Investigating the relationship between liver enzymes and incidence of hypertension: A population-based cohort study in Kharameh, a city in the South of Iran
Abstract
Background and Aims
Hypertension and abnormal liver enzymes are common health issues that frequently coexist, and recent research has suggested a possible association between them, warranting further investigation. Therefore, the aim of this study is to explore the relationship between liver enzymes and hypertension.
Methods
This prospective cohort study utilized data from the Kharameh cohort study, which is a branch of the Prospective Epidemiological Studies in Iran (PERSIAN) database. The study included 7710 participants aged between 40 and 70 years. Hypertension was defined in accordance with the European guidelines for hypertension management, and the association between liver enzymes and hypertension was modeled and predicted using Firth logistic regression.
Results
This study investigated the association between liver enzymes and hypertension risk in a cohort of 7710 individuals aged 40–70 years. The results showed that higher levels of alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), and alkaline phosphatase (ALP) were associated with an increased risk of hypertension, and this relationship remained significant even after adjusting for potential confounding factors. Additionally, separate analyses within age subgroups revealed a significant association between ALP concentration and high blood pressure in certain age ranges.
Conclusion
The study demonstrated a significant association between high levels of ALT, GGT, and ALP and an increased risk of hypertension, regardless of other risk factors. These results suggest that monitoring liver enzymes, specifically ALT, GGT, and ALP, could serve as a useful tool to predict hypertension risk and identify individuals who could benefit from early intervention. Overall, these findings underscore the importance of monitoring liver function in preventing and managing hypertension.
1 INTRODUCTION
Hypertension is a chronic medical condition that affects millions of people worldwide.1 It is often referred to as a silent killer due to its association with cardiovascular disease, stroke, and premature mortality.2 Approximately one in four adults globally suffers from hypertension, making it a significant public health concern.3 It serves as a common risk factor for various diseases such as cardiovascular disease, cerebrovascular and renal disease, and is a leading preventable cause of premature death and disability worldwide.4-6 Research indicates that hypertension is responsible for 8.5 million deaths worldwide, attributed to stroke, ischemic heart disease, other heart diseases, and kidney disease, and it accounts for 7% of disability-adjusted life years globally.7, 8 In 2008, the Eastern Mediterranean region reported a prevalence rate of approximately 30%, while Iran had a lower rate of 25% for both sexes. A study conducted in the southern region of Fars province found that women had a higher prevalence rate of 33.53%, compared to men with a rate of 21.44%.9, 10 These figures highlight the widespread nature of this condition and emphasize the necessity for effective prevention and treatment strategies.
Numerous studies have extensively researched the association between hypertension and various biochemical risk factors. While risk factors such as age, sedentary lifestyle, smoking, unhealthy dietary habits (such as excessive salt intake), ethnicity, and alcohol consumption have been identified for hypertension, the relationship between hypertension and liver parameters remains an area of limited information and conflicting outcomes.10-16
Previous research in this field has primarily focused on lipid profiles. Certain epidemiological studies have shown connections between liver enzymes (alanine aminotransferase [ALT] and gamma glutamyl transferase [GGT]) and metabolic syndrome, type 2 diabetes, and cardiovascular disease.17, 18 However, the specific link to hypertension requires further investigation. There is limited information available regarding the relationship between hypertension and liver parameters, and the results from various studies have produced conflicting outcomes.19, 20 Although previous research has examined the relationship between liver enzyme levels, these studies have typically focused on only one or two factors in a small population. Therefore, in this cohort study, we aim to investigate the relationship between liver enzymes and high blood pressure using Firth's regression model. Our goal is to provide a more comprehensive understanding of the potential underlying factors that contribute to the development of HTN.
2 MATERIALS AND METHODS
The data for this study were obtained from the Kharameh cohort, which is a part of the Prospective Epidemiological Studies in Iran (PERSIAN) project. Adhering to the principles of the Helsinki Declaration and Iranian national research ethics guidelines, this study holds the reference number IR.SUMS.SCHEANUT.REC.1401.136. In line with ethical standards, all participants have provided informed written consent before their engagement. Launched in 2014, the Persian Cohort Study encompasses 18 diverse regions in Iran and includes all significant ethnic groups. It stands as one of the most extensive cohort studies in the area, and its goals, rationale, and structure were previously published.21
2.1 Study design
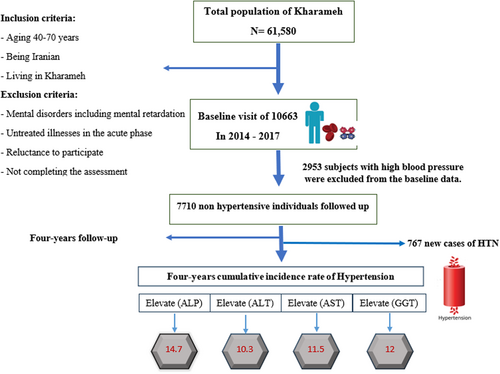
This prospective cohort study followed 7710 individuals aged 40 and above who did not have hypertension at the start of the study, from December 10, 2014 to February 28, 2017. Individuals who had been initially diagnosed with high blood pressure based on ICD-10 codes were excluded. The cohort was subsequently followed up for 4 years until 2021, as illustrated in Figure 1.
2.2 Outcome variable
Our research objective was to investigate the relationship between liver enzymes and hypertension. The diagnosis of hypertension was verified by two internists and followed the hypertension management recommendations outlined in the European guidelines, which define hypertension as having a systolic blood pressure of 140 mmHg or higher and a diastolic blood pressure of 90 mmHg or higher.
2.3 Measurement
In this research, the first step was to obtain written consent from participants. The researchers then collected data on participants’ demographic profiles, including age, gender, marital status, education level, occupation, place of residence, social and economic status, and behavioral factors, such as smoking, alcohol consumption, hookah use, drug use, and physical activity. This was done through a series of interviews, laboratory experiments, and physical examinations, using a standardized questionnaire administered by trained experts. The questionnaire had been previously tested and validated by the Persian cohort national team, as Kharameh cohort was part of the PERSIAN cohort.21 The study participants were asked to fast for 12 h before the laboratory experiments, which involved blood sampling. The researchers took measurements of the participants’ weight, height, and blood pressure, and calculated their body mass index (BMI). Based on their BMI, the participants were classified into one of four categories: less than 18.5, 18.5–24.9, 25–29, and greater than 30 kg/m2. Dyslipidemia was identified as cholesterol levels above 200 mg/dL or triglyceride serum levels above 150 mg/dL, while diabetes was identified as either a history of diabetes or a fasting blood sugar level above 126 mg/dL. Serum levels of ALT, aspartate aminotransferase (AST), GGT, and alkaline phosphatase (ALP) were deemed elevated in this study. The reference range for each biomarker was defined by the laboratory at the cohort center. Specifically, serum levels of ALT (serum glutamic pyruvic transaminase) and AST (serum glutamic-oxaloacetic transaminase) greater than 40 and 35 U/L, respectively, were considered elevated in males and levels greater than 35 and 40 U/L, respectively, were considered elevated in females. For GGT (gamma glutamyl transferase), elevated serum levels were defined as greater than 54 and 37 U/L in males and females, respectively. Serum levels of ALP (alkaline phosphatase) greater than 306 U/L were considered elevated in both genders. The study categorized participants with normal range levels into quartiles. For males, the quartiles for ALT levels were: ≤14 U/L, 15–19 U/L, 20–25 U/L, and 26–40 U/L, while for females the quartiles were: ≤12 U/L, 13–15 U/L, 16–20 U/L, and 21–35 U/L. For AST levels, the quartiles for males were: ≤16 U/L, 17–19 U/L, 20–23 U/L, and 24–40 U/L, while for females the quartiles were: ≤14 U/L, 15–16 U/L, 17–20 U/L, and 21–35 U/L. Similarly, GGT and ALP levels in the normal range were also divided into quartiles. The quartiles for GGT levels in men were: ≤18 U/L, 19–23 U/L, 24–31 U/L, and 32–54 U/L, while for women the quartiles were: ≤14 U/L, 15–18 U/L, 19–23 U/L, and 24–37 U/L. The quartiles for ALP levels in both genders included: ≤177 U/L, 178–209 U/L, 210–244 U/L, and 245–306 U/L.22
2.4 Statistical analysis
The study presented data using appropriate descriptive statistics. Normally distributed continuous variables were reported as mean ± standard deviation, while non-normally distributed variables were reported as median with the interquartile range (Q1–Q3). Qualitative variables were expressed as a number and percentage. The study used Chi-square tests to investigate relationships between categorical variables. To model and predict factors associated with hypertension, Firth's logistic regression analysis was used instead of traditional exact logistic regression analysis. This approach was chosen to address computational limitations and convergence issues that may arise from sparse data. Firth's logistic regression analysis provides unbiased and reliable estimates of coefficients, corresponding p values, and odds ratios in unbalanced data settings.
The statistical analyses were conducted using SPSS 23 and Stata 12 software, while graphs were created using Prism GraphPad version 8 software. Two-tailed p values were used, and the significance level was set at 0.05.
3 RESULTS
The baseline characteristics of the study population are presented in Tables 1 and 2. Of the 7710 participants, 48.8% were male with an average age of 51.38 ± 7.9 years, while 51.2% were female with an average age of 50.16 ± 8.1 years. More than half of the participants (55.3%) were overweight or obese, 8.7% were single, widowed, or divorced, 64.2% were illiterate, 59% lived in rural areas, and 42.3% were unemployed. Regarding substance use, the study found that 17.9%, 28.2%, and 4.2% of the participants reported using drugs, cigarettes, and alcohol, respectively. Moreover, 25.9% of the participants had pre-hypertension, and 9.4% had diabetes mellitus. The study also found that hypertension was more common among female participants, those aged 60–70 years, individuals with a BMI greater than 30.0 kg/m2, illiterate individuals, and those living in rural areas.
| Variable | All participant's (n=) n (%) | Incidence of hypertension (n=) n (%) | Normotension (n=) n (%) | p value |
|---|---|---|---|---|
| Age (years) | ||||
| 40–49 | 3893 (50.5) | 171 (22.3) | 3722 (53.6) | <0.001 |
| 50–59 | 2565 (33.3) | 296 (38.6) | 2265 (32.6) | |
| 60–70 | 1252 (16.2) | 300 (39.1) | 956 (13.8) | |
| Sex | ||||
| Male | 3759 (48.8) | 294 (38.3) | 3465 (49.9) | <0.001 |
| Female | 3951 (51.2) | 473 (61.7) | 3478 (50.1) | |
| BMI (kg/m2) | ||||
| <18.4 | 368 (4.8) | 19 (2.5) | 349 (5) | <0.001 |
| 18.5–24.9 | 3075 (39.9) | 221 (28.8) | 2854 (41.1) | |
| 25–29.9 | 3101 (40.2) | 344 (44.9) | 2757 (39.7) | |
| >30 | 1166 (15.1) | 183 (23.9) | 983 (14.2) | |
| Marital status | ||||
| Unmarried | 172 (2.2) | 9 (1.2) | 163 (2.3) | <0.001 |
| Widowed or divorced | 503 (6.5) | 86 (11.2) | 417 (6) | |
| Married | 7035 (91.2) | 672 (87.6) | 6363 (91.6) | |
| Education level | ||||
| Illiterate | 3672 (47.6) | 495 (64.5) | 3177 (45.8) | <0.001 |
| Diploma and below | 3577 (46.4) | 245 (31.9) | 3332 (48) | |
| Academic | 461 (6) | 27 (3.5) | 434 (6.3) | |
| Living place | ||||
| Urban | 3157 (41) | 271 (35.3) | 2886 (41.6) | <0.001 |
| Rural | 4549 (59) | 496 (64.7) | 4053 (58.4) | |
| Employed | ||||
| No | 3260 (42.3) | 400 (52.2) | 2860 (41.2) | <0.001 |
| Yes | 4450 (57.7) | 367 (47.8) | 4083 (58.8) | |
| Physical activity | ||||
| Light | 1694 (22) | 179 (23.3) | 1515 (21.8) | 0.007 |
| Moderate | 1893 (24.6) | 221 (28.8) | 1672 (24.1) | |
| High | 1942 (25.2) | 175 (22.8) | 1767 (25.5) | |
| Severe | 2177 (28.3) | 192 (25) | 1985 (28.6) | |
| Wealth score index | ||||
| Low income | 4088 (53) | 459 (59.8) | 3629 (52.3) | <0.001 |
| Low- middle income | 1764 (22.9) | 169 (22) | 1595 (23) | |
| Middle-high income | 1720 (22.3) | 132 (17.2) | 1588 (22.9) | |
| High income | 138 (1.8) | 7 (0.9) | 131 (1.9) | |
| Blood pressure | ||||
| Hypotension | 591 (7.7) | 8 (1) | 583 (8.4) | <0.001 |
| Normotensive | 5120 (66.4) | 316 (41.2) | 4804 (69.2) | |
| Pre-hypertension | 1999 (25.9) | 443 (57.8) | 1556 (22.4) | |
| Diabetes | ||||
| No | 6982 (90.6) | 666 (86.8) | 6316 (91) | 0.001 |
| Yes | 728 (9.4) | 101 (13.2) | 627 (9) | |
| Family history of hypertension | ||||
| First degree | ||||
| No | 4029 (52.3) | 352 (45.9) | 3677 (53) | <0.001 |
| Yes | 3681 (47.7) | 415 (54.1) | 3266 (47) | |
| Second degree | ||||
| No | 6448 (83.6) | 645 (84.1) | 5803 (83.6) | 0.71 |
| Yes | 1262 (16.4) | 122 (15.9) | 1140 (16.4) | |
| High cholesterol level | ||||
| No | 4692 (60.9) | 393 (51.24) | 4299 (61.96) | <0.001 |
| Yes | 3013 (39.1) | 374 (48.76) | 2639 (38.04) | |
| High triglyceride level | ||||
| No | 5641 (73.21) | 505 (65.84) | 5136 (74.03) | <0.001 |
| Yes | 2064 (26.79) | 262 (34.16) | 1802 (25.97) | |
| HDL (mg/dL) | ||||
| <40 for males and <50 for females | 3332 (43.3) | 341 (44.5) | 2991 (43.2) | 0.26 |
| ≥40 for males and ≥50 for females | 4364 (56.7) | 426 (55.5) | 3938 (56.8) | |
| Opium use | ||||
| No | 6327 (82.1) | 675 (88) | 5652 (81.4) | <0.001 |
| Yes | 1383 (17.9) | 92 (12) | 1291 (18.6) | |
| Hookah use | ||||
| No | 7362 (95.5) | 741 (96.6) | 6621 (95.4) | 0.12 |
| Yes | 348 (4.5) | 26 (3.4) | 322 (4.6) | |
| Smoking | ||||
| No | 5538 (71.8) | 613 (79.9) | 4925 (70.9) | <0.001 |
| Yes | 2172 (28.2) | 154 (20.1) | 2018 (29.1) | |
| Alcohol consumption | ||||
| No | 7384 (95.8) | 752 (98) | 6632 (95.5) | <0.001 |
| Yes | 326 (4.2) | 15 (2) | 311 (4.5) | |
| Glomerular filtration rate (mL/min) | 76.6 (69.59, 8373) | <0.001 | ||
- Abbreviation: BMI, body mass index.
More than 40% of participants had high ALT levels. This value for AST, GGT and ALP was 39.9%, 26.9%, and 21.6%, respectively (Table 2).
| Variable | All participant's n | HTN | NTH | p value |
|---|---|---|---|---|
| ALT | ||||
| Quartile 1 | 989 (12.8) | 72 (9.4) | 917 (13.2) | 0.03 |
| Quartile 2 | 1372 (17.8) | 133 (17.3) | 1239 (17.9) | |
| Quartile 3 | 1975 (25.6) | 215 (28) | 1760 (25.4) | |
| Quartile 4 | 2416 (31.4) | 250 (32.6) | 2166 (31.2) | |
| Elevated | 951 (12.3) | 97 (12.6) | 854 (12.3) | |
| AST | ||||
| Quartile 1 | 945 (12.3) | 92 (12) | 853 (12.3) | 0.30 |
| Quartile 2 | 1234 (16) | 104 (13.6) | 1130 (16.3) | |
| Quartile 3 | 2449 (31.8) | 248 (32.3) | 2201 (31.7) | |
| Quartile 4 | 2776 (36) | 288 (37.5) | 2488 (35.9) | |
| Elevated | 300 (3.9) | 35 (4.6) | 265 (3.8) | |
| GGT | ||||
| Quartile 1 | 2749 (35.7) | 231 (30.1) | 2518 (36.3) | 0.004 |
| Quartile 2 | 1497 (19.4) | 144 (18.8) | 1353 (19.5) | |
| Quartile 3 | 1378 (17.9) | 155 (20.2) | 1223 (17.6) | |
| Quartile 4 | 1443 (18.7) | 161 (21) | 1282 (18.5) | |
| Elevated | 634 (8.2) | 76 (9.9) | 558 (8) | |
| ALP | ||||
| Quartile 1 | 2669 (34.7) | 202 (26.3) | 2467 (35.6) | <0.001 |
| Quartile 2 | 1872 (24.3) | 197 (25.7) | 1675 (24.2) | |
| Quartile 3 | 1497 (19.4) | 160 (20.9) | 1337 (19.3) | |
| Quartile 4 | 1209 (15.7) | 141 (18.4) | 1068 (15.4) | |
| Elevated | 455 (5.9) | 67 (8.7) | 388 (5.6) |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase.
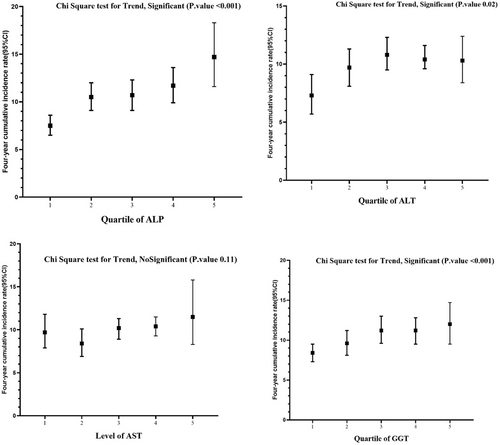
The 4-year cumulative incidence rate of HTN for participants with high levels of ALP was 14.7 (95% confidence interval [CI]: 11.6, 18.3), for GGT it was 12 (95% CI: 9.5, 14.7), for AST it was 11.5 (95% CI: 8.3, 15.8), and for ALT it was 10.3 (95% CI: 8.4, 12.4) (Figures 1 and 2).
The Chi-Square test for trend was conducted, revealing a significant association for ALP and GGT (p < 0.001), ALT (p = 0.02), and a non-significant association for AST (p = 0.11) (Figure 2).
3.1 Association of liver enzymes with incidence of hypertension
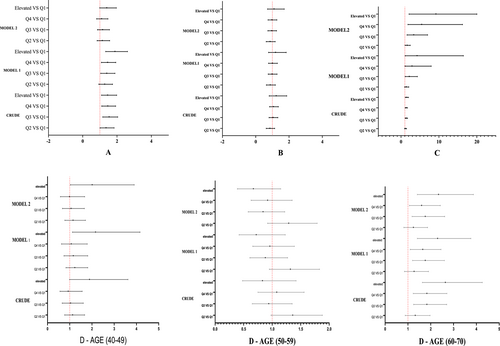
Table 3 and Figure 3 presents findings indicating that higher levels of ALT, GGT, and ALP are associated with an increased risk of hypertension. This relationship remained significant in a multivariate-adjusted logistic model. Specifically, in model 1, age and sex were adjusted, and ALT, ALP, and GGT demonstrated significant associations with hypertension. In model 2, adjustments were made for BMI, fasting blood glucose, fatty liver, physical activity, smoking and opium use, and alcohol use. Both models showed a significant positive association between ALT, ALP, and GGT and hypertension. Due to an interaction between ALP levels and age, separate analyses were conducted within age subgroups. The results indicate a significant association between ALP concentration and high blood pressure in the age ranges of 40–49 and 60–70.
| Variable | ALT (SGPT) | AST (SGOT) | GGT | ALP | ||
|---|---|---|---|---|---|---|
| Age | ||||||
| 40–49 | 50–59 | 60–70 | ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Crude | ||||||
| Quartile 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quartile 2 | 1.36 (1.01, 1.83) | 0.85 (0.63, 1.14) | 1.16 (0.93, 1.44) | 1.13 (0.77, 1.67) | 1.36 (0.98, 1.88) | 1.32 (0.88, 1.97) |
| Quartile 3 | 1.54 (1.17, 2.04) | 1.04 (0.8, 1.33) | 1.38 (1.11, 1.71) | 1.04 (0.66, 1.61) | 0.94 (0.65, 1.35) | 1.83 (1.25, 2.70) |
| Quartile 4 | 1.46 (1.11, 1.92) | 1.06 (0.83, 1.36) | 1.37 (1.10, 1.69) | 0.93 (0.56, 1.56) | 1.08 (0.75, 1.56) | 1.83 (1.23, 2.71) |
| Elevated | 1.45 (1.05, 1.98) | 1.23 (0.81, 1.86) | 1.50 (1.13, 1.96) | 1.89 (0.99, 3.62) | 0.83 (0.48, 1.42) | 2.64 (1.63, 4.26) |
| Model 1 | ||||||
| Quartile 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quartile 2 | 1.28 (0.94, 1.74) | 0.87 (0.64, 1.18) | 1.39 (0.95, 2.04) | 1.23 (0.83, 1.81) | 1.32 (0.95, 1.83) | 1.27 (0.85, 1.90) |
| Quartile 3 | 1.4 (1.05, 1.88) | 0.98 (0.75, 1.28) | 2.17 (1.09, 4.30) | 1.16 (0.74, 1.81) | 0.88 (0.61, 1.27) | 1.76 (1.20, 2.60) |
| Quartile 4 | 1.45 (1.09, 1.93) | 1.0 (0.77, 1.30) | 2.89 (1.05, 7.96) | 1.07 (0.63, 1.79) | 0.96 (0.66, 1.39) | 1.65 (1.10, 2.45) |
| Elevated | 1.88 (1.34, 2.61) | 1.19 (0.77, 1.83) | 4.22 (1.08, 16.45) | 2.16 (1.12, 4.16) | 0.72 (0.42, 1.23) | 2.30 (1.41, 3.76) |
| Model 2 | ||||||
| Quartile 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quartile 2 | 1.14 (0.84, 1.57) | 0.86 (0.63, 1.17) | 1.61 (1.07, 2.43) | 1.15 (0.78, 1.71) | 1.29 (0.92, 1.79) | 1.24 (0.82, 1.86) |
| Quartile 3 | 1.16 (0.86, 1.56) | 0.96 (0.73, 1.25) | 3.34 (1.59, 7.0) | 1.06 (0.67, 1.66) | 0.84 (0.58, 1.22) | 1.76 (1.18, 2.61) |
| Quartile 4 | 1.1 (0.81, 1.47) | 0.97 (0.74, 1.27) | 5.43 (1.82, 16.22) | 0.99 (0.58, 1.67) | 0.92 (0.63, 1.35) | 1.60 (1.06, 2.42) |
| Elevated | 1.4 (0.99, 1.96) | 1.10 (0.71, 1.71) | 9.2 (2.12, 39.8) | 2.01 (1.03, 3.90) | 0.67 (0.39, 1.15) | 2.34 (1.41, 3.87) |
- Note: Crude: unadjusted; Model 1: adjusted for age and sex; Model 2: Model 1 plus, BMI, glucose, fatty liver, physical activity, smoking and opium use, alcohol user.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; GGT, gamma glutamyl transferase; OR, odds ratios; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
4 DISCUSSION
The aim of this study was to investigate the relationship between liver enzymes and hypertension among individuals aged 40–70 years in Kharameh, a city in southern Iran. Our main findings indicate that levels of ALT, GGT, and ALP, which are liver enzymes, are associated with an increased risk of developing hypertension. These findings provide valuable insights into the potential factors associated with hypertension and may have important implications for the prevention of this condition.
The present study revealed a relationship between high blood pressure and ALT levels, indicating a higher risk of hypertension in individuals with elevated ALT levels. These findings are consistent with previous research; for instance, Rahman et al.20 reported a higher prevalence of ALT in people with hypertension. Other studies have also reported a significant association between ALT and high blood pressure, even after controlling for confounding factors such as age.23, 24 However, in the study of Stranges et al.,25 no association was observed between high ALT levels and hypertension. Although our cohort study confirms these previous findings, the precise mechanism underlying this relationship remains unclear, and there is no simple explanation to account for it. Prior studies have proposed different interpretations of the causes of this relationship; for example, Hong et al.26 suggested that hypertensive patients may develop non-alcoholic fatty liver disease after a prolonged period of elevated blood pressure, which may account for this relationship. Other studies have mentioned the role of confounding factors in this relationship, although in our cohort study, we adjusted for these factors, measured interaction effects, and controlled for liver diseases, yet still found an association. This result suggests that ALT may independently contribute to the development of hypertension. Although the precise mechanism behind this association remains unclear, some researchers suggest that ALT may cause oxidative stress, faster heart rate, increased arterial stiffness, and inflammation, which can damage blood vessels and lead to the development of hypertension.23, 27-30 Previous studies have shown that elevated GGT levels are a risk factor for high blood pressure. In their study, Stranges et al. reported that GGT was a strong predictor of hypertension incidence over a 6-year follow-up period in a dose-response relationship.25, 31 A meta-analysis of 13 prospective studies showed a significant positive association between GGT levels and the risk of hypertension.19 Our study findings confirmed these results and demonstrated that high GGT levels remain a risk factor for high blood pressure even after controlling for confounding factors such as BMI, fasting blood glucose, fatty liver, physical activity, smoking, opium use, and alcohol consumption. As with ALT, the exact mechanism of GGT with hypertension is still not fully understood, however, based on previous studies, there are several possible reasons for this association. Research suggests that GGT is positively associated with inflammation markers such as fibrinogen, C-reactive protein (CRP), and F2-isoprostanes, as seen in longitudinal studies.18, 32 Furthermore, GGT has been found to be expressed in human atherosclerotic lesions, colocalizing with ox-LDL and foam cells, indicating its possible contribution to the progression of atherosclerosis.33 Finally, GGT interacts with iron and plays a crucial role in the generation of free radical species, leading to oxidative stress.34 Taken together, these findings suggest that elevated GGT levels may be indicative of inflammation and oxidative stress, which are key features of hypertension.19 Some studies have supported the hypothesis that fatty liver may be the mechanism of association between GGT and hypertension. However, in our study after adjusting for fatty liver disease, this association still existed.25 Our study found that there was an interaction between ALP levels and age. Specifically, we observed that an increase in ALP levels was significantly positively associated with the risk of developing high blood pressure in individuals aged 40–49 and 60–70 years. In contrast, among individuals aged 50–59, we observed a non-significant inverse relationship. In our research, the cause for this opposing connection among individuals aged 50–59 might stem from the fact that, in this area, women tend to consume herbal teas due to menopause, and men due to co-morbidities. It appears that the intake of these teas leads to alterations in ALP levels. A previous study by Shimizu et al.35 reported a significant positive association between ALP levels and high blood pressure in nondrinkers but no association in drinkers. In our study, we found no interaction between alcohol consumption and ALP levels. After adjusting for the effects of alcohol consumption, we still observed a significant positive relationship between ALP levels and high blood pressure. In contrast to our study, no significant relationship between ALP levels and hypertension was observed in the study of Rahman et al.20 The exact mechanisms behind the positive association of higher ALP levels with the risk of hypertension are not fully understood, but there are some proposed explanations. One potential explanation is that ALP could play a role in regulating vascular calcification, which is a crucial contributor to the development of hypertension. Elevated levels of ALP have been linked to increased arterial stiffness and calcification.36, 37 Another proposed mechanism involves the connection between ALP and inflammation. Several studies have demonstrated that higher ALP levels are correlated with elevated levels of inflammatory markers, including CRP, which could contribute to hypertension.9, 37, 38 Although the precise mechanisms are not yet fully understood, the positive correlation between higher ALP levels and hypertension risk may be due to ALP's involvement in vascular calcification or inflammation.30, 39, 40
5 CONCLUSION
In conclusion, the results of the study suggest that there is a significant association between elevated levels of liver enzymes, such as ALT, GGT, and ALP, and an increased risk of developing hypertension. The study findings are consistent with previous research, and while the exact mechanisms underlying the relationship are not fully understood, several possible explanations have been proposed, including inflammation, oxidative stress, and vascular calcification. The study's results highlight the importance of monitoring liver enzyme levels as a potential predictor of hypertension risk, particularly in individuals with underlying liver diseases or risk factors for liver disease, such as obesity and excessive alcohol consumption. Given the high prevalence of hypertension and its associated health complications, including cardiovascular disease and stroke, these findings may have important implications for the prevention and management of hypertension. Further research is needed to elucidate the exact mechanisms underlying the association between liver enzyme levels and hypertension risk and to explore potential interventions for reducing this risk. Nonetheless, the present study provides valuable insights into the potential factors associated with hypertension and underscores the importance of monitoring liver enzyme levels as part of routine health assessments for individuals at risk of developing hypertension.
6 LIMITATION AND STRENGTH
Our study has several strengths. First, it is a prospective cohort study that addresses the limitations of cross-sectional studies, such as the issue of temporality. Second, we used Firth's regression model to analyze the data. This model, known as Penalized Maximum Likelihood logistic regression, can provide unbiased estimates even in the presence of rare and unbalanced events. Third, we attempted to study multiple variables to control for confounding and examine their effects simultaneously. Finally, experienced experts collected the data, and two internal specialists confirmed cases of high blood pressure. However, our study also has some limitations. First, due to the stigma associated with drug and alcohol use in Iran, participants may have concealed their behaviors, potentially causing bias. Second, we measured the hepatic enzymes at the baseline level which may not represent the long-term profile. Last, the study did not measure hepatitis B and C infection among the participants which could have an effect on elevated hepatic enzymes.
AUTHOR CONTRIBUTIONS
Najibullah Baeradeh: conceptualization; formal analysis; investigation; methodology; resources; writing—original draft. Mozhgan Seif: formal analysis; methodology; project administration; software; writing—original draft. Abbas Rezaianzadeh: conceptualization; methodology; supervision; writing—original draft. Seyed Vahid Hosseini: validation; writing—review & editing.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to the dedicated experts who actively participated in this project. Their invaluable expertise, insightful contributions, and unwavering commitment have greatly enhanced the quality and impact of our research. We are grateful for their time, support, and valuable discussions, which have contributed significantly to the success of this project. This article is based on a specialized PhD thesis, which was supported by Shiraz University of Medical Sciences. However, we confirm that Shiraz University of Medical Sciences and any financial relationships had no involvement in the study's design, data collection, analysis, interpretation, report writing, or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Mozhgan Seif affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.