Preoperative urine neutrophil gelatinase-associated lipocalin predicts mortality in colorectal cancer patients after laparoscopic surgery: A single-center study
Long Huynh Thanh and Quyen Dao Bui Quy shared their first authorship.
Abstract
Purpose
To determine the rate of acute kidney injury (AKI) after laparoscopic colorectal cancer (CRC) surgery and the predictive value of urine neutrophil gelatinase-associated lipocalin (uNGAL) for postoperative AKI and mortality during 3 years of follow-up.
Methods
A total of 216 CRC patients who had undergone laparoscopic surgery were included in our study. We divided all patients into two groups, including group 1 (n = 31) with postoperative AKI and group 2 (n = 185) without postoperative AKI. Urine NGAL was measured using the ELISA technique. Clinical and laboratory data were collected the day before surgery. Postoperative AKI included events occurring within 7 days of the index operation, and mortality was obtained during 3 years of follow-up.
Results
The ratio of postoperative AKI was 14.35% (31/216 patients). The urine NGAL level in group 1 was significantly higher than in group 2, p < 0.001. At cut-off value = 14.94 ng/mL, uNGAL has a predictive value for AKI (area under the curve [AUC] = 0.858, p < 0.001). After 3 years of follow-up, the total mortality rate was 7.9%. The mortality rate in group 1 (45.2%) was significantly higher than in group 2 (1.6%) with p < 0.001). At cut-off value = 19.85 ng/mL, uNGAL has a predictive value for mortality (AUC = 0.941, p < 0.001).
Conclusions
The rate of acute kidney injury after laparoscopic CRC surgery was 14.35%. Preoperative urine NGAL has a good predictive value for postoperative acute kidney injury and mortality during 3 years of follow-up.
1 INTRODUCTION
Colorectal cancer (CRC) is the most common malignancy worldwide, with a high mortality rate.1, 2 According to GLOBOCAN 2018, CRC accounts for the third-highest incidence and the second-highest mortality rates worldwide.3 By 2030, the incidence of CRC is predicted to increase by 60% in developing countries.4 Colon cancer is divided into right colon cancer, including cancers of the cecum, ascending colon, transverse colon, and left colon cancer (descending colon and sigmoid colon). Cancer that is located within 15 cm of the anal sphincter is called rectal cancer. Most CRCs are adenocarcinomas, less commonly mucinous adenocarcinoma, adenosquamous carcinoma, signet-cell carcinoma, and medullary carcinoma.5 The laparoscopic CRC surgery technique is widely applied in clinical practice due to the advantages of a small incision, quick recovery, and shorter hospital stay compared to open surgery.6-8 Compared with other surgical techniques, its short-term outcomes and long-term efficacy have been well established, as documented by a series of large, multicenter, randomized controlled trials.9-12 However, laparoscopic surgery has complications such as bleeding, intestinal obstruction, and acute kidney injury (AKI). These complications are often related to elderly patients and cancer on both sides of the colon and rectum.9
AKI is a common postoperative complication that is defined as an abrupt decrease in kidney function, including decreased urine output and elevated serum creatinine levels.13 Postoperative AKI is a severe complication that increases patient morbidity and mortality.14 AKI occurs in 5%–10% of all hospitalized patients, 4%–13.4% of patients who have undergone major abdominal surgery, including CRC, and up to 60% of intensive care unit (ICU) patients.15-17 Human neutrophil gelatinase-associated lipocalin (NGAL) is a 25 kDa glycosylated protein from the lipocalin family.18 Neutrophils, monocytes/macrophages, and adipocytes are cells with abundant NGAL expression.19 Evidence shows that NGAL is secreted not only in the proximal tubules but also in the distal nephrons. In the proximal tubule, NGAL is colocalized, at least in part, with proliferating epithelial cells.20 These findings suggest that NGAL may be expressed by damaged tubular epithelium. Urinary NGAL has been considered a diagnostic biomarker for early AKI when serum creatinine is not yet elevated.21, 22
In this paper, we want to determine the rate of AKI after laparoscopic CRC surgery. We hypothesize that preoperative urine NGAL has a predictive value for postoperative AKI and mortality after 3 years of follow-up.
2 MATERIALS AND METHODS
2.1 Study design
A total of 216 CRC patients who had undergone laparoscopic complete mesocolic excision CME with lymph node dissection from November 2011 to November 2017 at Nguyen Tri Phuong Hospital, Ho Chi Minh City, Vietnam, were enrolled. We excluded patients below 16 years of age, patients with chronic kidney disease (CKD) or abnormal total urine analysis test (positive hematuria or proteinuria), and pregnant women. All patients with anuria were excluded from the study. Written informed consent and the Hospital's Ethics Committee clearance were obtained before the study's participants' recruitment.
Pre-existing co-morbidities such as diabetes mellitus and hypertension and medications were noted. Diabetes mellitus was identified according to a physician's diagnosis, antidiabetic drug treatment, or two subsequent analyses demonstrating fasting blood glucose levels of >126 mg/dL or >7.0 mmol/L. Hypertension was defined as the regular use of antihypertensive drugs for controlling blood pressure or at least two blood pressure measurements of >140/90 mmHg. Demographic data such as age, gender, and comorbid conditions…were collected before surgery. On the day before surgery, the patient was given a catheter to collect urine for 24 h. After 24 h, measure the urine volume, take 5 mL of urine to determine the NGAL level, and then calculate the 24-h urine NGAL concentration. Urine NGAL was measured by the BioVendor Human Lipocalin-2/NGAL ELISA kit based on the sandwich enzyme immunoassay method.
2.2 Surgical procedure and outcomes
2.2.1 Surgical procedure
The procedure is summarized as follows: The mesocolon is made along the mesenteric axis proximal to the superior mesenteric vein for right-sided tumors. Next, dissection was performed along the superior mesenteric vein, exposing the body of Henle's stomach. Expose the middle colonic artery and sever it at the base of the right branch. Next, dissect and cut the middle colon vein and lymph node. After the cut is complete, an external shunt is performed. With the left tumor, the procedure begins by withdrawing the sigmoid dermis anteriorly, and the visceral peritoneum on the base of the sigmoid mesocolon is incised transversely with the sacral process. The incision continues up the ligament of Treitz, and the arterial, venous, and lymph node components are dissected and resected. After cutting, anastomosis is performed in the body through the anus.
2.2.2 Early outcomes
Major complications in surgery include bleeding and organ trauma. Postoperative complications include surgical site infection, bowel obstruction, anastomosis leak, bleeding, organ dysfunction (including AKI), and sepsis. Surgical outcomes and baseline complications were collected from the end of surgery until the patient was discharged. AKI was defined based on kidney disease: improving global outcomes (KDIGO) criteria23: Patients were in proportion to a 1.5–1.9 fold increase of SCr level or an acute rise in SCr of more than 26.5 µmol/l (0.3 mg/dL) within 48 h. Postoperative AKI included events occurring within 7 days of the index operation. Based on the status of AKI appearance, patients were divided into two groups: Group 1: Patients with AKI (n = 31), and Group 2: Patients with non-AKI (n = 185).
2.2.3 Long outcomes
Patients were followed up every 3 months for the first year after surgery and every 6 months for the next 2 years (3 years in total). They all underwent colonoscopy once a year, and whole-body computed tomography and/or abdominal ultrasound were performed every 6 months to monitor recurrence. The date of death, mortality causes, and other factors were collected and recorded in the database. Survival time was calculated from the date of surgery to the date of death.
2.3 Statistical methods
All the normal distribution and continuous data were represented by mean and standard deviation and were analyzed by analysis of variance (ANOVA) and student t-test. The skewed distributions were represented by the median (25%–75%), analyzed by the Mann–Whitney U and Kruskal–Wallis tests. Categorical data were presented by the frequency with percentage and were analyzed using the χ² test. Receiver operating characteristic (ROC) curves with the area under the curve (AUC) were calculated to predict AKI and mortality from patients after 3 years of follow-up. Multivariable adjusted regression analysis was performed to identify the predictors of hospital mortality. Survival curves were assessed using the Kaplan–Meier analysis and evaluated by the log-rank test. Statistical analysis was done using Statistical Package for Social Science (SPSS) version 20.0. A p-value < 0.05 was considered significant.
3 RESULTS
As the results in Table 1, the ratio of hypertension, diabetes, anemia, decreased serum albumin and prealbumin, increased CRP-hs, CEA, as well as the rate of lymph node metastasis, stage III TNM in group 1, was significantly higher compared with those of the group 2, p < 0.05 to p < 0.001. In particular, the urine NGAL concentration in group 1 was significantly higher than in group 2, with a p-value < 0.001.
| Clinical characteristics and laboratory parameters | Total (n = 216) | Group 1 (n = 31) | Group 2 (n = 185) | p |
|---|---|---|---|---|
| Ages (years) | 60.08 ± 14.03 | 61.81 ± 16.84 | 59.79 ± 13.54 | 0.460 |
| Number of males (n, %) | 125 (57.9) | 15 (48.4) | 110 (59.5) | 0.248 |
| Hypertension | ||||
| Yes (n, %) | 48 (22.2) | 19 (61.3) | 29 (15.7) | <0.001 |
| No (n, %) | 168 (77.8) | 12 (38.7) | 156 (84.3) | |
| Diabetic mellitus | ||||
| Yes (n, %) | 21 (14.4) | 17 (54.8) | 14 (7.6) | <0.001 |
| No (n, %) | 185 (85.6) | 14 (45.2) | 171 (92.4) | |
| BMI (kg/m2) | ||||
| <18.5 (n, %) | 34 (15.7) | 7 (22.6) | 27 (14.6) | |
| 18.5–22.9 (n, %) | 126 (58.3) | 18 (58.1) | 108 (58.4) | 0.432 |
| ≥ 23.0 (n, %) | 56 (25.9) | 6 (19.4) | 50 (27.0) | |
| Mean | 21.4 ± 2.78 | 20.72 ± 2.84 | 21.51 ± 2.76 | 0.145 |
| Anemia (n, %) | 73 (33.8) | 24 (77.4) | 49 (26.5) | <0.001 |
| Hemoglobin (g/L) | 130.96 ± 14.96 | 112.67 ± 13.79 | 134.03 ± 12.83 | <0.001 |
| Ure (mmol/L) | 5.1 (4.3–6.2) | 5.6 (4.33–6.28) | 5.0 (4.29–6.1) | 0.242 |
| Creatinine (µmol/L) | 76.9 ± 11.14 | 79.4 ± 12.76 | 76.48 ± 10.83 | 0.178 |
| Prealbumin (g/L) | ||||
| <0.2 (n, %) | 11 (5.1) | 5 (16.1) | 6 (3.2) | 0.011 |
| Mean | 0.37 ± 0.07 | 0.32 ± 0.09 | 0.38 ± 0.06 | 0.002 |
| Albumin (g/L) | ||||
| <35.0 (n, %) | 63 (29.2) | 15 (48.4) | 48 (25.9) | 0.011 |
| Mean | 37.81 ± 5.21 | 36.19 ± 5.26 | 38.08 ± 5.16 | 0.062 |
| Protein (g/L) | ||||
| <60.0 (n, %) | 48 (22.2) | 9 (29) | 39 (21.1) | 0.324 |
| Mean | 64.33 ± 6.02 | 63.74 ± 6.81 | 64.43 ± 5.89 | 0.556 |
| CRP-hs (mg/L) | ||||
| >5.0 (n, %) | 73 (33.8) | 23 (74.2) | 50 (27) | <0.001 |
| Mean | 3.7 (2.7–5.4) | 7.8 (4.2–8.9) | 3.3 (2.55–5.1) | <0.001 |
| CEA (ng/mL) | ||||
| >5.0 (n, %) | 126 (58.3) | 27 (87.1) | 99 (53.5) | <0.001 |
| Median | 6.36 (2.73–15.6) | 34.6 (21.39–56.36) | 6.0 (2.42–9.28) | <0.001 |
| Urine NGAL (ng/mL) | 11.63 (7.56–18.51) | 23.50 (17.17–27.68) | 10.74 (6.95–17.13) | <0.001 |
| Tumor size after surgery (cm) | ||||
| <3.0 (n, %) | 63 (29.2) | 6 (19.4) | 57 (30.8) | |
| 3.0 - < 7.0 (n, %) | 133 (61.6) | 19 (61.3) | 114 (61.6) | 0.075 |
| ≥ 7.0 (n, %) | 20 (9.3) | 6 (19.4) | 14 (7.6) | |
| Median | 3 (2–5) | 3 (3–6) | 3 (2–5) | 0.472 |
| The differentiation of the tumor | ||||
| Highly differentiated (n, %). | 23 (10.6) | 2 (6.5) | 21 (11.4) | 0.013 |
| Moderately differentiated (n, %) | 156 (72.2) | 18 (58.1) | 138 (74.6) | |
| Poorly differentiated (n, %) | 37 (17.1) | 11 (35.5) | 26 (14.1) | |
| Metastasis | ||||
| Lymph node metastasis (n, %) | 108 (50) | 23 (74.2) | 85 (45.9) | 0.004 |
| Distant metastasis (n, %) | 0 (0) | (0) | (0) | N/A |
| Nodule size (mm) | ||||
| <5.0 (n, %) | 216 (100) | 31 (100) | 185 (100) | N/A |
| 5.0–10.0 (n, %) | 0 (0) | 0 (0) | 0 (0) | |
| >10.0 (n, %) | 0 (0) | 0 (0) | 0 (0) | |
| TNM stage | ||||
| I (n, %) | 79 (36.6) | 6 (19.4) | 73 (39.5) | 0.009 |
| II (n, %) | 32 (14.8) | 2 (6.5) | 30 (16.2) | |
| III (n, %) | 105 (48.6) | 23 (74.2) | 82 (44.3) |
- Note: Italic values indicate significant differences with p-value < 0.05.
- Abbreviations: BMI, body mass index; CRP-hs, C reactive protein-high sensitive; CEA, carcinoma embryonic antigen; NGAL, neutrophil gelatinase-associated lipocalin; TNM, tumor nodes metastasis.
Table 2 showed that Group 1 had a longer surgery time and a higher rate of surgical site infections, especially the mortality rate after 3 years of follow-up was higher than that of group 2 (from p < 0.01 to p < 0.001)
| Clinical characteristics and laboratory parameters | Total (n = 216) | Group 1 (n = 31) | Group 2 (n = 185) | p |
|---|---|---|---|---|
| Surgery time (minute) | ||||
| Mean | 136.94 ± 34.08 | 157.1 ± 42.04 | 133.57 ± 31.45 | 0.005 |
| Min | 80 | 80 | 80 | |
| Max | 220 | 220 | 220 | |
| Time to have a medium again (day) | ||||
| Mean | 1.53 ± 0.63 | 1.77 ± 0.66 | 1.49 ± 0.62 | 0.022 |
| Min | 1 | 1 | 1 | |
| Max | 3 | 3 | 3 | |
| Early complications | ||||
| Surgical site infection (n, %) | 18 (8.3) | 9 (29) | 9 (4.9) | <0.001 |
| Early bowel obstruction (n, %) | 3 (1.4) | 2 (6.5) | 1 (0.5) | 0.055 |
| Length of stay in treatment (day) | ||||
| Mean | 7 (6–8) | 7 (6–11) | 7 (6–7.5) | 0.768 |
| Min | 4 | 5 | 4 | |
| Max | 16 | 16 | 16 | |
| Mortality (n, %) | 17 (7.9) | 14 (45.2) | 3 (1.6) | <0.001 |
- Note: Italic values indicate significant differences with p-value < 0.05.
Using multivariate logistic regression analysis in Table 3, we found that hypertension, diabetic mellitus, urine NGAL, and early complications were the independent factors related to AKI in CRC patients after laparoscopic surgery, p < 0.05–0.001.
| Variable | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Diabetic mellitus | 9.26 | 1.84–46.55 | 0.007 |
| Hemoglobin | 0.91 | 0.86–0.95 | <0.001 |
| Urine neutrophil gelatinase-associated lipocalin | 1.35 | 1.19–1.52 | <0.001 |
| Early complications | 65.06 | 9.43–448.59 | <0.001 |
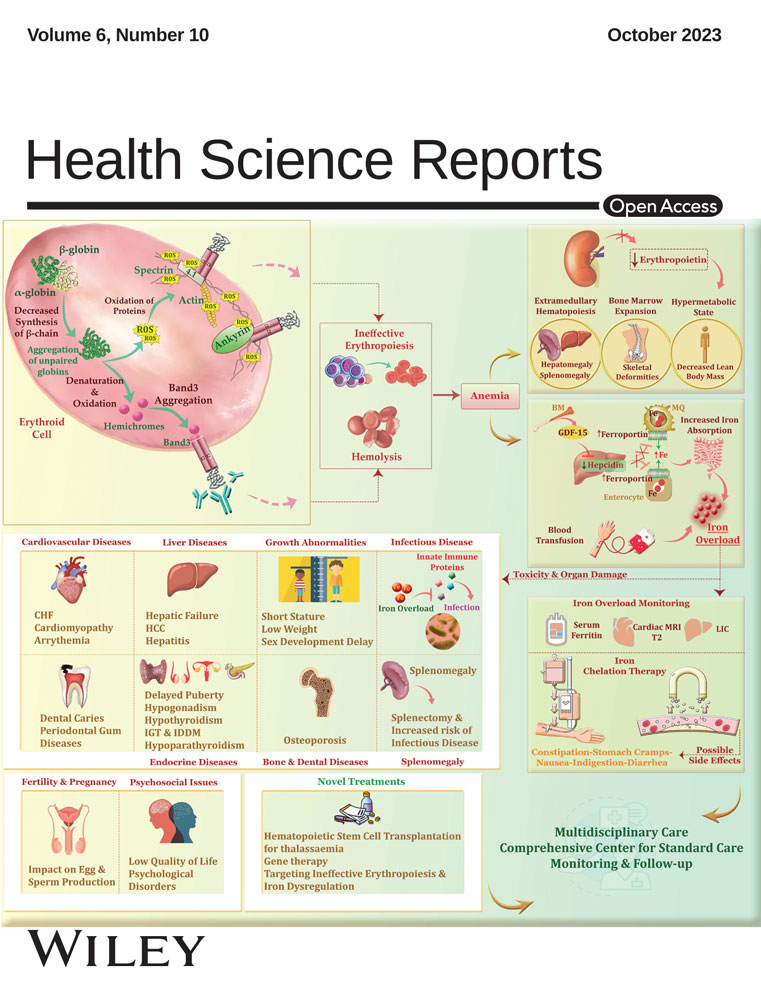
The results of the ROC curve model in Figure 1 showed that CRP-hs, CEA, and urine NGAL were good factors in predicting AKI in CRC patients after laparoscopic surgery, p < 0.001.
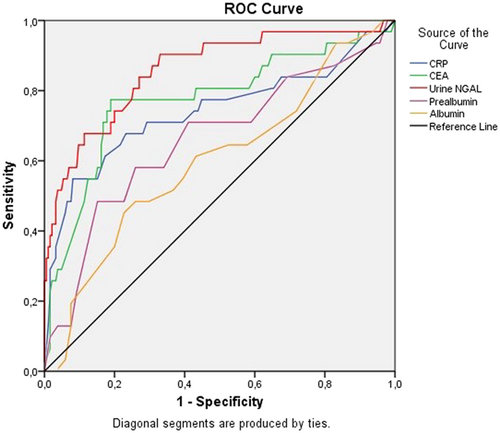
Table 4 showed that urine NGAL, CRP-hs, and CEA were the independent factors related to mortality in CRC patients after laparoscopic surgery, p < 0.05.
| Variable | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Urine NGAL | 1.71 | 1.20–2.44 | 0.003 |
| CEA | 1.13 | 1.03–1.24 | 0.011 |
| CRP-hs | 4.03 | 1.42–11.40 | 0.009 |
- Abbreviations: CEA, carcinoma embryonic antigen; CRP-hs, C reactive protein-high sensitive; NGAL, neutrophil gelatinase-associated lipocalin.
We found that CRP-hs, CEA, and urine NGAL were good factors in predicting mortality after 3 years of follow-up in CRC patients receiving laparoscopic surgery, p < 0.001 (Figure 2).
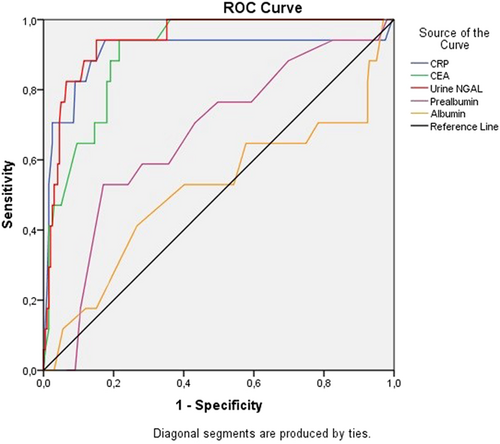
The CRC patients with AKI after laparoscopic surgery (red line) exhibited a significantly higher death rate compared to that of non-AKI patients (green line) with a p-value < 0.001 (log-rank test) based on the results of Kaplan–Meier analysis in Figure 3.
4 DISCUSSION
4.1 Ratio of AKI and predictive factors in CRC patients after laparoscopic surgery
The rate of AKI after laparoscopic surgery of CRC patients in our study is 14.35% (31/216 patients). O'Connor et al.15 reviewed 4287 articles, of which 19 mentioned the AKI rate in 82,514 patients undergoing abdominal surgery; the overall AKI rate was also 13.4%. In patients undergoing emergency colorectal surgery without prior CKD, Miyake et al.24 found that up to 21.6% of patients (541/2504) developed AKI after surgery. In particular, on the same subject with CRC, Sim et al.17 reported that the AKI rate in the group of patients after laparoscopic surgery was 8.8% (87/987 patients) and in the group of patients after open surgery was 9.1% (242/2650 patients). Thus, AKI is a complication that needs attention after laparoscopic CRC surgery. The rate of AKI in different studies is different because AKI is related to many factors, such as preoperative patient characteristics, anesthesia status, surgery time, and accompanying early postoperative complications.15-17
We compared preoperative patient characteristics between the AKI and non-AKI groups to investigate preoperative factors associated with AKI. The results show that many factors are involved (Table 1), but when multivariate analysis, only anemia, and diabetes are independent factors related to AKI after surgery (Table 3). James MT et al.25 suggested that diabetes was a risk factor for AKI in general surgery patients. In the study of Sim et al.17 on CRC patients undergoing laparoscopic surgery, diabetes was also found to be an independent risk factor for AKI complications after surgery. This may explain the high risk of AKI in patients with diabetes, which are common complications in the above condition that can lead to AKI even when the patient does not have CKD.26
Among the factors related to colorectal tumor and postoperative factors, we found that there are many factors associated with the occurrence of AKI when using univariate analysis, such as rate of lymph node metastasis, TNM stage (Table 1) as well as long operative time, early postoperative complications (Table 2). However, in multivariate analysis, the occurrence of postoperative complications was an independent factor related to AKI (Table 3). Slagelse et al.27 also confirmed many postoperative factors associated with AKI, including postoperative complications such as bleeding or infection. AKI most frequently occurred during the initial days following surgery. Major surgery carries the risk of intravascular blood and fluid loss, fluid drainage from the vascular compartment, the consequent reduction in circulating volume, decreased renal blood flow, renal ischemia, and eventual AKI.27 Patients with early complications, such as intestinal obstruction and infection, worsen the shortage of circulating fluid and are more likely to have AKI complications.
The role of biomarkers in AKI prognosis has been confirmed. There have been many biomarkers used to assess and predict AKI including neutral gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), liver-type fatty acid-binding protein (L-FABP), interleukin-18 (IL-18), insulin-like growth factor-binding protein 7 (IGFBP7), tissue inhibitor of metalloproteinase 2 (TIMP-2), and calprotectin (S100A8)/9). Among them, uNGAL is a widely used marker because it appears earliest in the presence of AKI and does not require intervention during sampling.21 In this study, we examined the predictive role of urinary NGAL in AKI in CRC patients before surgery. The results showed that urine NGAL has a good predictive value for AKI (Figure 1), AUC = 0.858, p < 0.001. Using urine or serum, assess the role of established renal biomarkers. Each sample material has its advantages and disadvantages. Plasma is less susceptible to bacterial contamination and easily obtainable in oligo-/anuric patients. However, changes in serum biomarker concentrations are sometimes associated with decreased renal function and as the product of a systemic reaction.28, 29
In contrast, the advantage of urine specimen collection is that it is noninvasive, may have a higher specificity for renal injury, and is unaffected by systemic disease.30 Vanmassenhove et al.31 speculated that urinary biomarkers more faithfully reflected tubular injury, while serum marker levels were better at assessing changes in glomerular clearance. uNGAL is believed to be better at predicting AKI in various patient populations.32-34 Again, in CRC patients, urine NGAL can be used to predict AKI after complete laparoscopic resection of the tumor.
4.2 Ratio of mortality and predictive factors after 3 years follow-up
We followed up with 216 patients for 3 years. The cumulative mortality rate was 7.9% (17/216 patients), in which the AKI group had a higher mortality rate than the non-AKI group, p < 0.001 (Table 2). When considering preoperative biochemical factors related to mortality, we found that CEA, CRP-hs, and uNGAL levels were independent factors, p < 0.05 (Table 4). Incredibly, these three biomarkers all have predictive mortality values in which uNGAL has a good predictive value with AUC = 0.941, p < 0.001 (Figure 2). Several previous studies have mentioned the role of serum CRP-hs in the prognosis of metastasis and mortality in CRC patients.35-37 CEA level in CRC is related to preoperative tumor grade, recurrence, and survival rate.38, 39 The role of NGAL in cancer in general and CRC, in particular, has been mentioned in some previous studies.40, 41
Besides the prognostic role of biomarkers in mortality, our results show that the 3-year mortality rate in the AKI group is significantly higher than in the non-AKI group (Table 2). Seriously, the CRC patients with AKI after laparoscopic surgery exhibited a substantially higher death rate than non-AKI patients (log-rank test, p < 0.001) in Figure 3. Postoperative AKI is associated with CKD progression and increased late mortality after cardiac and other organ surgery.42 The STARSurg Collaborative43 recently confirmed that postoperative AKI in patients undergoing gastrointestinal surgery increased mortality after 1 year of observation. The role of AKI in the causal relationship with mortality has not been established. AKI may be associated with postoperative mortality that may be a direct factor in mortality risks, such as progression to CKD or cardiovascular events. The results of this study may remind surgeons of the need for good postoperative care, risk stratification, and prevention of AKI after surgery. Since AKI is still diagnosed by oliguria/anuria or elevated serum creatinine until now, it is considered that AKI occurred a few days before surgery.23 A preoperative urine biomarker such as NGAL could be a good predictor of AKI and late mortality in patients after laparoscopic CRC surgery.
Our study has some of the following limitations. First, we only quantified uNGAL concentrations the day before surgery, not many times before. Therefore, we cannot determine which time of uNGAL before surgery has the best value in predicting AKI that appears after surgery. Second, the type of AKI that occurs after surgery has not been analyzed so that we can further investigate the factors affecting the occurrence of AKI after surgery and have an early intervention plan to reduce the AKI and AKI severity rate.
5 CONCLUSION
The rate of AKI after laparoscopic CRC surgery was 14.35%. After 3 years of follow-up, preoperative urine NGAL has a good predictive value for postoperative AKI and mortality.
AUTHOR CONTRIBUTIONS
Long Huynh Thanh: Conceptualization; investigation; methodology; project administration; resources; writing—original draft. Quyen Dao Bui Quy: Conceptualization; investigation; methodology; resources; validation; writing—original draft. Khiem Nguyen Manh: Data curation; investigation; resources; visualization. Dung Nguyen Huu: Methodology; supervision; writing—review and editing. Kien Nguyen Trung: Data curation; formal analysis; writing—review and editing. Thang Le Viet: Conceptualization; investigation; methodology; supervision; writing—original draft.
ACKNOWLEDGMENTS
All authors have read and approved the final version of the manuscript. The corresponding author had full access to all of the data in this study and took complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Animals did not participate in this research. All human research procedures followed the committee's ethical standards responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Ethical Committee of Vietnam Military Medical University (No.73/QĐ-HVQY) and Nguyen Tri Phuong Hospital (No.112/QĐ-BVNTP).
TRANSPARENCY STATEMENT
The lead author Thang Le Viet Thang affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Authors can provide additional relevant original data underpinning their research if requested by the Editor or reviewers.