Association of vaccination status with the clinicobiochemical profile, hospital stay, and mortality in COVID-19: A case–control study
Abstract
Background and Aims
The effectiveness of coronavirus disease 2019 (COVID-19) vaccines in reducing symptoms, disease advancement, complications, and mortality in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been well-established. This case-control study aimed to compare different blood parameters, and prognostic and survival outcomes of COVID-19 patients based on vaccination status.
Methods
We performed a case-control study that included hospitalized patients with COVID-19 at Tribhuvan University Teaching Hospital, Kathmandu, Nepal. Individuals who received vaccination were designated as cases and unvaccinated individuals as controls. Demographics, co-morbidity, clinical data, laboratory data, and disease outcomes were recorded for both groups. Multivariate, Cox, and linear regression were used for analysing blood parameters, hospital admission, survival, and hospital stay, respectively, between cases and controls.
Results
Out of 100 participants enrolled, 46 were vaccinated, and 54 weren't. At admission, ferritin and erythrocyte sedimentation rate (ESR) were significantly lower in cases. At discharge, cases showed a higher monocyte than controls. Ferritin, ESR, and d-imer showed excellent performance in determining the severity of symptoms. Significant correlation and regression of ferritin and ESR with the length of hospital stay was observed. Length of hospital stay was significantly lower in cases than in controls. No significant differences between cases and controls were observed in mortality.
Conclusion
COVID-19 vaccines effectively reduced hospitalization duration. Ferritin and ESR were significantly lower in vaccinated individuals and showed the best utility in monitoring the disease.
1 INTRODUCTION
The World Health Organization (WHO) declared severe acute respiratory syndrome (SARS-CoV-2) a global pandemic on March 11, 2020.1 It affected the population worldwide with more than 627 million cases by mid-October 2022.2 Preventive measures such as physical distancing, face masks, strict isolation, and quarantines have been employed as control measures to prevent viral transmission.3 Medications like Remdesivir, Sarilumab, and others has been employed; however, they have limited benefits.4 The risk of post-covid syndrome is also high.5 Additionally, several coronavirus disease 2019 (COVID-19) vaccines have been developed to overtake the pandemic. An efficient and secure vaccine against SARS-CoV-2 has proven invaluable in halting the spread and lowering COVID-19-associated morbidities and fatalities.6 These vaccines are produced based on different techniques and components, such as RNA, viral vectors, protein subunits, dead virions, and live attenuated viruses. The SARS-CoV-2 spike protein or its portions are expressed in majority of COVID-19 vaccines to elicit an immune response.7 Currently, the WHO has approved 11 vaccines with different compositions of each type of vaccine, including mRNA technology, recombinant DNA technology, and attenuated virus.8-10 Vaccines' efficacy vary in terms of reducing the chances of morbidity and mortality.11 It largely depends on the individual's immune response to vaccination, subsequently, on the host's intrinsic factors (age, sex, nutritional status, comorbidities, etc.) and vaccine factors (components, adjuvants, etc.).12-14
Nepal began its first vaccination campaign against COVID-19 in mid-2021, initially prioritizing frontline healthcare workers (HCWs), then extending it to people over the age of 60 and people with comorbidities between the ages of 45 and 60, and finally to everyone over the age of 45. Later, vaccines were available to all adults and children above 12 years of age.15, 16 The Indian pharmaceuticals regulator approved Covishield (AstraZeneca formulation), a recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 spike glycoprotein, and Covaxin (inactivated whole virions grown in Vero cells) to be used as an emergency. To date, nearly 88% of all doses administered in Nepal are Covishield vaccines.17 For symptomatic and laboratory-tested COVID-19, this vaccine possessed a protective efficacy of 67% (95% confidence interval [CI]: 57%–74%) and nearly 100% (95% CI: 72%–100%) efficacy for preventing hospitalization and severe infection, starting 21 days after the second dose. These protective efficacy rates were based on pooled data from four trials.
The effectiveness of these vaccines has been largely unexplored and not explicitly documented in the Nepalese setting to date despite their importance in overcoming vaccine hesitancy and increasing vaccine acceptance to prevent and control the transmission of COVID-19. Therefore, in this study, we aimed to analyse the effectiveness of COVID-19 vaccines in minimizing the mortality associated with COVID-19 infection in Nepal. We also compared the levels of different blood parameters in vaccinated (cases) and unvaccinated (controls) COVID-19 hospitalized patients.
2 METHODOLOGY
2.1 Patient involvement
This study has been reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.18
All first-time SARS-CoV-2-positive patients diagnosed by reverse transcriptase polymerase chain reaction (RT-PCR) technique and hospitalized in Tribhuvan University Teaching Hospital, a tertiary health facility in Kathmandu, Nepal, between January 30, 2021, and March 15, 2021, were included in the study. A case-control setting was employed where hospitalized COVID-19 patients who received the COVID-19 vaccine were enrolled as cases, and unvaccinated COVID-19 hospitalized patients were enrolled as controls. Convenience sampling was used for the selection of the study participants. The cases and controls were matched by age and gender. Recently vaccinated patients (<14 days) were also included in cases, as preliminary evidence suggests that a mortality benefit is observed even within 14 days of vaccination.19 This has also been described for other infections.20
2.2 Ethical approval and consent
Ethical approval for the study was provided by the Institutional Review Committee (IRC) of the Institute of Medicine (IOM) [Reference number 66 (6-11) E2 078/079]. Permission was taken from the hospital administration. The study's objectives were shared, and written informed consent was obtained from each participant. We ensured voluntary participation, and the participants' confidentiality was considered. Informed consent was signed by the study participants. The consent from critically ill patients was obtained from their respective family members.
2.3 Data collection
COVID-19 infection was defined as the detection of SARS-CoV-2 RNA in a combined nasopharyngeal and throat swab using the RT-PCR technique. Clinical and laboratory data, including demographics, comorbidities, hospital admission details, vaccination status, and COVID-19 test results, were collected by trained personnel via medical visits and medical report reviews and compiled in a pre-specified data tabulation sheet on Microsoft Excel®. Vaccination status, including date of vaccination and vaccine type, was verified by the vaccination certificate of the participant. Values of a wide range of blood parameters, including hematological, biochemical, and inflammatory markers, were collected at admission and at discharge. Participants were followed up until all of them reached an outcome: death or discharge.
2.4 Outcomes
The primary outcome was the all-cause death of the index SARS-CoV-2–positive sample. Secondary outcomes included a requirement for hospital admission (within 14 days of the positive test) and length of stay during the index hospitalization only for patients who survived their access. Prespecified subgroup analysis was performed for patients with infections ≥14 days after vaccination and by vaccination type. Biochemical analysis among the cases and controls was also included in the outcomes.
2.5 Statistical analysis
Statistical analysis was performed using SPSS version 27 (IBM Corp). After matching, groups were univariably compared using the Fischer exact, Pearson χ2, or Mann–Whitney U test as appropriate. Considering the normal distribution of the levels of blood parameters, their levels were expressed as mean and standard deviation. Length of hospital stay, mortality, and mean levels of blood parameters between vaccinated and unvaccinated groups were compared using a two-tailed independent sample t-test. Receiver operating characteristics (ROC) analysis was performed to evaluate the diagnostic performance of blood parameters, and the area under the curve (AUC) was statistically compared. An AUC value of 0.5–0.6 was considered unsatisfactory, 0.6–0.7 as satisfactory, 0.7–0.8 as good, and >0.8 as excellent performance. Correlation, linear regression, and Cox proportional hazard analysis were performed to investigate the association of blood parameters to the length of hospital stay. ROC and hazard ratios from the co-regression analysis were expressed in terms of 95% confidence interval (CI). Differences of 0.2 or less in confounding variables were considered acceptable. Otherwise, they were inserted into the multivariable model.21 A p-value of ≤ 0.05 was considered a statistically significant finding.
3 RESULTS
3.1 Participants characteristics
A total of 100 COVID-19 patients (46 cases and 54 controls) were enrolled in the study. Table 1 outlines the descriptive characteristics of cases and controls. The percentage of females was higher in controls (55.3%–33/54), and that of males was higher in cases (46.8%–29/46). Participants above 60 years of age were predominant in cases (54.5%–24/46), while participants less than 60 years of age were the majority in controls (60.7%–34/54). Higher proportions of the cases were current smokers, while most controls were nonsmokers. In contrast, the majority of patients were nonalcoholics, while a majority of controls consumed alcohol. The higher proportion of cases and controls were nonhypertensive, non-diabetic, and did not report chronic obstructive pulmonary disease and pulmonary tuberculosis. Although, there was not statistically significant, a higher proportion of vaccinated individuals had mild COVID-19, whereas the unvaccinated group consisted of a higher proportion of patients with moderate to severe COVID-19.
| Characteristics | Case (n = 46) | Control (n = 54) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 29 (46.8) | 33 (53.2) | 0.8 |
| Female | 17 (44.7) | 21 (55.3) | |
| Age | |||
| Less than 60 years | 22 (39.3) | 34 (60.7) | 0.1 |
| 60 years and above | 24 (54.5) | 20 (45.5) | |
| Occupation | |||
| Independent | 21 (48.8) | 22 (51.2) | 0.6 |
| Dependent | 25 (43.9) | 32 (56.1) | |
| Current smoker | |||
| Yes | 11 (52.4) | 10 (47.6) | 0.5 |
| No | 35 (44.3) | 44 (55.7) | |
| Current alcohol consumption | |||
| Yes | 12 (44.4) | 15 (55.6) | 0.8 |
| No | 34 (46.6) | 39 (53.4) | |
| Hospital stay | |||
| General ward | 23 (46.9) | 26 (53.1) | |
| HDU | 11 (37.9) | 18 (62.1) | |
| ICU | 12 (54.5) | 10 (45.5) | 0.4 |
| Hypertension | |||
| Yes | 12 (37.5) | 20 (62.5) | |
| No | 34 (50.0) | 34 (50.0) | 0.2 |
| Diabetes | |||
| Yes | 9 (47.4) | 10 (52.6) | |
| No | 37 (45.7) | 44 (54.3) | 0.8 |
| COPD | |||
| Yes | 2 (22.2) | 7 (77.8) | 0.1 |
| No | 44 (48.4) | 47 (51.6) | |
| Pulmonary TB | |||
| Yes | 2 (66.7) | 1 (33.3) | 0.4 |
| No | 44 (45.4) | 53 (54.6) | |
| COVID-19 severity | |||
| Mild | 17 (60.7) | 11 (39.3) | 0.1 |
| Moderate | 12 (37.5) | 20 (62.5) | |
| Severe | 17 (42.5) | 23 (57.5) | |
- Note: Data expressed as n (%) and mean ± SD.
- Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HDU, high dependency unit; ICU, intensive care unit; SD, standard deviation; TB, tuberculosis.
3.2 Hospital stay and clinical outcomes
Duration of hospital stay was significantly lower in cases (vaccinated) as compared to controls (unvaccinated) (mean: 9.4 and 14.9 days, respectively, p = 0.002). Two cases died, whereas no deaths were recorded in the control group. No significant differences between cases and controls were observed in mortality (p = 0.122). Table 2 displays the outcome results between cases and controls.
| Outcome | Cases (n = 46) | Control (n = 54) | |
|---|---|---|---|
| Hospital stay days | 9.4 ± 5.1 | 14.9 ± 10.4 | 0.002* |
| Outcomes | |||
| Survived n (%) | 44 (95.6%) | 54 (100%) | 0.122 |
| Dead n (%) | 2 (4.3%) | 0 (0%) | |
- * Significant.
3.3 Blood parameters of study participants
Table 3 compares the levels of different blood parameters between cases and controls at admission and then at discharge. At admission, ferritin and ESR were significantly lower in vaccinated individuals (p < 0.001 for each). Two individuals who died were excluded from the statistical comparison of blood parameters at discharge. We found a significantly higher proportion of monocytes in vaccinated individuals during discharge (p = 0.03). Changes in other blood parameters with regard to vaccination status were statistically insignificant.
| Laboratory parameters mean (SD) | At admission | At discharge | ||||
|---|---|---|---|---|---|---|
| Cases (n = 46) | Controls (n = 54) | p-Value | Cases (n = 44) two patients who died were excluded | Controls (n = 54) | p-Value | |
| Hemoglobin (g/dL) | 12.7 (2.7) | 13.1 (2.8) | 0.5 | 13.0 (2.1) | 13.1 (2.2) | 0.8 |
| TLC (cells/mm3) | 11,911.1 (15,890.3) | 10,630.7 (14,644.0) | 0.7 | 12,920.4 (19,262.6) | 9342.4 (12,812.9) | 0.2 |
| Neutrophils (%) | 71.9 (11.7) | 73.2 (13.6) | 0.6 | 71.5 (6.8) | 70.2 (5.5) | 0.3 |
| Lymphocytes (%) | 21.4 (9.2) | 21.4 (11.8) | 0.9 | 21.6 (6.0) | 6.0 (4.9) | 0.7 |
| Monocytes (%) | 4.2 (2.6) | 3.6 (2.5) | 0.2 | 4.2 (1.9) | 3.4 (1.6) | 0.03* |
| ESR (/1st hour) | 13.9 (5.6) | 24.5 (5.9) | <0.001* | - | - | - |
| Platelets (cells/mm3) | 190,413.1 (73,014.0) | 211,009.3 (72,687.0) | 0.8 | 187,704.5 (73,020.1) | 211,009.3 (72,687.0) | 0.1 |
| PT (s) | 14.4 (7.3) | 13.8 (1.8) | 0.6 | 12.7 (1.4) | 14.2 (13.4) | 0.4 |
| INR | 1.3 (1.3) | 1.1 (0.2) | 0.2 | 1.02 (0.2) | 0.9 (0.1) | 0.4 |
| Ferritin (µg/L) | 276.7 (159.2) | 680.1 (180.3) | <0.001* | - | - | - |
| d-dimer (mg/L) | 1.5 (6.8) | 0.8 (0.1) | 0.5 | - | - | - |
| Sodium (mmol/L) | 136.7 (3.5) | 135.3 (4.4) | 0.1 | 137.7 (2.7) | 137.7 (3.1) | 0.8 |
| Potassium (mmol/L) | 4.7 (4.8) | 4.5 (4.6) | 0.8 | 4.6 (4.5) | 3.9 (0.3) | 0.2 |
| RBS (mmol/L) | 6.0 (7.9) | 6.9 (17.1) | 0.7 | 4.9 (1.1) | 4.6 (0.5) | 0.1 |
| Urea (mmol/L) | 9.1 (7.9) | 11.6 (16.6) | 0.3 | 6.2 (3.2) | 8.2 (7.3) | 0.09 |
| Creatinine (µmol/L) | 182.8 (310.0) | 190.8 (269.8) | 0.8 | 125.1 (101.5) | 192.1 (26.1) | 0.5 |
| Total bilirubin (mg/dL) | 12.9 (6.7) | 16.6 (14.7) | 0.1 | 13.8 (6.8) | 19.1 (23.5) | 0.1 |
| Direct bilirubin (mg/dL) | 2.9 (2.6) | 3.8 (3.3) | 0.1 | 2.6 (1.5) | 6.3 (15.5) | 0.1 |
| AST (U/mL) | 52.3 (50.4) | 60.5 (58.0) | 0.4 | 41.4 (33.9) | 39.9 (16.5) | 0.7 |
| ALT (U/mL) | 48.6 (43.9) | 63.3 (65.6) | 0.2 | 41.9 (10.9) | 44.7 (12.1) | 0.2 |
| Albumin (g/dL) | 35.1 (6.2) | 33.7 (4.6) | 0.2 | 38.2 (10.4) | 35.6 (3.8) | 0.08 |
| Total protein (g/L) | 71.3 (8.4) | 68.3 (12.4) | 0.1 | 73.4 (6.8) | 73.4 (7.2) | 0.9 |
| LDH (U/mL) | 500.3 (215.1) | 583.2 (294.4) | 0.1 | 405.8 (110.3) | 409.2 (109.7) | 0.8 |
| ALP (IU/mL) | 114.7 (99.9) | 119.9 (133.4) | 0.8 | 104.0 (71.7) | 98.1 (61.6) | 0.6 |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ESR, erythrocyte sedimentation rate; g/dL, gram per deciliter; g/L, grams per milliliter; INR, international normalized ratio; IU/mL, international units per milliliter; µg/L, micrograms per liter; mg/L, milligrams per liter; mmol/L, millimoles per liter; LDH, lactate dehydrogenase; PT, prothrombin time; RBS, random blood sugar; SD, standard deviation; TLC, total leukocyte count; U/mL, units per milliliter.
- * Statistically significant; -: not available.
3.4 Diagnostic performance of blood parameters in COVID-19 infection
We performed the ROC analysis of all blood parameters to compare the diagnostic performance of each one in COVID-19. The ROC curve in Figure 1 illustrates the AUC for each parameter. The curves of ferritin, ESR, and d-dimer were prominently observed to cover the highest AUCs (0.919, 0.901, and 0.894), and this finding was statistically significant (p < 0.001 for each), which corroborates the diagnostic superiority of these selected markers in the monitoring of COVID-19 patients. Table 4 displays the AUC values of each blood parameter along with statistical components.
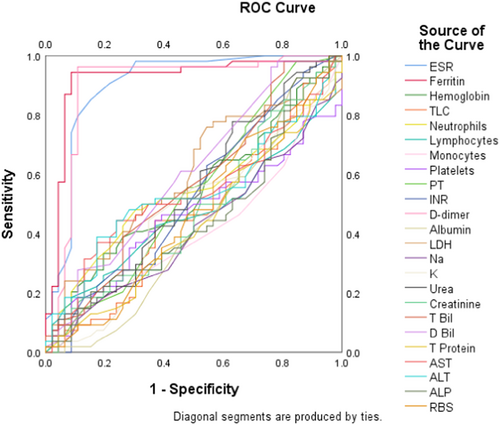
| Test result variable(s) | AUC | 95% CI | p-Value |
|---|---|---|---|
| ESR | 0.901 | 0.933–0.969 | <0.001* |
| Ferritin | 0.919 | 0.855–0.984 | <0.001* |
| Hemoglobin | 0.540 | 0.427–0.645 | 0.5 |
| TLC | 0.462 | 0.348–0.577 | 0.5 |
| Neutrophils | 0.541 | 0.427–0.655 | 0.5 |
| Lymphocytes | 0.470 | 0.356–0.584 | 0.6 |
| Monocytes | 0.410 | 0.298–0.522 | 0.1 |
| Platelets | 0.462 | 0.348–0.576 | 0.5 |
| PT | 0.537 | 0.422–0.653 | 0.5 |
| INR | 0.527 | 0.412–0.642 | 0.6 |
| d-dimer | 0.894 | 0.816–0.972 | <0.001* |
| Albumin | 0.426 | 0.311–0.541 | 0.2 |
| LDH | 0.590 | 0.477–0.702 | 0.1 |
| Na | 0.419 | 0.307–0.530 | 0.1 |
| K | 0.473 | 0.358–0.588 | 0.6 |
| Urea | 0.538 | 0.423–0.653 | 0.5 |
| Creatinine | 0.480 | 0.365–0.594 | 0.7 |
| Total bilirubin | 0.564 | 0.450–0.678 | 0.2 |
| Direct bilirubin | 0.605 | 0.493–0.717 | 0.07 |
| Total Protein | 0.432 | 0.319–0.544 | 0.2 |
| AST | 0.537 | 0.423–0.651 | 0.5 |
| ALT | 0.537 | 0.423–0.652 | 0.5 |
| ALP | 0.453 | 0.338–0.568 | 0.4 |
| RBS | 0.469 | 0.354–0.584 | 0.6 |
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COVID-19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin time; RBS, random blood sugar; ROC, receiver operating characteristic; TLC, total leukocyte count.
3.5 Cox proportional hazard model
Cox proportional hazard model (Table 5) revealed that unvaccinated COVID-19 patients had a higher hazard of longer hospital stay than the vaccinated group (hazard ratio [HR]: 1.20, 95% CI = 1.00–1.043, p = 0.05). After obtaining significant results from the ROC analysis for ferritin, d-dimer, and ESR in COVID-19, we correlated these selected parameters with the length of hospital stay (LOS) and detected a significant positive correlation of ferritin (r = 0.213, p < 0.001) and ESR (r = 0.391, p < 0.001) with LOS. There was no significant correlation of d-dimer with LOS (r = 0.009, p = 0.9), therefore, we excluded it from downstream analysis. The association of ferritin and ESR with LOS was further investigated using a linear regression model which revealed significant results for both ferritin (r2 = 0.045, p = 0.03) and ESR (r2 = 0.153, p < 0.001). Subsequent analysis from the Cox regression model revealed a significantly higher HR for ferritin and ESR elevations with unvaccinated status (HR: 1.01, 95% CI = 1.00–1.013, p = 0.05 and HR: 1.38, 95% CI = 1.10–1.73, p = 0.02) values.
| Factor | Vaccinated | Unvaccinated | p-Value |
|---|---|---|---|
| Higher length of hospital stay | 1 (Ref) | HR: 1.20 (1.00–1.043) | 0.05 |
| Higher ferritin | 1 (Ref) | HR: 1.01 (1.00–1.013) | 0.05 |
| Higher ESR | 1 (Ref) | HR: 1.38 (1.10–1.73) | 0.02 |
- Abbreviations: ESR, erythrocyte sedimentation rate; HR, hazard ratio.
4 DISCUSSION
Our study primarily aimed to evaluate the efficacy measures: length of hospital stay and mortality of vaccine in COVID-19. We found a significantly lower hospital stay duration in the vaccinated group; however, the mortality rate was statistically indifferent. We also compared the levels of different blood parameters with regard to vaccination status. At admission, we found that ferritin and ESR were significantly reduced in the vaccinated group. At discharge, the vaccinated group had a statistically higher monocyte percentage than the unvaccinated group. Our ROC analysis revealed an excellent diagnostic performance of ferritin, ESR, and d-dimer in monitoring COVID-19, however, the latter didn't vary based on vaccination.
Our findings on reduced ferritin levels in vaccinated COVID-19 patients align with the study of Korishetter et al.,22 that compared the biochemical parameters in these patients with respect to vaccination status. Ferritin is an acute phase reactant and is known to contribute to the cytokine storm in COVID-19.23 Association of higher ferritin levels with increased incidence of acute respiratory distress, longer length of hospital stay, severity, and in-hospital mortality has been reported.23, 24 Following vaccination, the reduction in the severity and hospital stay could therefore account for the lower ferritin levels in the vaccinated group. Likewise, as an inflammatory marker, ESR has been found to increase significantly in severe COVID-19, and lower values have been reported in milder illness.25 Ferritin and ESR monitoring can therefore prove beneficial in foreseeing the prognosis of patients with COVID-19. d-dimer showed excellent statistics in the ROC analysis, however, the levels didn't vary in accordance with vaccination status, which was similar to the findings of two similar studies.26, 27 In contrast, d-dimer was found to be significantly higher in the vaccinated population.28 Interestingly, we found higher monocytes in vaccinated patients at discharge, which underlies the mechanism of higher monocyte activation following vaccination in COVID-19.29 Studies have also shown that disease severity scores and the degree of the rise of inflammatory markers are associated with a higher risk of mortality.30
The effectiveness of vaccination in reducing the severity of COVID-19 infection, including the need for hospitalization and death from COVID-19, has now been well-established by numerous studies.11, 31, 32 Our study involved patients hospitalized for COVID-19 during the pandemic; among them, the vaccinated group's average length of stay was significantly shorter than the unvaccinated group's (9.5 vs. 14.9 days) (p = 0.002). Patients in the vaccinated group (60.7%–17/46) had mild COVID-19 at admission, compared to those in the unvaccinated group (39.3%–11/54). Being tertiary care hospitals, ICU occupancy was higher because sicker patients were referred directly, and ICU admissions with high severity were similar between the two groups.
We found a statistically significant reduction in hospitalizations and length of hospital stay after vaccination, consistent with previous studies conducted in various parts of the world.33, 34 Comparative to studies that assessed patients at the community level, our population was primarily made up of multimorbid patients who arrived at the emergency room with COVID-19 symptoms and thus had a high baseline probability of admission. Additionally, our cohort included patients who had received their shots within the first 14 days, according to the literature, during which time the effects of vaccination were still relatively mild. This might have made our findings less clear.
A large number of people in Nepal were exposed to the COVID-19 risk during the second wave of the pandemic. There was an increase in the more recent delta variant (B.1.617.2) of the SARS CoV-2 during this time, which was responsible for about 87% of the COVID-19 cases in Kathmandu and the Northern part of Nepal in May 2021.35 The remainder of the infections were brought on by the lineages B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma), and a few others. Several individuals had contracted the illness despite receiving either a partial or full vaccination. This raised concerns about the effectiveness and defence provided by the vaccines offered in Nepal against this new strain. BNT162b2 (Pfizer) and mRNA1273 (Moderna) are two mRNA vaccines primarily used in the vaccination program in the United States. Clinical trials have already shown remarkably high vaccine efficacy rates in the American population (95% and 94.1% for BNT162b2 and mRNA1273, respectively).36 Covishield and Covaxin were the two vaccines that the vaccination campaign in Nepal began primarily with. Both of them are given intramuscularly twice, separated by 4–12 weeks. It has been demonstrated that Covishield (ChAdOx1 nCoV-19, AZD1222) has an efficacy rate of 70% (95.8% CI: 54.8%–80.6%). The effectiveness of Covaxin (BBV152) against various SARS-CoV-2 variants has been examined in vitro. Currently, 24.1% of Nepal have yet to receive their second dose, compared to 6.2% who have received it.
Since this was not an interventional study and previous infection histories were inconsistently documented, we did not account for baseline COVID-19 serology or prior infection histories. We wouldn't anticipate that many of our patients had prior episodes because the natural infection is linked to a high level of protection. We cannot speculate on the direction of potential confounding because it is also unclear whether prior infection would boost or lower vaccination uptake. Finally, some members of our cohort (between September 30 and December 7) missed their chance to get vaccinated, and as a result, they experienced postvaccination infection. Additionally, they were less likely to carry the B.1.1.7 lineage of infection. One of the limitations of our observational study is that it is only from one site in the country and cannot be called truly representative of the state or country. The study has also not factored in individual patients' various laboratory and imaging modalities to classify the severity of the COVID-19 illness. The study has not looked at the long-term effects of COVID-19 as the follow-up period was short and limited to 1 week after discharge or cure. Further genomic studies are warranted to assess how many infections, especially in cases of vaccine breakthroughs, are due to variant coronavirus strains. The clinical characteristics, hospital course, and short- and long-term outcomes are also of special interest in vaccine-breakthrough infections due to variants.
5 CONCLUSION
COVID-19 vaccination effectively minimized the hospitalization duration in COVID-19, however, there was no significant difference in mortality. Ferritin and ESR were significantly lower in vaccinated COVID-19 hospitalized patients, which advocates the application of these markers in monitoring COVID-19 infections and predicting prognosis. Our findings further show direction for further experimental research on the molecular effects of vaccines on inflammatory response against SARS-CoV-2.
AUTHOR CONTRIBUTIONS
Sangam Shah: Conceptualization, data curation; writing—original draft, writing—review and editing, supervision. Kiran Paudel: Investigation, methodology formal analysis, writing—original draft, writing—review and editing. Abhinav Bhattarai: Investigation; writing—original draft. Sangharsha Thapa: Formal analysis; investigation. Sandesh Bhusal: Writing—review and editing, validation. Yagya R. Adhikari: Investigation; methodology. Tara B. Adhikari: Supervision, writing—review, and editing. Nikita Bhatta: Data curation. Prince Mandal: Project administration. Pratima Sharma: Data curation. Bishal Budha: Investigation. Shova Aryal: Investigation; validation. Santa K. Das: Supervision. Pankaj Pant: Supervision.
ACKNOWLEDGMENTS
No funding was received for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest to disclose.
TRANSPARENCY STATEMENT
The lead author Pankaj Pant affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
All the data required are in the manuscript itself. Data that are not available can be accessed from the corresponding author. All authors have read and approved the final version of the manuscript corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.