Clinical implication of children's depression rating scale-revised score: Linking the children's depression rating scale-revised score and clinical global impression using patients data from clinical trials
Abstract
Background and Aims
The Children's Depression Rating Scale-Revised (CDRS-R) score has been widely used to assess the severity of major depression in children and adolescents; however, the clinical implications of changes in the CDRS-R score remain unclear. We evaluated these clinical implications by assessing the relationship between changes in the CDRS-R score and changes in the Clinical Global Impression of Improvement (CGI-I), in clinical research on major depression.
Methods
We used data from four clinical trials involving two antidepressants and evaluated the relationship between CDRS-R score changes and the CGI-I score using the equipercentile linking method.
Results
CDRS-R score changes corresponding to a minimally improved (score of 3) CGI-I score was approximately 14 points.
Conclusion
Our findings from the linking analyses are useful for interpreting the clinical implications of changes in the CDRS-R score.
Key points
-
Equipercentile linkage analysis was performed using patients data from clinical trials to evaluate the relationship between Children's Depression Rating Scale-Revised (CDRS-R) and Clinical Global Impression of Improvement (CGI-I).
-
The results of this study will be useful in understanding the clinical meaning of a given change in CDRS-R.
-
Changes in the CDRS-R total score corresponding to the minimally improved CGI-I (score of 3) were approximately 14 points.
1 INTRODUCTION
The Children's Depression Rating Scale-Revised (CDRS-R), modeled after the Hamilton Depression Rating Scale (HAMD), is the standard outcome measure for assessing the severity of major depression in children and adolescents; nevertheless, there have been no reports yet on what should be defined as a “clinically relevant change” in the CDRS-R score. In this investigation, we aimed to evaluate “clinically relevant change” in the CDRS-R score by comparing with clinical global impression of improvement (CGI-I), which is a tool for investigating clinical significance of the improvement in psychiatric disorders,1 using the equipercentile linking analyses. There is a general consensus on the definition of “response” (≥30%−50% improvement in the CDRS-R total score), based on previous clinical trials.2 This analysis of the absolute change is significant because most recent clinical trials of pediatric depression have the mean absolute change as the primary endpoint, rather than the percentage change. Previously, the relationship between changes in the CGI and those in the Brief Psychiatric Rating Scale and Positive and Negative Syndrome Scale in the treatment of acute schizophrenia was examined using the equipercentile linking method.3 Moreover, the relationship between changes in the HAMD and those in the CGI in the treatment of major depression was investigated using an empirical method4 and equipercentile linking method.5 Linking analyses between change in CDRS-R and CGI-I can provide magnitude of the change in CDRS-R score corresponds to each CGI improvement level, which will be useful in understanding the clinical meaning of a given change in CDRS-R.
The purpose of this investigation was to evaluate changes in the CDRS-R score corresponding to the “clinically relevant change” in the CGI-I. To know the amount of changes in CDRS-R corresponds to CGI-I could provide better understanding of the clinical implications of CDRS-R score. In this investigation, patients data from four clinical studies of venlafaxine and sertraline in children and adolescents with major depression6, 7 were utilized.
2 MATERIALS AND METHODS
We investigated the “clinically relevant change” in the CDRS-R total score based on the CGI-I score using equipercentile linking.8 The analysis was performed using the SAS program EQUIPERCENT.9 The absolute change from the baseline CDRS-R total score was linked to the absolute value of CGI-I. The CGI-I scores corresponding to changes in the CDRS-R total score from the baseline were plotted for each time point. The bootstrap method10 (SURVEYSELECT procedure and UNIVARIATE procedure in SAS) was used to check the accuracy of the estimation.
We pooled the clinical trial data on the treatment of major depression in children and adolescents, for which Viatris is the marketing authorization holder of the products (sertraline, venlafaxine) and original patient data were available. Data from four multicenter, randomized, double-blind, placebo-controlled clinical studies on the treatment of major depression in children and adolescents (0600B1-382-US, 0600B1-394-US, A0501001, and A0501017) were used for the analyses. To explore the relationship between change in CDRS-R and CGI-I, we conducted equipercentile linking analyses using all the observed data in the four studies (Week 1: N = 674, Week 2: N = 652, Week 3: N = 629, Week 4: N = 613, Week 6: N = 549, Week 8: N = 524, respectively). Table 1 shows details of the clinical studies. Studies 0600B1-382-US and 0600B1-394-US were conducted to evaluate the efficacy and safety of venlafaxine extended release (ER) in outpatients ages 7–17 years with major depressive disorder.6 The designs of these trials were similar and patients were randomized to receive venlafaxine ER or placebo once daily for 8 weeks, followed by a taper period up to 14 days. Studies A0501001 and A0501017 were conducted to evaluate the efficacy and safety of sertraline compared with placebo in treatment of outpatients aged 6–17 years with major depression.7 The designs of these trials were similar and patients were randomly assigned to double-blind receipt of either sertraline or placebo. The double-blind treatment phase was 10 weeks duration. Each of the four clinical trials was a powered study to detect a statistically significant treatment difference in the primary endpoint of CDRS-R compared with placebo and the sample size in each study is shown in Table 1. Further details of these clinical trials including eligibility criteria and study population are described in the previous reports.6, 7 The clinical studies were complied with the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by institutional review board of each center. Written informed consent was taken from all patients. The CGI-I consist of seven rating as follows: 1 = very much improved; 2 = much improved; 3 = minimally improved; 4 = no change; 5 = minimally worse; 6 = much worse; 7 = very much worse.
| Study | Antidepressants | Study title | Target patients | Sample size (n) | Treatment duration (weeks) |
|---|---|---|---|---|---|
| 0600B1-382-US | Venlafaxine | Double-blind, placebo-controlled study of venlafaxine ER in children and adolescents with major depression | Children and adolescents (7–17 years) with major depression | Treatment group: 78 Placebo group: 83 |
8 |
| 0600B1-394-US | Venlafaxine | Double-blind, placebo-controlled study of venlafaxine ER in children and adolescents with major depressive disorder | Children and adolescents (7–17 years) with major depression | Treatment group: 101 Placebo group: 92 |
8 |
| A0501001 | Sertraline | A multicenter 10-week randomized double-blind placebo-controlled flexible dose outpatient study of sertraline in children and adolescents with major depressive disorder | Children and adolescents (6–17 years) with major depression | Treatment group: 93 Placebo group: 88 |
10 |
| A0501017 | Sertraline | A multicenter 10-week randomized double-blind placebo-controlled flexible dose outpatient study of sertraline in children and adolescents with major depressive disorder | Children and adolescents (6–17 years) with major depression | Treatment group: 92 Placebo group: 91 |
10 |
In this investigation, the relationship between CDRS-R and CGI-I was evaluated by the graphical methods based on equipercentile linking, therefore no statistical testing was performed.
3 RESULTS
The demographic characteristics of patients involved in the four integrated studies are shown in Table 2. The mean baseline CDRS-R total score was approximately 60 in both active and placebo groups.
| Number of subjects | Active | Placebo | |
|---|---|---|---|
| N = 354 | N = 344 | ||
| Sex, n (%) | Female | 179 (50.6) | 160 (46.5) |
| Male | 175 (49.4) | 184 (53.5) | |
| Age (years), mean (SD) | 12.1 (2.86) | 12.1 (2.84) | |
| Race, n (%) | Arabic | 2 (0.6) | 0 |
| Asian | 29 (8.2) | 22 (6.4) | |
| Black | 27 (7.6) | 22 (6.4) | |
| Hispanic | 31 (8.8) | 31 (9.0) | |
| Other | 11 (3.1) | 6 (1.7) | |
| White | 254 (71.8) | 263 (76.5) | |
| Height (cm), mean ± SD (n) | 152.59 ± 15.799 (349) | 152.36 ± 16.065 (338) | |
| Weight (kg), mean ± SD (n) | 53.65 ± 20.543 (349) | 53.04 ± 20.452 (337) | |
| Duration of most recent episode (month), mean ± SD | 23.172 ± 23.0449 | 21.807 ± 22.1256 | |
| Baseline CDRS-R total score, mean ± SD | 60.5 ± 10.92 | 60.4 ± 10.79 | |
| Baseline CGI-S score, mean ± SD (n) | 4.5 ± 0.63 (351) | 4.5 ± 0.66 (339) | |
| Baseline HAMD score, mean ± SD (n)a | 18.2 ± 5.65 (166) | 18.2 ± 5.65 (163) | |
| Baseline MADRS score, mean ± SD (n)a | 25.6 ± 6.67 (166) | 25.1 ± 6.16 (163) |
- Abbreviations: CDRS-R, Children's Depression Rating Scale-Revised; CGI-S, Clinical Global Impressions-Severity; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; SD, standard deviation.
- a Data collected from two studies (0600B1-382-US study and 0600B1-394-US study).
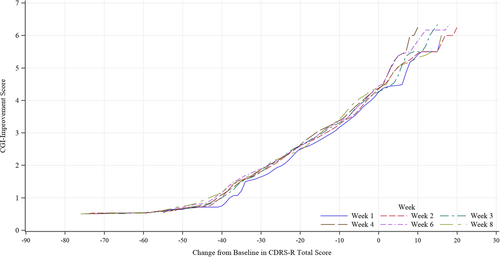
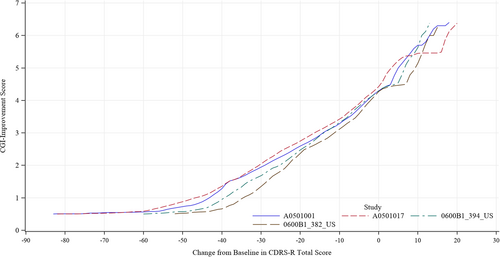
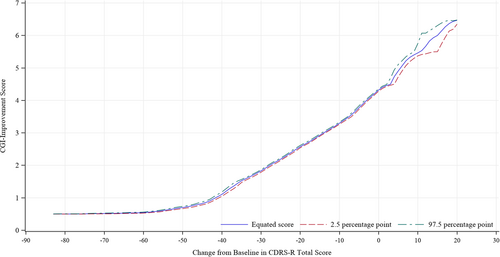
We visually investigated the four trials to determine the relationship between changes in the CDRS-R total score and absolute CGI-I score by constructing graphs using the equipercentile linking for each time point (Figure 1) and each study (Figure 2). The profiles were similar among the time points and four studies; therefore, we integrated the four studies and all time points for analysis (Figure 3). Changes in the CDRS-R total score corresponding to the minimally improved CGI-I (score of 3) were approximately 14 points. The relationship was similar between children and adolescent (Figure 4A,B).
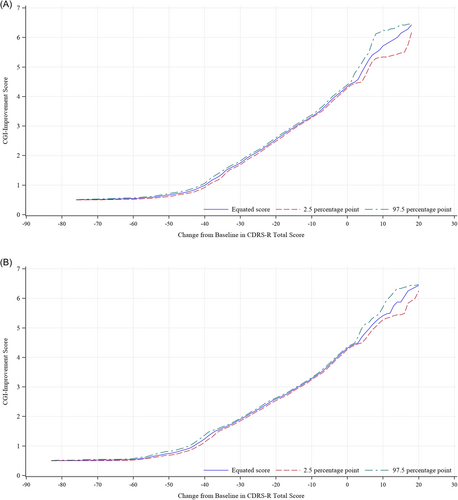
4 DISCUSSION
CGI-I scores are used as evaluation indices of improvement of condition in clinical studies of mental and psychiatric disorders. Using the equipercentile linking method, we examined changes in the CDRS-R score corresponding to “clinically relevant change” in the CGI-I scores.
The present study enables us to better interpret the changes in the CDRS-R in patients with major depression, as well as the results of clinical trials. Although CDRS-R has been used in clinical trials to determine outcome measures for assessing the severity of major depression in children and adolescents, clinically meaningful change in CDRS-R is unclear. Previously, Leucht et al.3 investigated the clinical implication of PANSS and BPRS by conducting equipercentile linking with CGI and reported the absolute reduction of the PANSS and BPRS which corresponded to a CGI change of “minimally improved.” In this study, we aimed to find the magnitude of change in CDRS-R score which is equivalent with each CGI-I score by equipercentile linking. In our equipercentile linking analyses with the data from four clinical trials for major depression in children and adolescents, the change in the CDRS-R score corresponding to the minimally improved CGI-I (score of 3) was around 14 points, suggesting that a reduction of approximately 14 points in CDRS-R is necessary for patients to achieve improvement corresponding to the minimal improvement in CGI. The equipercentile linking analyses by subgroups of children and adolescent showed that this relationship between the change in CDRS-R and CGI-I was almost the same regardless of children and adolescent.
Our findings may also be useful in determining the clinically meaningful difference used in the sample size calculation in subsequent trials. However, it seems to be difficult to expect a 14-point treatment difference of the CDRS-R for antidepressants compared with that with placebo, according to the previous clinical trials that demonstrated the efficacy of antidepressants in children and adolescent patients with major depression. Regarding approved antidepressants for treatment of pediatric depression in the United States, the difference in the change from baseline in the CDRS-R total score between the treatment group and the placebo group after at least 8 weeks of treatment were approximately 9.611 and 7.112 for fluoxetine, approximately 7.613 for combination of fluoxetine with cognitive-behavioral therapy, and 3.414 for escitalopram, respectively. Based on our findings, the magnitude of the treatment difference for fluoxetine and fluoxetine with cognitive-behavioral therapy compared with that for placebo was half or more of the CDRS-R improvement, corresponding to minimal improvement in the CGI. The treatment difference for escitalopram was relatively low among these trials. Generally, some improvement is expected even in the placebo group in clinical trials of patients with major depression. Indeed, the observed changes from baseline of the CDRS-R in placebo groups were around 15 in the above studies, indicating that minimal improvement was achieved in the placebo group. Considering the severity of major depression and its negative outcomes for patients, even small differences in efficacy can be important from a public health perspective.
It should be noted that there are some limitations to the conclusion that can be drawn from the current equipercentile linking analyses. Since we used patients data which were collected from four clinical studies with two antidepressants as treatment groups (venlafaxine and sertraline), our results were obtained from patients with limited characteristics rather than from a wide range of patients with depression. The patients included in these trials were restricted by eligibility criteria. In addition, efficacy of antidepressant was not demonstrated compared with placebo in all the studies. Therefore, it is unclear whether these results can be generalized to different patient populations or patients in real clinical setting. However, the relationship between change in CDRS-R and CGI-I was generally similar regardless of the timepoints and studies in our investigation, supporting the robustness of our finding. Furthermore, our investigation was conducted using large number of patients data (includes approximately 700 patients), which should make the results rather robust.
Despite the limitations, we showed the change in the CDRS-R score corresponding to the “clinically relevant change” in CGI-I scores, using the data from four clinical trials for patients with major depression in children and adolescent. Our investigation should contribute to a better understanding of the clinical meaning of change in CDRS-R.
5 CONCLUSIONS
The clinical implications of changes in the CDRS-R score were interpreted based on the equipercentile linking analysis between CDRS-R score and CGI subscale. A difference of approximately 14 points in the CDRS-R score was found to be equivalent to the minimal improvement in the CGI.
AUTHOR CONTRIBUTIONS
Hiroki Yoshimatsu: Conceptualization; formal analysis; methodology; writing—original draft; writing—review and editing. Takayuki Imaeda: Conceptualization; formal analysis; methodology; writing—original draft; writing—review and editing. Shingo Higa: Funding acquisition; writing—original draft; writing—review and editing. Keisuke Nomoto: Writing—original draft; writing—review and editing.
ACKNOWLEDGMENTS
Conduct of statistical analysis and support on medical writing was provided by EPS Corporation and was funded by Viatris. The authors would like to thank them for their support. This research was sponsored and funded by Viatris. The funder was involved in interpretation of data, writing of the report, and the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Hiroki Yoshimatsu affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Viatris Inc. but restrictions apply to the availability of these data, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Viatris Inc.