HCM | 2 studies | 235 | 2.23 (1.22–4.08)/0.009 | 0.00/0.995 | NA |
| Major arrhythmic events | |||||
| DCM | 2 studies | 84 | 4.11 (0.39–42.4)/0.235 | 87.7/0.004 | NA |
| HCM | 5 studies | 235 | 3.21 (2.04–5.06)/<0.0001 | 0.51/0.400 | 0.46/0.34 |
| ARVC | 2 studies | 149 | 2.40 (0.26–21.9)/0.438 | 83.5/0.014 | NA |
- Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; CI, confidence interval; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; NA, not applicable; NO, number.
4 DISCUSSION
FQRS pattern on ECG is linked to rhythm disturbances, which can potentially increase the susceptibility of the heart to life-threatening ventricular arrhythmias. There is accumulated evidence indicating that fQRS is associated with a poor prognosis in various types of cardiomyopathies, such as NICMs, and could serve as a risk factor and predictor of adverse outcomes in this patient population.49 In this way, the present systematic review and meta-analysis aimed to explore the relationship between fQRS and two significant outcome measures, including all-cause mortality and major arrhythmic events (MAEs) in patients with nonischemic cardiomyopathy. Our meta-analysis indicated that the risk of all-cause mortality as well as MAEs in nonischemic cardiomyopathy patients with fQRS was about twice that of those without fQRS (RR: 1.920 and RR: 2.041, respectively). In addition, the pooled HR of all-cause mortality and MAEs in patients with cardiomyopathy and fQRS was 1.729 and 3.626, respectively. This meta-analysis suggests that the presence of fQRS is significantly associated with an increased risk of all-cause mortality and development of MAEs in patients with nonischemic cardiomyopathy. Therefore, fQRS seems to be predictive for poor cardiac outcomes and mortality. In addition, the subgroup analysis indicated the strongest association between fQRS presence and increased risk of MAEs was in HCM patients (RR: 3.44/HR: 3.21).
Over recent decades, various imaging modalities such as echocardiography, cardiac magnetic resonance (CMR) or positron emission tomography (PET) have been used to assess myocardial function and fibrosis and evaluate the prognosis of cardiomyopathies. CMR technique, which provides a comprehensive myocardial tissue characterization and detects myocardial fibrosis and scarring, has shown prognostic value in subjects with ischemic and nonischemic cardiomyopathy.50, 51 However, CMR is usually expensive and difficult to access and apply. In this way, the importance of ECG findings, as a noninvasive, cost-effective, and convenient tool, is being investigated to predict the prognosis of patients with cardiomyopathy. The notable association between the presence of fQRS on the surface ECG and higher risk of MAEs and mortality has been increasingly reported by recent studies in various cardiovascular conditions,52-55 including various types of cardiomyopathies.17, 18, 44 This meta-analysis revealed that the presence of fQRS was significantly associated with an elevated risk of all-cause mortality and MAEs in patients with nonischemic cardiomyopathies. Therefore, fQRS could be considered as a valuable prognostic marker for patients during clinical management, potentially enhancing decision-making processes for clinicians.
Similar to our findings, a meta-analysis found a strong relationship between the presence of fQRS on baseline ECG and an increased risk of all-cause mortality and MAEs (RR: 1.63, 95% CI: 1.22–2.19 and RR: 1.74, 95% CI: 1.09–2.80, respectively) in HF patients with reduced ejection function (EF). These correlations were more pronounced in those who had not received an implantable cardioverter-defibrillator (ICD), compared with those who had received ICD.16 In line with our results, the findings of Rosengarten and colleagues meta-analysis, which consisted of 12 studies with 5009 patients, revealed the increased risk of all-cause mortality (RR: 1.71 (CI 1.02–2.85)) and SCD (RR: 2.20 (CI 1.05–4.62)) was linked with fQRS in patients with coronary artery disease or nonischemic cardiomyopathy.56 Another meta-analysis has similarly identified a significant association between fQRS complex on ECG and intraventricular dyssynchrony, especially in patients with nonischemic cardiomyopathy (OR: 19.97, CI: 12.12–32.92, p < 0.001), which may indicate poor outcomes.57 Also, fQRS was independently associated with nonresponse to cardiac resynchronization therapy, suggestive a worse prognosis.57 Consistently, another meta-analysis of 5 studies involving 673 HCM patients (205 with fQRS and 468 without fQRS) conducted by Rattanawong and colleagues, reported that fQRS was significantly associated with MAEs (RR: 7.29, 95% CI: 4.00–13.29).58 Taken together, the current evidence suggests that fQRS may have prognostic value for predicting worsened outcomes in patients with cardiomyopathies, which is in line with the findings of this study.
The pathogenesis of cardiomyopathy and the cause of fQRS formation play an essential role in explaining the relationship between fQRS and worse prognosis in cardiomyopathy. FQRS is generally known as additional notches and/or spikes within a narrow QRS complex. It has been demonstrated that fQRS reflects an abnormality in intraventricular depolarization and myocardial activation, which result from heterogeneous conduction characteristics of injured myocardium due to the formation of scar and/or fibrous tissue.59, 60 Accumulating studies have suggested that fQRS could be considered a novel ECG marker for the detection of myocardial scar and fibrosis with more sensitivity and less specificity than Q wave, in a varied spectrum of cardiac disorders.13, 61, 62
Fibrosis of the ventricular myocardium is the main pathologic characteristic in various types of nonischemic cardiomyopathies, including DCM, ARVC, LVNC, HCM, and RCM,63, 64 and is independently related to poor prognosis.65, 66 Fibrosis and scar tissue, which have no electrical and contractile function, results in heterogenicity in the ventricular myocardium, which contributes to promoting contractile dysfunction as well as rhythm disturbances.67 Myocardial fibrosis and/or scarring cause changes in intercellular impedance, localized conduction blocks, impaired impulse propagation, and conduction velocity delay in damaged myocardium. Therefore, these areas could act as a potential substrate for arrhythmogenic reentrancy and maintain the arrhythmia circuit, leading to an increased risk of automaticity and susceptibility to developing malignant ventricular arrhythmias and SCD.68 Therefore, the extent and location of the scar and/or fibrosis in the ventricular myocardium create a diverse QRS vector during myocardial depolarization, which could result in fQRS.59
In patients with various ischemic and nonischemic cardiovascular disorders, the myocardial fibrosis or scarring formation, which promotes ventricular stiffness, can contribute to ventricular dyssynchrony,69 systolic and diastolic dysfunction and subsequent decreased ejection fraction (EF)70; thereby leading to the development of HF, which can cause poor prognosis. EF was observed to be significantly negatively related to the presence of fQRS in the patients with acute myocardial infarction,14 takotsubo cardiomyopathy,71 and nonischemic dilated cardiomyopathy.36 Furthermore, the correlation between fQRS and elevated risk of ventricular tachyarrhythmias was found to be more notable in patients with low LVEF (≤50%).72 Also, a study demonstrated that fQRS was a potential predictor of HF and hospitalization due to HF in patients with HCM, likely indicating poor prognosis.45
As another explanation, fQRS was reported to be significantly associated with the thickness of epicardial adipose tissue (EAT).73 EAT is a metabolically active tissue, that could promote myocardial fibrosis via the release of adipo-fibrokines.74 Furthermore, the presence of fQRS in sarcoidosis is likely a sign of advanced myocardial damage and active inflammatory lesions.62 Therefore, it seems that the association between worsened outcomes and presence of fQRS on surface ECG in cardiovascular disorders may be related to ventricular arrhythmias, SCD, and ventricular dysfunction secondary to myocardial fibrosis and scarring.75
4.1 Limitations
There were some limitations in our meta-analysis that should be noted. First of all, included studies had a qualitative evaluation of fQRS by visual inspection, which can be subject to interpretation. It is important to note that qualitative evaluation of QRS fragmentation may miss more subtle deflections in the QRS complex that may also be significant indicators of prognosis. So quantitative methods have been introduced but are not used in most of studies.19, 76 An additional limitation is the use of different definitions of fQRS across studies. Second, subgroup analysis was used to identify the source of heterogeneity, but because we used raw data for our meta-analysis, the effects of other potential sources of heterogeneity, such as participant age, country of residence, and baseline comorbidities, were not considered when calculating pooled RR. In addition, there was evidence of publication bias in the outcome measure of risk ratio of major arrhythmic events. Because of the possibility of bias in pooling the estimates, the findings should be interpreted with caution. Third, we only focused on all-cause mortality and did not take specific-cause mortality such as cardiovascular mortality into account. Fourth, the relationship between other ECG parameters such as prolonged QTc, which can influence mortality and MAEs occurrence, and fQRS was not determined. Fifth, included studies have chosen different indicators for MAEs and had different follow-up times to detect these events so the weight and effect size strength of similar articles were different. Finally, all non-English articles were excluded that can lead to bias in the results.
4.2 Future directions
Altogether, further studies would be conducted to establish the role of fQRS in predicting poor prognosis in patients with nonischemic cardiomyopathy and other cardiac diseases. It seems that several large-scale and multicenter cohort studies, which use a standard and similar definition for fQRS, are required.
5 CONCLUSION
In conclusion, the current meta-analysis suggests that the presence of fQRS on ECG significantly increased the risk of all-cause mortality and MAEs in patients with various types of nonischemic cardiomyopathies, particularly HCM. Therefore, fQRS might be considered an available prognostic factor in the risk stratification of nonischemic cardiomyopathy patients.
AUTHOR CONTRIBUTIONS
Moein Zangiabadian: Conceptualization; formal analysis; methodology; software; writing—original draft. Mohammad Sharifian Ardestani: Conceptualization; investigation; validation. Malihe Rezaee: Writing—original draft. Elahe Saberi Sharbabaki: Investigation; writing—original draft. Mahdi Nikoohemmat: Data curation; investigation. Mohammad Eslami: Writing—original draft. Kian Goudarzi: Software. Mojgan Sanjari: Validation. Mohammad Hasan Namazi: Project administration. Mohammad Ali Akbarzadeh: Methodology; validation; writing—review & editing. Azadeh Aletaha: Writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Mohammad Ali Akbarzadeh, Azadeh Aletaha affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/Supporting Information. Further inquiries can be directed to the corresponding authors.