Immune profile of healthcare professionals six months after vaccination or exposure to SARS-CoV-2 in Angola
Cruz S. Sebastião and Euclides Sacomboio contributed equally.
Abstract
Background and Aims
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a public health concern. Until 2021, more than 2 million cumulative deaths were reported worldwide. Herein, we investigated the immune profile of healthcare professionals 6 months after vaccination or exposure to SARS-CoV-2 in Angola.
Methods
This was a prospective study conducted with 1068 Angolan healthcare professionals between August and December 2021. Participants were screened for the presence of IgG and IgM against SARS-CoV-2.
Results
About 9.6% and 98.2% of the participants had prior exposure to SARS-CoV-2 or vaccination against it, respectively. Participants aged between 20 and 40 years (11.2%), female (12.4%), with higher educational level (12.8%), from Luanda (60.3%), and nonhealthcare professionals (8.1%) were the most affected by the SARS-CoV-2. Gender, education, and local residence were related to SARS-CoV-2 exposure (p < 0.05). About 7.3% and 98% of the exposed population developed IgM and IgG after 3 months of exposure, respectively. The AstraZeneca vaccine was the most used, followed by the Jonhson & Johnson and Sputinik. Almost all (98%) participants vaccinated with AstraZeneca had immunity >3 months. Individuals who received only the first dose regardless of the type of vaccine had a higher immunity duration (>3 months) than those who received two doses. For individuals who received the Sputnik and Johnson, the average immunity was lower (<3 months), especially among those who were older (over 40 years old) and exposed to SARS-CoV-2.
Conclusion
We observed a high adherence rate to vaccination and a long immunity duration. The immunity duration depended on the type of vaccine. Further studies on the immunity profile in the population exposed to SARS-CoV-2 must be carried out in the general population from Angola to assess antibody-waning periods.
1 INTRODUCTION
Since December 2019, there was worldwide detection of viral infection caused by the new coronavirus, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), initially identified in Wuhan, one of the provinces of China, where the first human infection was reported.1 The infection caused by SARS-CoV-2 had proven to be a real destabilizer of health systems worldwide. Infected individuals may experience fever, cough, nasal congestion, sore throat, diarrhea, or other generally mild symptoms.2-4 Since the beginning of the pandemic and until 2021, more than 110 million cases of SARS-CoV-2 infection and 2 million deaths reported had been related to SARS-CoV-2. During the same period, more than 19,000 cases of SARS-CoV-2 infection and 457 deaths were recorded in Angola.5
Healthcare professionals are at increased risk of infection due to their high exposure to infected people as well as in constant contact with the community, however, once in case a healthcare professional is infected, this may constitute a risk for the individual, co-workers, the healthcare unit environment, and patients, in case the health professional is infected. Therefore, infection control and assessment of the immunization rate against SARS-CoV-2 is necessary for this group which is crucial for combating the coronavirus disease 19 (COVID-19) pandemic.6
Previous studies reported that the incidence of infections by SARS-CoV-2 among health professionals around the world reached about 3.9% of all cases.7-10 Usually, after infection, there is viral replication and production of antibodies such as immunoglobulin M (IgM) and immunoglobulin G (IgG) within a few weeks of SARS-CoV-2 infection.11 These antibodies have been detected in blood samples from infected individuals from the first few weeks after infection or when they present any symptoms related to the infection.7 However, the duration of immunity after exposure to SARS-CoV-2 is still poorly understood and requires further investigation, mainly in resource-limited countries.8, 9 Numerous types of COVID-19 vaccines, including AstraZeneca, Jonhson & Johnson, and Sputinik, were administered to the Angolan population during the year 2021. These vaccines can protect recipients from a SARS-CoV-2 infection by the formation of antibodies and provide immunity against a SARS-CoV-2 infection.10 In general, all vaccines appear to be safe and effective tools to prevent severe COVID-19, hospitalization, and death against all variants of concern, but the quality of evidence varies greatly depending on the vaccines considered and there are still uncertainties about the duration of immunity depending on each vaccine administered.12 Currently, there are no published studies that describe the immunity profile of the population exposed to SARS-CoV-2 or vaccinated in Angola. Therefore, with this study, we intended to identify the immunological memory against SARS-CoV-2 in health professionals who recovered from the SARS-CoV-2 infection in Angola, to provide local and global data capable of determining the time interval of the duration of immunity who have recovered from the SARS-CoV-2 infection and the risk of being infected again in Angola.
2 MATERIAL AND METHODS
2.1 Study design and setting
This was a prospective study conducted with 1068 health professionals from different provinces of Angola, between August and December 2021. The study was carried out in the reference healthcare units of the provinces registered for the examination, namely, Luanda, Bengo, Huambo, Kwanza Norte, Zaire, Benguela, Cabinda, Malange, Cuando Cubango, and Uige. The study was coordinated by the National Institute for Health Research (INIS), located in Luanda. INIS is a public institution of the Angolan Ministry of Health (MoH), which develops research in the most diverse areas of health and its determinants, to contribute to strengthening public health policies in Angola. The study protocol was reviewed and ethically approved by the National Ethics Committee of the Angolan MoH (approval nr. 12/2021). Moreover, informed consent and questionnaires were used to collect sociodemographic information including the type of COVID-19 vaccine administered to all participants. Oral consent was obtained from all study participants before the collection of data and/or biological samples. The collected data (sociodemographic and clinical) were anonymized and used only for this study.
2.2 Sample collection and testing
An estimated volume of 5 mL of blood was collected in a tube containing ethylenediaminetetraacetic acid (EDTA) from all participants and immediately transported to the INIS serology laboratory, where they were subjected to screening for the presence of IgG and IgM antibodies against SARS-CoV-2. Initially, samples were centrifuged to get an aliquot of plasma and stored from 2°C to 8°C, until further analysis. All the sample preparation and processing were performed at the Immunoserology Laboratory of INIS. Qualitative detection of IgM/IgG antibodies against SARS-CoV-2 was performed with enzyme-linked fluorescence assays (ELFA) (bioMérieux SA), commercially available. This serological assay combines a two-step sandwich enzyme immunoassay method able to detect fluorescence at the end of the reaction. All processing was carried out on the mini VIDAS equipment (bioMérieux SA). The samples were processed following the manufacturer's instructions. The results were calculated and all samples with a test value less than one were considered negative, while samples with a value equal to or higher than one were considered positive. Positive and negative control provided by the manufacturer were included in all reactions. A questionnaire was used to collect sociodemographic information as well as the type of COVID-19 vaccine used in Angola.
2.3 Statistical analysis
The analysis was conducted in SPSS version 28 (IBM SPSS Statistics) and the generation of the graphs using SigmaPlot 12.0 (Systat Software, Inc). The descriptive analysis was presented with frequencies and percentages. The normal data distribution was presented as mean and standard deviation (SD). Chi-square (X2) test was performed to analyze the relationship between the qualitative variables. The reported p-value is two-tailed and was deemed statistically significant when p < 0.05.
3 RESULTS
3.1 Sociodemographic characteristics related to SARS-CoV-2 exposure or vaccine status in healthcare
Sociodemographic characteristics related to exposure to SARS-CoV-2 or vaccination status in healthcare professionals in Angola are summarized in Table 1. Of the 1068 professionals included in the study, 9.6% (n = 101/1068) were exposed to SARS- CoV-2, and of these 98.2% (n = 1049/1068) had already been vaccinated. Most professionals were aged between 20 and 40 years (71.1%, n = 546/1068), with an equal ratio of men to women (50% for each gender), with higher educational level (49.6%, n = 530/1068), from Luanda (60.3%, n = 644/1068) and nonclinical staff (53.0%, n = 566/1068). In professionals aged between 20 and 40 years, female, with higher education, from Huambo, Zaire, and Benguela and who worked in the clinical team, the incidence of SARS-CoV-2 was greater than 10%. It was found that there was a significant relationship between gender, educational level, and the province of residence with SARS-CoV-2 (p < 0.05), while age and occupation had no relationship with SARS-CoV-2 exposure (p > 0.05). None of the sociodemographic characteristics was related to vaccination against SARS-CoV-2 (p > 0.05).
| Characteristic participants | N (%) | SARS-CoV-2 exposure | COVID-19 vaccine | ||||
|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | p-value | No (%) | Yes (%) | p-value | ||
| Overall | 1068 (100) | 965 (90.4) | 103 (9.60) | 19 (1.80) | 1049 (98.2) | ||
| Age | |||||||
| <20 y | 5 (0.50) | 5 (100) | 0 (0.0) | 0.186 | 0 (0.0) | 5 (100) | 0.950 |
| 20-40 y | 546 (71.1) | 485 (88.8) | 61 (11.2) | 10 (1.80) | 536 (98.2) | ||
| >40 y | 517 (48.4) | 475 (91.9) | 42 (8.10) | 9 (1.70) | 508 (98.3) | ||
| Gender | |||||||
| Female | 534 (50.0) | 468 (87.6) | 66 (12.4) | 0.003 | 9 (1.70) | 525 (98.3) | 0.817 |
| Male | 534 (50.0) | 497 (93.1) | 37 (6.90) | 10 (1.90) | 524 (98.1) | ||
| Educational level | |||||||
| Basic | 111 (10.4) | 108 (97.3) | 3 (2.70) | <0.001 | 4 (3.60) | 107 (96.4) | 0.250 |
| Medium | 427 (40.0) | 395 (92.5) | 32 (7.50) | 8 (1.90) | 419 (98.1) | ||
| High | 530 (49.6) | 462 (87.2) | 68 (12.8) | 7 (1.30) | 523 (98.7) | ||
| Province | |||||||
| Luanda | 644 (60.3) | 586 (91.0) | 58 (9.0) | <0.001 | 18 (2.80) | 626 (97.2) | 0.337 |
| Bengo | 112 (10.5) | 104 (92.9) | 8 (7.10) | 0 (0.0) | 112 (100) | ||
| Huambo | 79 (7.40) | 69 (87.3) | 10 (12.7) | 1 (1.30) | 78 (98.7) | ||
| Cuanza-Norte | 60 (5.60) | 58 (96.7) | 2 (3.30) | 0 (0.0) | 60 (100) | ||
| Zaire | 54 (5.10) | 47 (87.0) | 7 (13.0) | 0 (0.0) | 54 (100) | ||
| Benguela | 43 (4.00) | 28 (65.1) | 15 (34.9) | 0 (0.0) | 43 (100) | ||
| Cabinda | 38 (3.60) | 36 (94.7) | 2 (5.30) | 0 (0.0) | 38 (100) | ||
| Malange | 23 (2.20) | 22 (95.7) | 1 (4.30) | 0 (0.0) | 23 (100) | ||
| Cuando-Cubango | 13 (1.20) | 13 (100) | 0 (0.0) | 0 (0.0) | 13 (100) | ||
| Uige | 2 (0.20) | 2 (100) | 0 (0.0) | 0 (0.0) | 2 (100) | ||
| Occupation | |||||||
| Clinic staff | 502 (47.0) | 445 (88.6) | 57 (11.4) | 0.075 | 7 (1.40) | 495 (98.6) | 0.371 |
| Non-Clinic staff | 566 (53.0) | 520 (91.9) | 46 (8.10) | 12 (2.10) | 554 (97.9) | ||
- Note: Bold numbers mean that results were statistically significant for Chi-square tests (p < 0.05).
- Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3.2 Immunity duration profile in health professionals in Angola
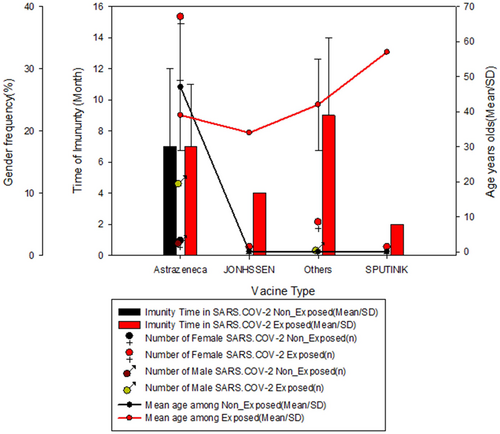
The immunity time profile of health professionals in Angola depending on the type of vaccine, exposure to SARS-CoV-2, infection condition, and the number of vaccine doses are shown in Figures 1-3. About 7.3% and 98% of the exposure population present IgM and IgG after three months of exposure to the virus, respectively. The CoviShield (AstraZeneca) vaccine was the most used, followed by the Jonhson & Johnson (Janssen Pharmaceuticals) and Sputinik (Gamaleya Institute). Almost all (98%) participants vaccinated with AstraZeneca had immunity over 3 months, compared to other vaccines (Results not shown). For individuals vaccinated with Astrazeneca the immunity time did not differ between exposed and unexposed, however, all other vaccines were administered in individuals exposed to SARS-CoV-2, where the longest time of immunity was observed among individuals who received other vaccines that were not the most common (AstraZeneca, Johnson, and Sputnik). No difference was observed between females and males regarding immunity duration, which led us to believe that gender did not influence the duration of immunity (Figure 1).
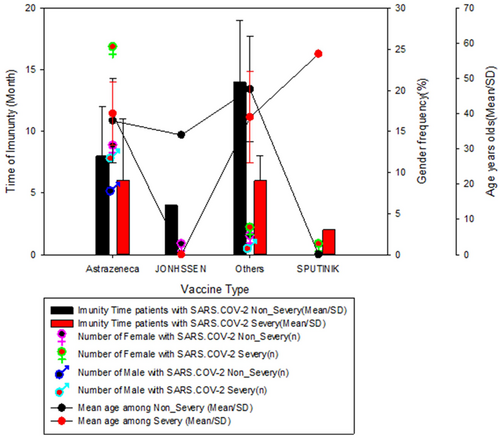
When we evaluated the duration of vaccine immunity among exposed individuals who had non-severe or severe SARS-CoV-2 infection, we found that for all types of vaccines administered, individuals who were exposed to non-severe infection developed longer-lasting immunity (>3 months) than those who developed a severe infection, especially those who had taken/exposed/administered other vaccines. The Johnson vaccine for some reason was only given to female subjects who were exposed to non-severe infection and had shorter immunity time among subjects exposed to non-severe infection, whereas the Sputnik vaccine was only given to males with non-severe disease. Females who were exposed to severe infection showed shorter immunity time between immunity among individuals who were exposed to severe infection. The highest mean age among vaccinates was observed in subjects who received the Sputnik vaccine (57 years), while the lowest mean age was observed in subjects who were vaccinated with the Johnson vaccine (34 years) (Figure 2).
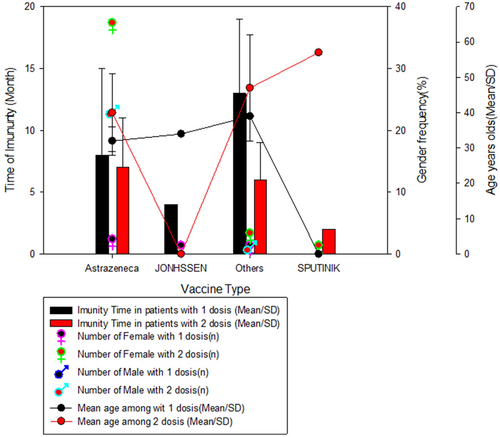
When we evaluated the duration of vaccine immunity in relation to the number of vaccine doses, we noticed that in all the vaccines administered, individuals who received only one dose had a longer immunity time (>3 months) than individuals who received two doses of the vaccine, especially for the other vaccines that were not from AstraZeneca, Jonhson, and Sputinik. As was to be expected, all individuals vaccinated with Johnson received only one dose of the vaccine, however, it was the one with the shortest duration among individuals who took only one dose. A curious fact was that all individuals vaccinated with Sputnik took the second dose, however, a short immunity duration was observed for these participants. These data were independent of gender or mean age between groups (Figure 3).
4 DISCUSSION
To the best of our knowledge, this was the first study to investigate immunological memory in the Angolan population after exposure to the SARS-CoV-2 or vaccination. The high rate of adherence to vaccines against SARS-CoV-2 observed in this population in Angola, might be a reflection of the success of the strategies such as (i) information in different media and (ii) awareness campaigns adopted by the Angolan Ministry of Health, as measures to reduce the spread and mortality of COVID-19 in the Angolan population. These measures also showed positive results in the spread of SARS-CoV-2 infection in low prevalence among the Angolan population residing in different settings, as reported by our research team in previous studies.13-17 A cohort study carried out in Italy, found that about 88% of the population was fully vaccinated which was slightly low compared with the 98% rate of vaccinates observed in our study (Table 1). Also, our study presents about 10% of the unvaccinated population and there were mainly non-healthcare professionals, showing the need to continue the information and awareness-raising strategies mainly targeting the nonmedical population with little knowledge of the different ways of spreading infections in the community. These findings appear to resemble a study developed in the United Kingdom, which also showed a high rate of adherence to measures to contain the spread of SARS-CoV-2 in healthcare professionals, compared to non-healthcare professionals.18 This high rate of adherence to measures to contain dissemination among healthcare professionals could be explained due to the high exposure to the virus that makes this group vulnerable. It can also be explained due to the awareness that in case of infection it can easily spread in the community due to the constant contact with the community.
Important statistically significant relationships were observed in this study between demographic characteristics and exposure to SARS-CoV-2. However, these data differ slightly from the data found in the general population, in a study carried out by our research team in 2020, where age and place of residence were statistically related to SARS-CoV-2 infection (p < 0.05).16 In the previous study, the risk of SARS-CoV-2 infection in the Angolan population was higher in individuals aged 60 years and over (odds ratio [OR]: 23.3 [95% confidence interval [CI]: 4.83–112], p < 0.001), in women (OR: 1.24 [95% CI: 0.76–2.04], p = 0.390), in individuals from Luanda province (OR: 7.40 [95% CI: 1.64–33.4], p = 0.009), and in healthcare professionals (OR: 1.27 [95% CI: 0.60–2.71], p = 0.529) that were now the focus of the present study.16 In the present study, the shortest duration of immunity was verified in individuals who received the Sputnik vaccine. This might be related to the fact that the average age of individuals who received the Sputnik vaccine was close to 60 years (Figure 1). A vaccine effectiveness review study showed that immunity decreased from 1 month to 6 months after complete vaccination by 21.0% (95% CI 13.9–29.8) in all ages and 20.7% (10.2 –36·6) among the elderly.19 We found that the shortest time of immunity was verified in individuals who had non-severe SARS-CoV-2 infection and developed a longer duration of immunity when compared with those who had immunity caused by severe SARS-CoV-2 infection, except for those who received the Jonhson vaccine. Moreover, among those who had a severe infection, the shortest duration of immunity was observed for those who were vaccinated with Sputnik, when compared with those who were vaccinated with AstraZeneca or Jonhson & Johnson vaccines (Figure 2). These findings corroborate the results of the review study evaluating the effectiveness of the vaccine, which verified that for individuals with the symptomatic disease of COVID-19, vaccine efficacy decreased by 24.9% (95% CI 13.4–41.6) at all ages and 32.0% (11.0–69.0) in older adults, among those with severe COVID-19 illness, vaccine efficacy or efficacy decreased by 10.0% (95% CI 6.1–15.4) at all ages and 9.5% (5.7–15.4) 14,6) in the elderly.19
The data of the present research shows that individuals who received until the moment of the study only the first dose, had a higher average immunity duration than individuals who performed the two doses of the vaccine (Figure 3). It is worth mentioning that, a small number of individuals who had the AstraZeneca vaccine and were not exposed to SARS-CoV-2, whereas all the other individuals studied were exposed to the virus (Figure 1). A review study related to the duration of immunity, showed that vaccine-induced protection against SARS-CoV-2 infections increases rapidly after the first dose of vaccines and peaks within 4–42 days after vaccination. The second dose begins to taper off in subsequent months, typically 3–24 weeks. Vaccine-induced antibody response levels varied across different demographic and population characteristics and were higher in people who reported no underlying health conditions compared with those with underlying health conditions, such as immunosuppressed.7
This study has some potential limitations. First, the small sample size limits the robustness of our analyzes as well as their significance. Second, close monitoring to assess other factors that might be promoting or inhibiting immunity time was not evaluated in the studied population, and are subject to further investigation in the future, mainly in resource-limited countries. Finally, genetic, behavioral, and dietary features capable of affecting the immune response were not evaluated. Despite these limitations, this study presents an important estimate of the immune response of the Angolan population to SARS-CoV-2 infection. We cannot forget that the Angolan population has its characteristics, such as genetic, behavioral, and eating habits which could, on the one hand, influence the spread of SARS-CoV-2 in the general population and, on the other hand, affect the immune response and the duration of immunity against infection. These characteristics may explain Angola's success when the low rate of infection and mortality by COVID-19, despite the health limitations typical of a resource-limited country. Therefore, studies involving genetic characteristics, gut microbiome, behavioral and dietary habits related to SARS-CoV-2 infection, and immune response, should be considered for further studies in the Angolan population.
5 CONCLUSION
This study showed that the duration of vaccine-derived immunity depends on the type of vaccine administered. There seems to be no difference in the duration of immunity between the groups of female and male individuals. For individuals who received the Sputnik and Johnson & Johnson vaccines, the average immunity was lower, especially among those who were older (over 40 years) and those who were exposed to severe SARS-CoV-2 infection. Individuals who received only one dose of the vaccine seem to develop longer immunity than those who received two doses. Follow-up studies and constant evaluation of the genetic, behavioral, and dietary features capable of affecting the immune response need to be done to obtain a better picture of the immunity profile of the population during the COVID-19 pandemic scenario.
AUTHOR CONTRIBUTIONS
Cruz S. Sebastião: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; validation; writing – original draft; writing – review & editing. Euclides Sacomboio: Conceptualization; data curation; formal analysis; investigation; supervision; validation; writing – original draft; writing – review & editing. Ngiambudulu M. Francisco: Investigation; writing – review & editing. Edson K. Cassinela: Writing – review & editing. António Mateus: Project administration. Zinga David: Project administration. Joana Paixão: Investigation; project administration; writing – review & editing. Jocelyne Neto de Vasconcelos: Project administration; writing – review & editing. Joana Morais: Conceptualization; project administration; writing – review & editing.
ACKNOWLEDGMENTS
The authors are grateful for the participation of all Angolan healthcare professionals enrolled in the study. Thanks also to the research team of INIS/CISA for the data collection, technical/administrative support, and testing. We also wish to express our gratitude to the Science and Technology Development Project Funding agreement 11/MESCTI/PDCT/2020 for the action entitled Building COVID-19 Response Capacity in Angola. All authors have read and approved the final version of the manuscript. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was reviewed and approved by the National Ethics Committee of the Angolan MoH (approval nr. 12/2021). Moreover, informed consent and questionnaires were used to collect sociodemographic information including the type of COVID-19 vaccine administered to all participants.
TRANSPARENCY STATEMENT
The lead author Cruz S. Sebastião, Joana Morais affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are within the article.