Antithrombotic treatment after intracerebral hemorrhage: Surveys among stroke physicians in Scandinavia and the United Kingdom
Abstract
Background and Aims
It is unclear whether patients with previous intracerebral hemorrhage (ICH) should receive antithrombotic treatment to prevent ischemic events. We assessed stroke physicians' opinions about this, and their views on randomizing patients in trials assessing this question.
Methods
We conducted three web-based surveys among stroke physicians in Scandinavia and the United Kingdom.
Results
Eighty-nine of 205 stroke physicians (43%) responded to the Scandinavian survey, 161 of 180 (89%) to the UK antiplatelet survey, and 153 of 289 (53%) to the UK anticoagulant survey. In Scandinavia, 19 (21%) stroke physicians were uncertain about antiplatelet treatment after ICH for ischemic stroke or transient ischemic attack (TIA) and 21 (24%) for prior myocardial infarction. In the United Kingdom, 116 (77%) were uncertain for ischemic stroke or TIA and 115 (717%) for ischemic heart disease. In Scandinavia, 32 (36%) were uncertain about anticoagulant treatment after ICH for atrial fibrillation, and 26 (29%) for recurrent deep vein thrombosis or pulmonary embolism. In the United Kingdom, 145 (95%) were uncertain about anticoagulants after ICH in at least some cases. In both regions combined, 191 of 250 (76%) would consider randomizing ICH survivors in a trial of starting versus avoiding antiplatelets, and 176 of 242 (73%) in a trial of starting versus avoiding anticoagulants.
Conclusion
Considerable proportions of stroke physicians in Scandinavia and the United Kingdom were uncertain about antithrombotic treatment after ICH. A clear majority would consider randomizing patients in trials assessing this question. These findings support the need for such trials.
1 INTRODUCTION
A majority of patients suffering from intracerebral hemorrhage (ICH) have risk factors for ischemic events, and many have suffered such events.1 Consequently, almost half of these patients have an indication for antithrombotic treatment, either antiplatelet or anticoagulant drugs, or use such treatment at the time of the bleeding.2-5 After ICH, the risk of subsequent ischemic events may be at least as high as the risk of recurrent ICH.6-9 However, a recurrent ICH is also highly feared, and it is unknown whether antithrombotic drugs is beneficial in patients with indication for such treatment, in the long term after ICH.
Observational studies have indicated that antiplatelet or anticoagulant treatment after ICH is associated with a lower risk of ischemic events without an increase in the risk of major bleeding.5, 10-12 These studies have a high risk of confounding by indication, and the question is better addressed in randomized controlled trials. RESTART, a randomized trial, indicates that antiplatelet treatment after ICH is safe, because the potential increase in the risk of recurrent ICH is probably too small to exceed the benefits of antiplatelet treatment for secondary prevention.13 These results need to be confirmed by other adequately powered randomized trials. A feasibility trial randomizing ICH survivors with atrial fibrillation to non-vitamin K oral anticoagulants or aspirin, was too small to provide conclusions about anticoagulant drugs after ICH.14 SoSTART, a randomized trial, aimed to establish whether starting oral anticoagulation was non-inferior to avoiding oral anticoagulation for survivors of intracranial hemorrhage who have atrial fibrillation. The rates of recurrent intracranial hemorrhage were lower than expected, and the results were inconclusive.15 Another randomized trial, APACHE-AF, aimed to estimate the rates of nonfatal stroke or vascular death in patients with atrial fibrillation who survive an anticoagulation-associated ICH when treated with apixaban compared with avoiding anticoagulation. They found that the risk of nonfatal stroke or vascular death was high in both groups, and they underline the need of large randomized trials.16 No other randomized trials investigating effects of long-term antithrombotic treatment after ICH have been published.17 The lack of evidence is reflected in the current guidelines, which give weak and varying recommendations.18, 19
The uncertainty regarding this treatment dilemma has been shown in prior surveys in other countries.20, 21 The results indicated a wide variation in clinical practice. To our knowledge, similar surveys have not previously been conducted in Scandinavia or in the United Kingdom. In the preparations of randomized trials of antithrombotic treatment after ICH in these regions, we conducted surveys to assess stroke physicians' opinions regarding antithrombotic treatment after ICH, and their views on randomizing ICH survivors in trials assessing this question.
2 METHODS
2.1 Study design
We conducted web-based surveys among stroke physicians in Scandinavia and the United Kingdom. In Scandinavia, we conducted one survey in 2016 for both antiplatelet and anticoagulant treatment. In the United Kingdom, we conducted two surveys, one in 2011 for antiplatelet treatment and one in 2015 for anticoagulant treatment. The three surveys were customized as preparation for randomized trials (STATICH in Scandinavia, RESTART and SoSTART in the United Kingdom),13, 15, 22 which was the reason for the different approaches. The wording of the questions in the three surveys were different, but we were generally interested to measure the level of uncertainty among stroke physicians regarding this treatment dilemma.
2.2 Participants
In Scandinavia, e-mail invitations were sent to 205 stroke physicians. In Norway and Sweden, stroke physicians were identified through the national stroke registries (Norsk hjerneslagregister and RiksStroke, respectively). In Denmark, we contacted stroke physicians working at centers reporting to the national stroke registry (Dansk Apopleksiregister). E-mail invitations to the UK antiplatelet survey were sent to 180 collaborators in the Third International Stroke Trial (IST-3)23 and the Clots in Legs Or sTockings after Stroke (CLOTS) trial,24 and to all regional leads in the UK Stroke Research Networks. E-mail invitations to the UK anticoagulant survey were sent to all 289 investigators in the Restart or Stop Antithrombotics Randomized Trial (RESTART).25 Up to three email reminders were sent to nonresponders.
2.3 Survey questions
To simplify the presentation of the results, the questions in all three surveys are grouped into three broad parts.
The first part addressed different indications for antithrombotic drugs and whether they affect the treatment decision after ICH. In the Scandinavian survey, we asked if the stroke physicians would start or avoid antiplatelet or anticoagulant drugs in ICH survivors with specific indications, or if they were uncertain. In the UK antiplatelet survey, we asked which specific indications would make the stroke physicians uncertain about starting antiplatelet drugs in ICH survivors. In the UK anticoagulant survey, we asked if the stroke physicians were uncertain about starting anticoagulant drugs in some or all ICH survivors, or if they were not uncertain in any cases.
The second part addressed different ICH locations and whether they affect the treatment decision. In the Scandinavian survey, we asked if the stroke physicians would start or avoid antiplatelet or anticoagulant drugs in patients with lobar supratentorial, deep supratentorial, and infratentorial bleeding, or if they were uncertain. In the UK antiplatelet survey, we asked which of the ICH locations would make the stroke physicians uncertain about restarting antiplatelet drugs. The UK anticoagulant survey did not address ICH location.
The third part addressed stroke physicians' views on randomizing ICH survivors in trials comparing starting versus avoiding antithrombotic drugs. In the Scandinavian survey and the UK antiplatelet survey, we asked if the stroke physicians would randomize ICH survivors with an indication for antiplatelet or anticoagulant treatment in such trials. In the UK anticoagulant survey, we asked if the stroke physicians would consider randomizing ICH survivors in such a trial.
In the Scandinavian survey, we also asked if the stroke physicians would randomize ICH survivors with atrial fibrillation in a trial comparing left atrial appendage occlusion versus best medical treatment. This question was not addressed in the UK surveys. The Scandinavian survey also had an option for free text comments.
3 RESULTS
Eighty-nine of 205 stroke physicians (43%) responded to the Scandinavian survey, 161 of 180 (89%) to the UK antiplatelet survey, and 153 of 289 (53%) to the UK anticoagulant survey.
In the Scandinavian survey of 89 stroke physicians, 82 (92%) were 35 years old or more, 76 (85%) had more than 5 years of experience in stroke medicine, and they were evenly distributed between departments of neurology (45, 51%) and internal medicine (40, 45%). Characteristics of the UK stroke physicians were not collected.
The following results are grouped into the three survey parts mentioned above and reported separately for antiplatelet and anticoagulant treatment. We focus on the level of uncertainty among the stroke physicians regarding the treatment decision.
3.1 Views on antithrombotic treatment in ICH survivors with different indications for antithrombotic drugs
3.1.1 Antiplatelet treatment
In the Scandinavian survey, 19 (21%) were uncertain about antiplatelet treatment in ICH survivors with ischemic stroke or transient ischemic attack (TIA) of non-cardioembolic cause, 21 (24%) were uncertain in ICH survivors with myocardial infarction >1 year ago, 32 (36%) were uncertain in ICH survivors with peripheral arterial disease, 2 (2%) were uncertain in ICH survivors with atrial fibrillation, and 5 (6%) were uncertain in ICH survivors with myocardial infarction with PCI and stenting during the last year (Table 1 and Figure 1A).
| Start n (%) | Avoid n (%) | Uncertain n (%) | |
|---|---|---|---|
| Antiplatelet treatment | |||
| Scandinavia N = 89 | |||
| Ischemic stroke or TIA (of non-cardioembolic cause) | 55 (62) | 15 (17) | 19 (21) |
| Myocardial infarction >1 year ago | 41 (46) | 27 (30) | 21 (24) |
| Myocardial infarction with PCI and stenting during the last year | 80 (90) | 4 (4) | 5 (6) |
| Peripheral arterial disease | 20 (22) | 37 (42) | 32 (36) |
| Atrial fibrillation | 10 (11) | 77 (87) | 2 (2) |
| UK N = 150 | |||
| Ischemic stroke or TIA | 116 (72) | ||
| Ischemic heart disease | 115 (71) | ||
| Peripheral arterial disease | 119 (74) | ||
| Atrial fibrillation | 118 (73) | ||
| Anticoagulant treatment | |||
| Scandinavia N = 89 | |||
| Atrial fibrillation with CHA2DS2-VASc score >1 | 50 (56) | 7 (8) | 32 (36) |
| Recurrent deep vein thrombosis or pulmonary embolism | 60 (67) | 3 (3) | 26 (29) |
| Other cardiac conditions with increased risk of embolisma | 26 (29) | 23 (26) | 40 (45) |
| Mechanical heart valve | 87 (98) | 0 (0) | 2 (2) |
| UK N = 153 | |||
| Some cases | 100 (65) | ||
| All cases | 45 (29) | ||
| No cases | 8 (5) | ||
- Abbreviations: ICH, intracerebral hemorrhage; ITA, transient ischemic attack; UK, United Kingdom.
- a For example, severe dilated cardiomyopathy, left ventricular aneurysm, or other.
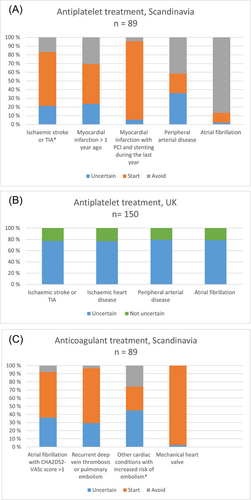
In the UK antiplatelet survey, 116 (77%) were uncertain about antiplatelet treatment in ICH survivors with ischemic stroke or TIA, 115 (77%) were uncertain in ICH survivors with ischemic heart disease, 119 (79%) were uncertain in ICH survivors with peripheral arterial disease, 118 (79%) were uncertain in ICH survivors with atrial fibrillation, (Table 1 and Figure 1B). Eleven skipped this question.
3.1.2 Anticoagulant treatment
In the Scandinavian survey, 32 (36%) were uncertain about anticoagulant treatment in ICH survivors with atrial fibrillation with CHA2DS2-VASc score >1, 26 (29%) were uncertain in ICH survivors with recurrent deep vein thrombosis or pulmonary embolism, 40 (45%) were uncertain in ICH survivors with other cardiac conditions with increased risk of embolism (e.g., severe dilated cardiomyopathy, left ventricular aneurysm, or other), and 2 (2%) were uncertain in ICH survivors with mechanical heart valve (Table 1 and Figure 1C).
In the UK anticoagulant survey, 100 (65%) were uncertain about anticoagulant treatment after ICH in some cases, 45 (29%) were uncertain in all cases. Eight (5%) UK stroke physicians were not uncertain in any cases, which means that 145 (95%) were uncertain about anticoagulant treatment after ICH in at least some cases (Table 1).
3.2 Views on antithrombotic treatment in ICH survivors with different bleeding locations
3.2.1 Antiplatelet treatment
In the Scandinavian survey, 22 (25%) were uncertain about antiplatelet treatment after a lobar supratentorial bleeding, 16 (18%) were uncertain after a deep supratentorial bleeding, and 33 (37%) were uncertain after an infratentorial bleeding (Table 2 and Figure 2A).
| Start n (%) | Avoid n (%) | Uncertain n (%) | |
|---|---|---|---|
| Antiplatelet treatment | |||
| Scandinavia N = 89 | |||
| Lobar supratentorial | 43 (48) | 24 (27) | 22 (25) |
| Deep supratentorial | 64 (72) | 9 (10) | 16 (18) |
| Infratentorial | 43 (48) | 13 (15) | 33 (37) |
| UK N = 161 | |||
| Lobar supratentorial | 141 (88) | ||
| Deep supratentorial | 124 (77) | ||
| Infratentorial | 136 (84) | ||
| No answer | 12 (7) | ||
| Anticoagulant treatment | |||
| Scandinavia N = 89 | |||
| Lobar supratentorial | 43 (48) | 21 (24) | 25 (28) |
| Deep supratentorial | 60 (67) | 6 (7) | 23 (26) |
| Infratentorial | 41 (46) | 11 (12) | 37 (42) |
- Abbreviation: ICH, intracerebral hemorrhage.
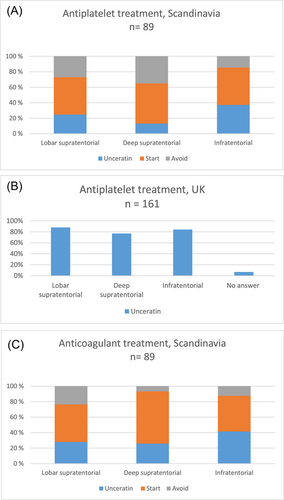
In the UK antiplatelet survey, 141 (88%) were uncertain about antiplatelet treatment after a lobar supratentorial bleeding, 124 (77%) were uncertain after a deep supratentorial bleeding, 136 (84%) were uncertain after an infratentorial bleeding, and 12 (7%) skipped this question (Table 2 and Figure 2B).
3.2.2 Anticoagulant treatment
In the Scandinavian survey, 25 (28%) were uncertain about anticoagulant treatment after a lobar supratentorial bleeding, 23 (26%) were uncertain after a deep supratentorial bleeding, and 37 (42%) were uncertain after an infratentorial bleeding (Table 2 and Figure 2C).
3.3 Views on randomizing ICH survivors in trials comparing starting versus avoiding antithrombotic treatment
3.3.1 Antiplatelet treatment
In the Scandinavian survey, 55 (62%) would randomize ICH survivors in a trial comparing starting versus avoiding antiplatelet treatment, 11 (12%) would not, and 23 (26%) were uncertain (Table 3).
| Antiplatelet treatment versus no antiplatelet treatment | |||
|---|---|---|---|
| Scandinavia N = 89 | Yes n (%) | No n (%) | Uncertain n (%) |
| Would randomize | 55 (62) | 11 (12) | 23 (26) |
| UK N = 161 | Yes n (%) | No n (%) | No answer n (%) |
| Would randomize | 136 (84) | 12 (7) | 13 (8) |
| Anticoagulant treatment versus no anticoagulant treatment | |||
| Scandinavia N = 89 | Yes n (%) | No n (%) | Uncertain n (%) |
| Would randomize | 49 (55) | 14 (16) | 26 (29) |
| UK N = 153 | Yes n (%) | No or no answer n (%) | |
| Would consider randomizinga | 127 (83) | 26 (17) | |
| Left atrial appendage occlusion versus best medical treatment for atrial fibrillation | |||
| Scandinavia N = 89 | Yes n (%) | No n (%) | Uncertain n (%) |
| Would randomize | 58 (65) | 10 (11) | 21 (24) |
- Abbreviation: ICH, intracerebral hemorrhage.
- a Patients with atrial fibrillation and CHA2DS2-VASc score >1.
In the UK antiplatelet survey, 136 (84%) would consider randomizing ICH survivors in a trial comparing starting versus avoiding antiplatelet treatment, 12 (7%) would not, and 13 (8%) skipped this question (Table 3).
3.3.2 Anticoagulant treatment
In the Scandinavian survey, 49 (55%) would randomize ICH survivors in a trial comparing starting versus avoiding anticoagulant treatment, 14 (16%) would not, and 26 (29%) were uncertain (Table 3).
In the UK anticoagulant survey, 127 (83%) would consider randomizing ICH survivors in a trial comparing starting versus avoiding anticoagulant treatment if the patient had atrial fibrillation with CHA2DS2-VASc score >1 (Table 3).
In the Scandinavian survey, 58 (65%) would randomize ICH survivors with atrial fibrillation in a trial comparing left atrial appendage occlusion versus best medical treatment (antithrombotic treatment or not), 10 (11%) would not, and 21 (24%) were uncertain (Table 3).
3.4 Free text comments
In the Scandinavian survey's free text area, many stroke physicians addressed the need for more clinical information than the questionnaires provided. Many also emphasized that the treatment decision must be based on the overall assessment of the individual patient's risk factors, such as age, blood pressure, comorbidities, as well as imaging findings like bleeding size, and the presence of microbleeds.
4 DISCUSSION
We surveyed stroke physicians in Scandinavia and the United Kingdom and found that considerable proportions were uncertain about antiplatelet and anticoagulant treatment after ICH in the standard (and non-compelling) indications for these drugs. In addition, we found that a clear majority of stroke physicians were positive about randomizing ICH survivors in trials assessing effects of antiplatelet or anticoagulant treatment. The current findings demonstrate equipoise about antiplatelet and anticoagulant treatment after ICH before the results of the first phase 2 trials of the effect to start or avoid antithrombotic treatment after ICH. The findings are supported by surveys in other parts of the world that have found wide variations in clinical practice and that the treatment decision is often based on the physicians' own preferences, as well as the patient's individual risk factors.4, 20, 21, 26
More stroke physicians in the United Kingdom than in Scandinavia seemed to be uncertain about antiplatelet treatment after ICH, both in specific indications and in different bleeding locations. These differences might be partially explained by the time gap between the two surveys, but we believe they are best explained by the different wordings of the survey questions. In the UK antiplatelet survey, “uncertain” (yes/no) was the only answer option, which has likely increased the proportion that answered “uncertain” in the United Kingdom compared to Scandinavia.
In Scandinavia, more than half of the stroke physicians would randomize patients in trials of starting versus avoiding antiplatelet or anticoagulant treatment after ICH. The equivalent numbers were even higher in the United Kingdom, which might be explained by the UK national clinical trials strategy to include patients into trials poststroke. In addition, the Scandinavian survey had “uncertain” as an option, which might have reduced the proportion that answered “yes” in Scandinavia compared to the United Kingdom.
One could argue that there is a discrepancy between the percentage of respondents from Scandinavia that were uncertain regarding starting antithrombotic treatment after ICH and the percentage that were willing to include patients into studies. This may reflect that physicians have established their own way of handling this therapeutic dilemma, but they know that the practice is not evidence-based, and therefore they are willing to randomize patients into studies.
The free-text comments in the Scandinavian survey confirm physicians' need for more clinical information about the individual patient's risk factors to guide the decision. Current guidelines recommend that several factors are evaluated, such as age, blood pressure, bleeding location, history of recurrent ICH, presence of cerebral microbleeds, and other signs of cerebral amyloid angiopathy.18, 27 Although lobar ICH and CAA-related ICH are shown to have higher recurrence risk,6, 28 and presence of cerebral microbleeds indicates a higher risk of both ICH and ischemic stroke,29, 30 the impact of these findings on the effects of antithrombotic treatment is not known.31, 32 In our surveys, it seems that stroke physicians were more uncertain about antithrombotic treatment after lobar and infratentorial than after deep bleedings. This may be an argument for including patients with all these intracerebral bleeding locations into randomized trials.
There are strengths of our study. The stroke physicians were unselected and drawn from the national stroke registries in the Scandinavian countries, as well as from the nation-wide trials within the National Institute of Health Research Stroke Research Network in the United Kingdom.33 The Scandinavian participants were experienced stroke physicians. Although we did not collect characteristics of the UK stroke physicians, we believe that the current findings are representative for the views of stroke physicians in these regions. Our study also has limitations. First, different wordings of the questions in the three surveys complicated the comparison of the results. Despite the differences, we believe the results are more valuable coming from several European countries. Second, only about half of the invitees responded to the Scandinavian and the UK anticoagulant survey, which may have introduced selection bias. Third, the questions about anticoagulant treatment did not differentiate between vitamin K antagonists and non-vitamin K antagonist oral anticoagulants, which may have affected the results. Fourth, the surveys were performed some years ago, and three randomized trials on this subject have recently been published.1314, 16 The RESTART trial may have increased the proportion of stroke physicians who would give antiplatelet treatment after ICH.13 Due to small size of NASPAF-ICH and inconclusive results from SOSTART and APACHE-AF, the trials are unlikely to have changed the opinions of stroke physicians regarding choice of treatment, but it might have increased the proportion who would randomize ICH survivors in trials assessing effects of anticoagulant drugs.14-16 Other than that, we believe the current results reflect stroke physicians' main opinions of today.
Current guidelines strongly recommend enrollment of ICH survivors into randomized trials investigating effects of antithrombotic treatment.27 Several such randomized trials are ongoing. STATICH and RESTART-France are currently investigating starting versus avoiding antiplatelet treatment after ICH, STATICH,22 ENRICH-AF,34 ASPIRE,35 PRESTIGE-AF,36 and A3ICH37 are investigating starting versus avoiding anticoagulant treatment for atrial fibrillation after ICH. In addition, STROKECLOSE38 is comparing left atrial appendage occlusion to the best medical treatment in ICH survivors with atrial fibrillation. The current findings support the need for these trials, which will enable physicians to make evidence-based decisions for their patients. The findings that most stroke physicians are positive about randomizing ICH survivors, are reassuring for the progress of these important trials.
5 CONCLUSION
Considerable proportions of stroke physicians in Scandinavia and the United Kingdom were uncertain about antithrombotic treatment after ICH. A clear majority would consider randomizing patients in trials assessing this question. These findings support the need for such trials.
AUTHOR CONTRIBUTIONS
Elisabeth Forfang: conceptualization; data curation; formal analysis; investigation; methodology; project administration; writing – original draft; writing – review & editing. Kristin Tveitan Larsen: writing – review & editing. Rustam Al-Shahi Salman: conceptualization; investigation; methodology; project administration; supervision; writing – review & editing. Simon M. Bell: data curation; formal analysis; investigation; writing – review & editing. Per Wester: conceptualization; investigation; methodology; writing – review & editing. Eivind Berge: conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; writing – review & editing. Torgeir Bruun Wyller: supervision; writing – review & editing. Ole Morten Rønning: conceptualization; investigation; methodology; supervision; writing – review & editing.
ACKNOWLEDGMENTS
This paper is dedicated to the memory of our coauthor Professor Eivind Berge who sadly deceased on February 6, 2020. He had the idea, and planned for this study. We would like to thank all participating physicians for answering the surveys. S. M. B. would like to acknowledge funding from the National Institute of Health Research (NIHR) as he supported by a NIHR clinical fellowship when performing this study. NIHR had no involvement in in study design, performing the surveys, or writing the report.
CONFLICTS OF INTEREST
R. A. S. received funding from the British Heart Foundation, paid to the University of Edinburgh, for the RESTART and SoSTART trials. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Since the surveys collected information about opinions and no clinical data, approvals from ethical committees were not considered necessary. Participants in the surveys were informed about the plan to publish the results.
TRANSPARENCY STATEMENT
The lead author Elisabeth Forfang affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The survey questions are available as a supplement. The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.