Mapping trends and hotspots in research on global influenza vaccine hesitancy: A bibliometric analysis
Zhengyu Zhang and Songjia Tang have contributed equally to this work and shared the first authorship.
Abstract
Background and Aims
Influenza is one of the most widespread respiratory infections and poses a huge burden on health care worldwide. Vaccination is key to preventing and controlling influenza. Influenza vaccine hesitancy is an important reason for the low vaccination rate. In 2019, Vaccine hesitancy was identified as one of the top 10 threats to global health by the World Health Organization. However, there remains a glaring scarcity of bibliometric research in that regard. This study sought to identify research hotspots and future development trends on influenza vaccine hesitation and provide a new perspective and reference for future research.
Methods
We retrieved publications on global influenza vaccine hesitancy from the Web of Science Core Collection database, Scopus, and PubMed databases from inception to 2022. This study used VOSviewer and CiteSpace for visualization analysis.
Results
Influenza vaccine hesitancy-related publications increased rapidly from 2012 and peaked in 2022. One hundred and nine countries contributed to influenza vaccine hesitation research, and the United States ranked first with 541 articles and 7161 citations. Vaccines-Basel was the journal with the largest number of published studies on influenza vaccine hesitations. MacDonald was the most frequently cited author. The most popular research topics on influenza vaccine hesitancy were (1) determinants of influenza vaccination in specific populations, such as healthcare workers, children, pregnant women, and so on; (2) influenza and COVID-19 vaccine hesitancy during the COVID-19 pandemic.
Conclusions
The trend in the number of annual publications related to influenza vaccine hesitancy indicating the COVID-19 pandemic will prompt researchers to increase their attention to influenza vaccine hesitancy. With healthcare workers as the key, reducing vaccine hesitancy and improving vaccine acceptance in high-risk groups will be the research direction in the next few years.
1 INTRODUCTION
Influenza is one of the most widespread respiratory infections and poses a major threat to health worldwide. According to the World Health Organization (WHO), there are 3–5 million cases of severe influenza and about 290,000–650,000 deaths globally each year.1 Current evidence suggests that certain populations, including young children, the elderly, and chronically ill patients, are at high risk for influenza-related deaths.2 Vaccines are key to preventing and controlling influenza. The 2019–2020 influenza vaccination program is thought to have prevented 7.5 million influenza infections, 3.7 million influenza-related medical appointments, 105,000 influenza-related hospitalizations, and 6300 influenza-related deaths.3 However, despite recommendations by the WHO and the US Centers for Disease Control and Prevention (CDC), influenza vaccination rates exhibit significant heterogeneity across different regions globally.4 An increasing body of evidence suggests that vaccination rates among the general public are very low, with many countries reporting coverage levels below 50% of the targeted populations.5 Moreover, vaccination rates are low among high-risk groups such as pregnant women6 and children.5
In recent years, significant emphasis has been placed on identifying potential barriers to vaccine acceptance. Factors such as insufficient awareness of the disease risk,7 concerns about vaccine safety and reports of vaccine adverse events,7 lack of vaccination,8 lack of regular health care sources, availability and affordability of vaccines,9 and lack of recommendations from medical personnel,10 have been identified as reasons for vaccine hesitancy. In 2019, the World Health Organization (WHO) officially defined vaccine hesitancy as the delay or refusal to vaccinate due to a lack of knowledge about vaccine safety, effectiveness, and the diseases they protect against. This designation positioned it as one of the top 10 global health threats.11
Schmid et al.4 highlighted that influenza vaccines have special characteristics compared to other standard vaccines, such as vaccine effectiveness varies annually and the requirement for yearly vaccination. Despite the declaration of influenza vaccination as a priority healthcare goal in many countries, it is typically recommended to target only specific risk groups, such as children. Thus, influenza vaccine hesitancy exhibits distinct features. Additionally, the emergence of the COVID-19 pandemic in late 2019 disrupted healthcare services globally and heightened concerns about vaccines in general.12, 13 Despite sporadic outbreaks of seasonal influenza in numerous countries, the persistent threat posed by influenza vaccine hesitancy remains a significant public health concern.
To our knowledge, no bibliometric studies have been conducted regarding influenza vaccine hesitation. Accordingly, based on the Web of Science Core, Scopus, and Pubmed databases, we conducted a bibliometric analysis of the existing literature on influenza vaccine hesitancy. This study utilized VOSviewer and CiteSpace analytical software to (I) conduct bibliometric analyses of influenza vaccine hesitancy studies; (II) generate visual knowledge maps; (III) present the current research landscape of influenza vaccine hesitancy; (IV) identify research hotspots and development trends; and (V) provide a valuable reference for future research in this area.
2 MATERIALS AND METHODS
2.1 Data sources and data extraction
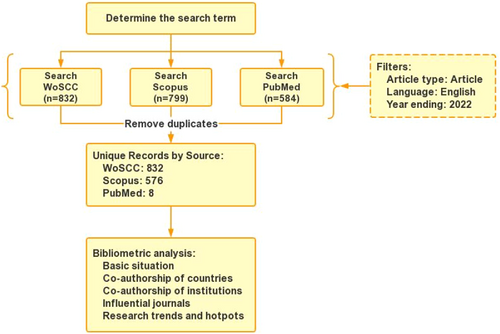
In this study, data were retrieved from the Web of Science Core Collection database (WoSCC), Scopus, and PubMed databases from inception until December 31, 2022. The search strategy used was a topic-based search using the keywords “influenza,” “vaccine,” and “hesitancy.” We restricted the document type to “Article.” Articles not published in English were also excluded, with no time limit. Two authors (ZZ and YY) worked together to assess the search results, and if necessary, a third author (HQ) was consulted for arbitration. After removing duplicates, we obtained 1416 articles. All retrieved records were downloaded in “plain text” format, and relevant information was extracted for further analysis. The data used in this study came from a public database; thus, the approval of the ethical committee was not required. The flowchart is shown in Figure 1.
2.2 Data analysis and visualization
VOSviewer (version 1.6.19) was used for visualization analysis in this study. It is well-established that VOSviewer can provide a variety of visualization modes (network visualization, overlay visualization, and density visualization), which were used for the coauthorship of countries, institutions, authors, and co-occurrence of keywords in this study. CiteSpace software was used to conduct a bibliometric analysis of the publications on influenza vaccine hesitancy, calculate betweenness centrality (BC) for countries, institutions, and authors, and the bursts of keywords. Moreover, Microsoft Word 2019 was used for descriptive statistics, including ranking, frequency, and citation analysis. The web tool MapInSeconds (https://www.mapinseconds.com/) was used to visualize the geographical distribution of publications.
3 RESULTS
3.1 The trend of annual publications
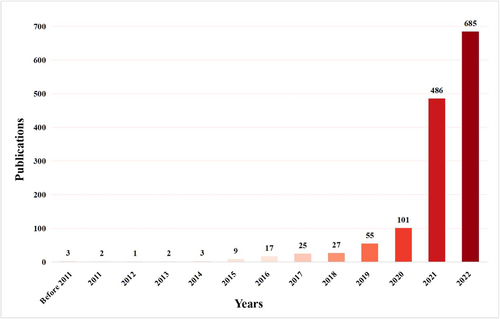
In August 1994, Wrenn et al. published the first article on Influenza vaccine hesitancy titled “Influenza and pneumococcal vaccination in the emergency department: is it feasible?.”10 The annual number of publications on influenza vaccine hesitancy is shown in Figure 2. It was found that the number of publications experienced a significant surge after 2019, with the number of publications in 2022 being 14 times higher than that in 2019.
3.2 Analysis of countries
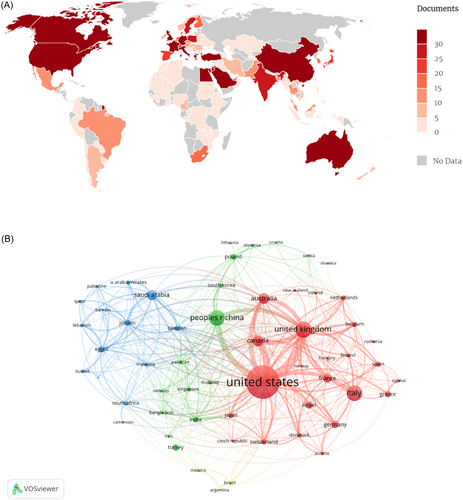
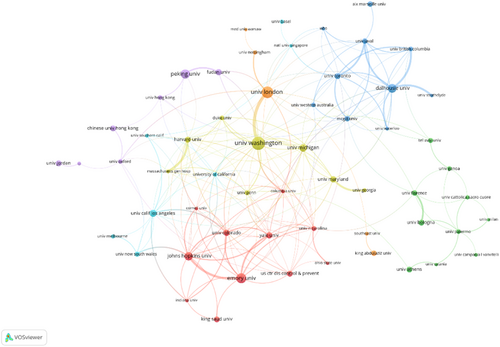
One hundred and nine countries participated in research on influenza vaccine hesitancy. Figure 3A depicts the coauthorship of countries, where areas in gray represent no published articles, while darker shades of red indicate a higher number of publications. The United States was the leading country in this field, with the highest number of publications (n = 541) and citations (n = 7161) (Table 1). BC is a measure that signifies the importance of nodes within a collaborative network, with BC values greater than 0.10 generally indicating a bridging role. The United States had a central role in the global cooperation network (BC = 0.28) and closely collaborated with the United Kingdom and Canada, as depicted in Figure 3B.
| Rank | Country | Continent | Documents | Citations | BC |
|---|---|---|---|---|---|
| 1st | United States | North America | 541 | 7161 | 0.28 |
| 2nd | United Kingdom | Europe | 157 | 3611 | 0.17 |
| 3rd | People Republic of China | Asia | 151 | 2405 | 0.04 |
| 4th | Italy | Europe | 148 | 1737 | 0.11 |
| 5th | Canada | North America | 86 | 1200 | 0.02 |
| 6th | Auatralia | Asia | 78 | 815 | 0.04 |
| 7th | Saudi Arabia | Asia | 73 | 1002 | 0.05 |
| 8th | France | Europe | 57 | 1212 | 0.06 |
| 9th | Germany | Europe | 45 | 573 | 0.01 |
| 10th | Israel | Asia | 40 | 402 | 0.01 |
- Abbreviation: BC, betweenness centrality.
3.3 Analysis of institutions
The top 10 institutions were predominantly located in North America and Europe, with seven in the United States (Table 2). The University of Washington in the United States led in terms of the number of publications, with 38 publications and 1704 citations, followed by the University of London in the United Kingdom (33 publications, 1530 citations), Emory University in the United States (28 publications, 213 citations) (Table 3). The University of London (BC = 0.22) and the University of Washington (BC = 0.18) demonstrated significant BC and played crucial roles in this field (Figure 4).
| Rank | Institution | Country | Documents | Citations | BC |
|---|---|---|---|---|---|
| 1st | University of Washington | United States | 38 | 1704 | 0.18 |
| 2nd | University of London | United Kingdom | 33 | 1530 | 0.22 |
| 3rd | Emory university | United States | 28 | 213 | 0.08 |
| 4th | Peking university | People Republic of China | 26 | 279 | 0.02 |
| 5th | Dalhousie University | Canada | 25 | 574 | 0.06 |
| 6th | Johns Hopkins University | United States | 24 | 624 | 0.08 |
| 7th | University of Michigan | United States | 21 | 229 | 0.05 |
| 8th | University of California, Los Angeles | United States | 19 | 263 | 0.03 |
| 9th | University of Maryland | United States | 18 | 652 | 0.01 |
| 10th | University of Colorado | United States | 18 | 209 | 0.02 |
- Abbreviation: BC, betweenness centrality.
| Rank | Author | Country | Documents | Citations | BC |
|---|---|---|---|---|---|
| 1st | Pierre Verger | France | 10 | 477 | 0.00 |
| 2nd | Heidi J Larson | United States | 9 | 1311 | 0.00 |
| 3rd | Dube Eve | Canada | 9 | 558 | 0.01 |
| 4th | Justin Gatwood | United States | 8 | 84 | 0.00 |
| 5th | Freimuth Vicki S | United States | 7 | 582 | 0.00 |
| 6th | Quinn Sandra C | United States | 7 | 532 | 0.00 |
| 7th | Bonanni Paolo | Italy | 7 | 206 | 0.00 |
| 8th | Betsch Cornelia | Germany | 7 | 161 | 0.00 |
| 9th | Omer Saad B | United States | 7 | 105 | 0.00 |
| 10th | Peretti-Watel Patrick | France | 6 | 568 | 0.00 |
- Abbreviation: BC, betweenness centrality.
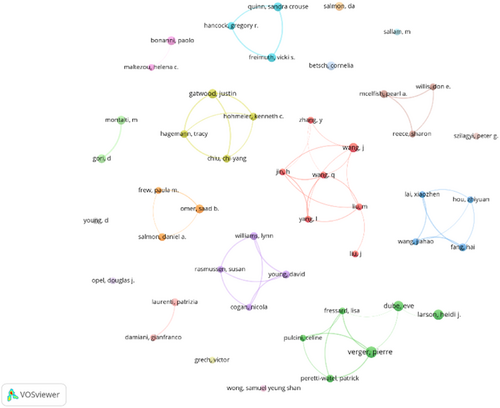
3.4 Analysis of authors
The top 10 prolific authors in the field of influenza vaccine hesitancy are shown in Table 3. The most prolific author was Pierre Verger, with 10 published articles with 477 citations. In terms of total citations, Heidi J Larson ranked first with 1311 citations. Similar to the institutional rankings, all authors in the top 10 were from Europe and North America. It is worth noting that the author's cooperation network was relatively sparse and fragmented, with a lack of cooperation among research groups (Figure 5).
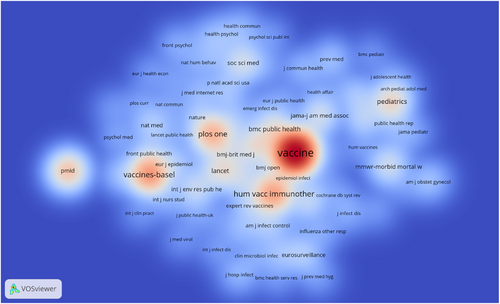
3.5 Analysis of journals
Table 4 lists the top 10 journals by number of publications and citations. Vaccines-Basel (267, 18.9%) was the most published journal, followed by Vaccine (167, 11.8%) and Human Vaccines & Immunotherapeutics (123, 8.9%). Nevertheless, the ranking of cited journals was not in accordance with the ranking by the number of publications. Vaccine was the most influential journal with 4213 citations, much higher than Vaccines-Basel (1306 citations). In contrast to the results of existing comparative journal studies,14, 15 among journals that published influenza vaccine hesitancy, non-OA journals had higher citation metrics and OA journals had higher output. The density visualization of the journal co-citation network is shown in Figure 6, where journals with higher citation density shown in red, and those with lower density appear in blue. Taking both the number of publications and citations into account, it can be inferred that Vaccine, Human Vaccines & Immunotherapeutics, and Vaccines-Basel were the most popular journals in the study of influenza vaccine hesitancy over the study period.
| Rank | Publication journal | Documents | IF | Cited journal | Citations | IF |
|---|---|---|---|---|---|---|
| 1st | Vaccines-Basela | 267 | 4.961 | Vaccine | 4213 | 4.169 |
| 2nd | Vaccine | 167 | 4.169 | Vaccines-Basela | 1306 | 4.961 |
| 3rd | Human Vaccines & Immunotherapeuticsa | 123 | 4.526 | Human Vaccines & Immunotherapeuticsa | 1198 | 4.526 |
| 4th | International Journal of Environmental Research and Public Healtha | 50 | 4.614 | PLoS Onea | 981 | 3.752 |
| 5th | PLoS Onea | 43 | 3.752 | Pediatricsa | 610 | 9.703 |
| 6th | Frontiers in Public Healtha | 33 | 6.461 | New England Journal of Medicine | 493 | 176.077 |
| 7th | BMC Public Healtha | 29 | 4.135 | Lancet | 483 | 202.728 |
| 8th | Expert Review of Vaccines | 22 | 5.683 | BMC Public Healtha | 472 | 4.135 |
| 9th | Vaccine:Xa | 14 | 0.000 | JAMA-Journal of the American Medical Association | 372 | 157.377 |
| 10th | Pediatricsa | 10 | 9.703 | Mmwr-Morbidity and Mortality Weekly Reporta | 357 | 35.301 |
- a Open Access.
3.6 Analysis of reference
It is well-established that when two or more references are cited by more than one article, the two references share a co-citation relationship. Publications with many citations are generally articles with high academic value and great professional influence. Of the 42,204 cited references, the top 10 most frequently cited references are shown in Table 5 and are valuable publications in the field. “Vaccine hesitancy: Definition, scope and determinants” published by MacDonald16 has been cited the most frequently, indicating that most scholars in this field have recognized the academic value of this literature.
| Rank | Author | Title | Journal | Year | Citation |
|---|---|---|---|---|---|
| 1st | MacDonald NE | Vaccine hesitancy: Definition, scope and determinants | Vaccine | 2015 | 291 |
| 2nd | Larson HJ | Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012 | Vaccine | 2014 | 143 |
| 3rd | Lazarus JV | A global survey of potential acceptance of a COVID-19 vaccine | Nature Medicine | 2021 | 122 |
| 4th | Schmid P | Barriers of Influenza Vaccination Intention and Behavior - A Systematic Review of Influenza Vaccine Hesitancy, 2005-2016 | PLoS Onea | 2017 | 110 |
| 5th | Dube E | Vaccine hesitancy: an overview | Human Vaccines & Immunotherapeuticsa | 2013 | 99 |
| 6th | Dror AA | Vaccine hesitancy: the next challenge in the fight against COVID-19 | European Journal of Epidemiology | 2020 | 98 |
| 7th | Paterson P | Vaccine hesitancy and healthcare providers | Vaccine | 2020 | 86 |
| 8th | Fisher KA | Attitudes Toward a Potential SARS-CoV-2 Vaccine: A Survey of U.S. Adults | Annals of Internal Medicine | 2016 | 83 |
| 9th | Sallam M | COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates | Vaccines-Basela | 2021 | 76 |
| 10th | Reiter PL | Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? | Vaccine | 2020 | 64 |
- a Open Access.
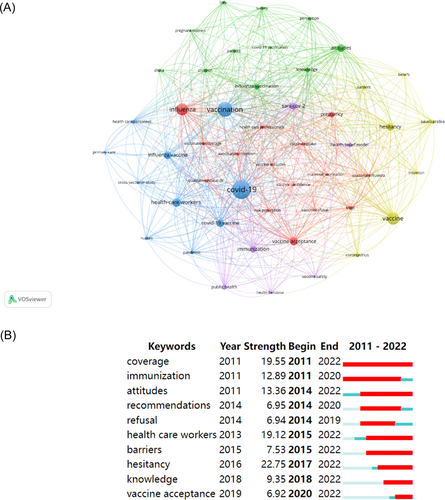
3.7 Analysis of keyword
Keywords can reflect the hotspots and frontiers in a particular field. In this analysis, the keyword clustering map generated by VOSviewer identified five clusters based on keywords that appeared more than 10 times in the literature (Figure 7). The largest cluster, represented in red, mainly investigated the determinants of influenza vaccination for specific populations, such as healthcare professionals and pregnant women. The second largest cluster in green focused on the attitudes of children and pregnant women toward COVID-19 vaccination. The blue cluster examined the awareness of influenza and COVID-19 vaccines among healthcare workers during the COVID-19 pandemic. The yellow cluster sought to improve vaccinators' knowledge and belief in vaccines and their vaccination intention. The purple cluster analyzed the impact of the health behaviors of vaccinators, the safety and immunization effectiveness of vaccines, and public health policies on COVID-19 vaccination.
4 DISCUSSION
In this study, we conducted a bibliometric analysis of publications related to influenza vaccine hesitancy to identify hotspots and trends in influenza vaccine hesitancy. Our analysis included an examination of the earliest literature on influenza vaccine hesitancy, which was published in 1994 and focused on vaccination rates, intentions, and reasons for refusal among emergency department patients and physicians. Interestingly, 57% of emergency department patients and 89% of emergency department physicians were not vaccinated against influenza due to the hesitancy among emergency department physicians about assuming the primary care task of providing such immunizations. This study highlighted the importance of healthcare providers in promoting vaccination.10 Before 2011, research on influenza vaccine hesitancy was scarce. The annual number of publications related to influenza vaccine hesitancy increased from 2012, with a significant surge after 2019, thus indicating that the study of influenza vaccine hesitancy is experiencing a rapid growth stage and represents a research hotspot for the coming years. The surge in research activity after 2012 can be attributed to the (H1N1) pdm09 epidemic, which was the most recent global influenza pandemic. According to estimates from the CDC, the (H1N1) pdm09 virus caused a significant number of fatalities, ranging from 151,700 to 575,400 worldwide during its initial year of circulation. This pandemic served as a catalyst for influenza vaccination campaigns in numerous countries.17-19 Additionally, there was an increased research focus on understanding vaccination intention during this period.20, 21 The increase in publications after 2019 may be attributed to the COVID-19 pandemic affecting influenza vaccination.22, 23
COVID-19 is an infectious respiratory disease that shares the same routes and means of transmission as the influenza virus, and the combination of COVID-19 and seasonal influenza in winter can lead to co-infection cases, leading to worse clinical outcomes.24, 25 Dadashi et al.26 reported that the prevalence of influenza infection among confirmed COVID-19 patients was 0.8%, the co-infection rate of influenza virus in China and the United States was 3.1% and 0.7%, and the overall influenza vaccine coverage rate for the 2019/2020 influenza season was 1.43% in China and 51.8% in the United States, respectively. Therefore, the co-infection of COVID-19 and seasonal influenza may be related to the higher prevalence of influenza in patients with low vaccination rates. Encouragingly, studies by Kong et al. demonstrated that the COVID-19 pandemic prompted a more active global influenza vaccination intention, with an increase observed in all regions in the 2020/2021 influenza season.23 However, influenza vaccine hesitancy still exists. The belief held by certain individuals who decline the influenza vaccine, that it is less important amidst the COVID-19 pandemic, adds to the hesitancy surrounding influenza vaccination. In addition, reports of adverse events from COVID-19 vaccines have increased concerns among the population about the immunization effectiveness and safety of other vaccines.12 Many countries have reported decreasing influenza infection rates during the 2020/2021 influenza season due to public health measures such as mandatory mask-wearing.25, 27, 28 Accordingly, low worry and perceived risk of vaccine-preventable diseases may be major factors accounting for vaccine hesitancy in the next influenza season. More worryingly, current evidence suggests that influenza vaccination rates in countries such as the United States and Italy have declined in the 2021/2022 and 2022/2023 influenza seasons.29-31 Influenza vaccination rates are inadequate, and the risk of influenza pandemic may increase. Hence, reducing influenza vaccine hesitancy and promoting vaccination should be priorities for the next influenza season.
Our study showed that the United States contributed the most to influenza vaccine hesitancy research in terms of the number of publications, total citations, and international cooperations in the field, which may be related to the significant public and private funding support and strong research cultural foundation in the United States.32 Europe and North America have been the leaders in research in the global influenza vaccine hesitancy field, with 9 out of the top 10 institutions located in North America and Europe and the University of Washington being the most important contributing institution, which may be due to several reasons. First, public health policies in North America and Europe have provided free influenza vaccination programs for high-risk populations, and well-established primary healthcare systems have protected the accessibility and convenience of vaccination.29, 33 Second, rich countries pay more attention to healthcare and are therefore more likely to provide funding to health development research infrastructure and fund research activities,32 positioning American and European institutions at the forefront of research on influenza and influenza vaccines.34 Third, close cooperation between Europe and North America facilitates the exchange of resources and methods to enhance their research influence. Fourth, democratic countries with broad freedom of speech, such as the United Kingdom and the United States, are more susceptible to anti-vaccine movements, which can contribute to public vaccine hesitancy and consequently attract increased attention from researchers in these countries.35 Despite this, limited cooperation among research groups suggests that progress in this area necessitates increased cross-disciplinary cooperation.
Vaccine, Vaccines-Basel, and Human vaccines & Immunotherapeutics are the most favored by researchers in the influenza vaccine hesitancy field. Academic achievements related to influenza vaccine hesitancy are more likely to be published in these journals, and researchers interested in influenza vaccine hesitancy are also more likely to find what they need to read in these journals. MacDonald et al.16 published an article in this journal titled “Vaccine hesitancy: Definition, scope and determinants,” which was the most cited article on the development of influenza vaccine hesitancy research. The article defines vaccine hesitancy as a delay in acceptance or refusal of vaccination despite the availability of vaccination services and divides the determinants of vaccine hesitancy into contextual, individual and group, and vaccine/vaccine-specific influences.
We found that articles about influenza vaccine hesitancy focused primarily on high-risk populations, such as children, older adults, and pregnant women, which are associated with an increased risk of death or adverse pregnancy outcomes if they contract influenza. The infection and prevalence rates of influenza virus are the highest among children, especially young children and children with high-risk diseases, who are more susceptible to pneumonia, asthma exacerbations, dehydration, and neurological complications.36-38 Moreover, children represent the most important transmitters of influenza,39 and vaccinating children can effectively reduce community transmission of influenza. Concerns about vaccine safety are the main reasons parents may refuse to vaccinate their children against influenza. Healthcare workers play a vital role in addressing childhood vaccine hesitancy, as their knowledge, confidence, and recommendation of vaccines have a positive impact in reducing hesitancy,40 helping to increase immunization coverage among children under 18 years of age.41, 42
Influenza vaccination for individuals aged 65 and older is a cost-effective strategy for preventing disease. It effectively reduces emergency hospital admissions, and hospitalizations related to influenza complications, and prevents deaths in this high-risk population, aged 65 and older, resulting in savings in social and public health costs. Consequently, North America and some European countries prioritize individuals over 65 for free influenza vaccination services. According to data from the OECD,43 only the United Kingdom and South Korea have achieved the target of 80% influenza vaccination coverage among people aged 65 and older between 2018 and 2022. Among this older age group with access to free vaccination services, the primary reason for influenza vaccine hesitancy is a low perception of disease severity and risk of infection.4, 44
Brittany et al reported that influenza infection in pregnant women led to an increased risk of hospitalization and aggravated pregnancy complications.45 The WHO recommends annual vaccination for pregnant women at any stage of pregnancy.1 However, previous studies have shown that influenza vaccination coverage is extremely low in pregnant women. In this respect, only 1.2% of pregnant women in France were vaccinated against influenza between 2009 and 2018,40 compared with more than 50% of people over 65 years old.43 The primary factor behind pregnant women not receiving the influenza vaccine is the absence of vaccination recommendations from healthcare providers. Research conducted by Im JH et al. demonstrated the positive effect of healthcare workers on influenza vaccination among pregnant women, highlighting their role in promoting vaccination in this vulnerable population. When healthcare workers' recommendations for influenza vaccination increased from 4.8% to 49.7%, the influenza vaccination rate among pregnant women increased from 4.0% to 59.3%.46
Healthcare workers encompass a broad category of professionals, including doctors (specialists, pediatricians, and general practitioners), nurses, various healthcare practitioners, and technicians.47 They face an elevated risk of contracting influenza, potentially serving as carriers of the virus to vulnerable patients. Healthcare workers play a pivotal role in diminishing the impact of disease outbreaks, serving as role models for preventive measures, and encouraging others to receive vaccinations. Consequently, healthcare workers were among the first to be recommended for immunization, prompting numerous countries to institute vaccination programs tailored to their needs. Despite these efforts, vaccination rates remain suboptimal, ranging from 8.84% to 83.6% among healthcare workers 48-53 among healthcare workers. Concerns about vaccine safety and perceived low risk of disease are major factors leading to vaccine hesitancy among healthcare workers. Apprehensions about vaccine safety and a perception of low disease risk are key factors contributing to vaccine hesitancy within this group. Notably, vaccine hesitancy among healthcare workers can diminish their willingness to recommend influenza vaccines to the public. This hesitancy among healthcare workers poses a significant obstacle to the public's access and acceptance of influenza vaccinations.21 Given the influential role of healthcare workers in shaping public vaccine acceptance, educational programs for healthcare workers should concentrate on addressing distrust and misconceptions regarding vaccination.
5 CONCLUSION
Through the visualization of bibliometrics, the research progress, research hotspots, and research frontiers on influenza vaccine hesitancy after 2011 were described. The findings of this study further add to the available body of evidence and provide a reference for influenza vaccine hesitancy. Due to the impact of the COVID-19 pandemic, the number of influenza vaccine hesitancy publications is currently increasing rapidly. The United States is the leader in the research on influenza vaccine hesitancy. The University of Washington and the University of London have worked closely on influenza vaccine hesitancy, are at the center of the global research collaborative network of influenza vaccine hesitancy, and are the preferred partner institutions in this research field. To date, the field of influenza vaccine hesitancy has not produced authors with global influence, and there has been a lack of cooperation between research groups at various institutions. Improving awareness of influenza and influenza vaccine among healthcare workers is key to reducing influenza vaccine hesitancy. The effect on influenza vaccine hesitancy caused by the Covid-19 pandemic on the subsequent influenza season, especially for high-risk groups, will be the focus of researchers.
This study also has some limitations. First, the data were retrieved from the WOSCC, Scopus, and PubMed databases, which primarily feature English-language journals. Consequently, there is a potential language bias in the findings, as publications on vaccine hesitancy from non-English-language journals may have been overlooked. Second, our study relies on published literature, and some developing countries might face challenges in conducting relevant research due to healthcare policies and limited resources. Third, there is a possibility of errors in the analysis stemming from variations in data formats across different databases. For instance, discrepancies in institution names, spellings, or abbreviations could have introduced errors when analyzing institution nodes. Moreover, considering the rapid growth of influenza vaccine hesitancy research, it is uncertain whether new high-quality articles have been excluded as the field continues to expand.
AUTHOR CONTRIBUTIONS
Zhengyu Zhang: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; visualization; writing—original draft. Songjia Tang: Data curation; formal analysis; investigation; methodology; resources; software; validation; visualization. Zhihui Huang: Data curation; investigation; methodology; resources; software; validation; visualization. Juntao Tan: Data curation; investigation; methodology; resources; software; validation; visualization. Xiaoxin Wu: Conceptualization; funding acquisition; project administration; supervision; writing—original draft; writing—review and editing. Qian Hong: Resources; software; validation; visualization; writing—original draft. Yuan Yuan: Data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; visualization; writing—review and editing.
ACKNOWLEDGMENTS
The authors thank the Home for Researchers editorial team (www.home-for-researchers.com) for language service. The corresponding author has obtained permission for them to be included in the Acknowledgments section of our article. This work was supported by grants from the Chongqing Municipal Health and Health Committee Maternal and Child Management Project (grant number 2023FY205), Huzhou Municipal Bureau of Science and Technology (grant number 2019GY85), and the Natural Science Foundation of Zhejiang Province (grant number LQ21H190004).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead authors Xiaoxin Wu, Qian Hong, and Yuan Yuan affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.