Anion-π Catalysis: Focus on Nonadjacent Stereocenters
Abstract
Anion-π interactions have been recently introduced to catalysis with the idea to stabilize anionic intermediates on π-acidic surfaces. Realized examples include enolate, enamine and iminium chemistry, domino processes and Diels–Alder reactions. Moving on from the formation of contiguous stereogenic centers on π-acidic surfaces, herein we report the first asymmetric anion-π catalysis of cascade reactions that afford nonadjacent stereocenters. Conjugate addition-protonation of achiral disubstituted enolate donors to 2-chloroacrylonitrile generates 1,3-nonadjacent stereocenters with moderate enantioselectivity and diastereoselectivity. The explored catalysts operate with complementary naphthalenediimide and fullerene surfaces with highly positive quadrupole moments and high polarizability, respectively, and proximal amine bases. We find that anion-π catalysts can increase the diastereoselectivity of the reaction beyond the maximal 1:4.0 dr with conventional catalysts to maximal 5.3:1 dr on the large fullerene surfaces. The enantioselectivity of anion-π catalysts, best on the confined naphthalenediimide surfaces with strong quadrupole moment, exceed the performance of conventional catalysts except for comparable results with a new, most compact, surprisingly powerful bifunctional control catalyst. Simultaneously increased rates and stereoselectivities compared to control catalysts without π-acidic surface support that contributions of anion-π interactions to the catalytic cascade process are significant.
Introduction
Complementary to cation-π interactions which have been widely accepted in chemistry and biology for a long time,1-8 anion-π interactions are much younger but are attracting rapidly increasing attention.9-18 Contributions from anion-π interactions have been reported for anion binding,19 sensing,20 transport,21 self-assembly22-25 and, since 2015, also significant catalysis.26 The integration of anion-π interactions into catalysis is based on the idea to stabilize anionic transition states or negatively charged reactive intermediates on π-acidic surfaces, thus decrease the transition state energy and accelerate the rate of the reaction.27 Moreover, by manipulating asymmetric auxiliaries, contributions from anion-π interactions could be expanded from chemoselectivity to stereoselectivity. These have been well exemplified by anion-π catalysis in asymmetric enamine28 and iminium chemistry,29 transamination,30 cascade processes31 and Diels–Alder reactions.32 Furthermore, biotin-streptavidin technology provided access to the first anion-π enzyme.33 Remote control of heterogeneous anion-π catalysis on electrodes by electric fields has been reported as well.34 However, the asymmetric transformations that have been realized with anion-π catalysis so far all generate contiguous stereogenic centers.28 29 31 Moving one step further, the next target was to introduce anion-π catalysis to the formation of more demanding nonadjacent stereocenters in acyclic molecules from achiral precursors.
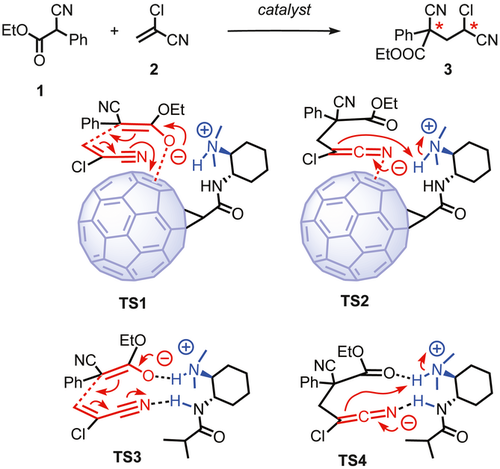
To construct two nonadjacent stereocenters in acyclic molecules, the typical strategy is to apply a multistep process which generates two stereocenters seperately.35 36 Direct creation of both stereocenters from achiral substrates in one step via an asymmetric cascade reaction is fundamentally more efficient and interesting.37 The catalytic domino conjugate addition-protonation reaction involing trisubstituted carbon donors 1 and α-substituted Michael acceptors 2 was attractive for anion-π catalysis not ony because the generated 1,3-tertiary-quaternary stereocenters in product 3 are widely present in numerous natural products38-40 but also because the stabilization of the anionic intermediates on π-acidic surfaces is promising and conceivable (Figure 1). In this report, this reaction is used to probe a series of different types of anion-π catalysts on their potential to expand anion-π catalysis to asymmetric cascade reactions that generate 1,3-nonadjacent stereocenters. The found diastereo- and enantioselectivities exceed those obtained with conventional catalysts, and increased rates, yields and stereoselectivities in the presence of π-acidic surfaces support contributions from anion-π interactions to the catalytic system.
Results and Discussion
To realize the direct formation of 1,3-nonadjacent stereocenters on π-acidic surfaces, catalysts 4 – 13 were prepared from commercially available starting materials following reported procedures (Figure 2).31 41 42 Among them, catalysts 4 – 8 were designed to covalently tether tertiary amine bases to the π-acidic surface of naphthalenediimides (NDIs) via suitable linkers. NDIs provide a privileged platform for anion-π catalysis due to their high intrinsic quadrupole moment perpendicular to the π-acidic surface and easy chemical modification of the substitutions in the core. A suitable linker, i.e., a fixed Leonard turn, is critical to connect a tertiary amine base to the NDI surface because this turn assures that the reaction occurs on the π-acidic surface and benefits from the contribution of anion-π interactions.41 Tertiary amines from classical cinchona alkaloids were also introduced to anion-π catalysts to investigate the influence of different bases on the reaction.
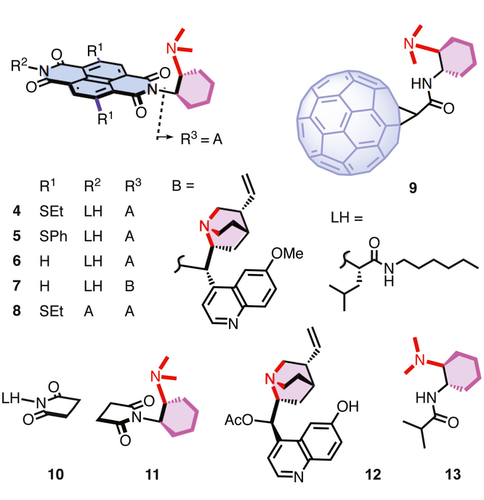
The recently introduced, somehow complementary fullerenes excel in anion-π catalysis because of their high polarizability and localized π holes on their surface.42 Anion-π catalysis on fullerenes is particularly attractive because the use of fullerenes in catalysis on the one hand43-45 and anion-π interactions on fullerenes on the other hand46-49 are both underrecognized general topics that deserve scientific attention. In fullerene anion-π catalyst 9, the preorganization from the chiral cyclohexyldiamine turn was used as in NDI catalysts 4 – 6 to position a tertiary amine base close to the active fullerene surface.42 This positioning has been shown to be essential to turn on anion-π interactions as soon as the negative charge is injected into the substrate by proton transfer to the base.42 The unique activity and selectivity identified so far with model reactions on anion-π fullerene catalyst 9 called for applications to more demanding reactions like the creation on nonadjacent stereocenters envisioned in this study.
Imides 10 and 11 are the fragmental versions of anion-π catalysts with preserved bases from chiral cyclohexyldiamine turns but without π surface. These controls were made for comparison to assess the contributions from anion-π interactions to the reaction. Other control catalysts include the original, conventional cinchona catalyst 12,37 and amide 13 as the fullerene-free version of catalyst 9.42
The reaction of interest employed ethyl 2-cyano-2-phenylacetate 1 as the carbon donor and 2-chloroacrylonitrile 2 as the Michael acceptor (Figure 1). In the presence of 10 mol% of anion-π catalyst 6, the formation of dicyanide 3 occurred within two to five days at ambient temperature (Figures 1 and 2, Table 1). Consistent with previous observations, different solvents influenced the performance of the NDI catalyst 6 significantly (Table 1, entries 1 – 10). More polar solvents naturally reduced activities, whereas aromatic solvents were more interesting because of possible interactions between the π-acidic surface of catalyst and the solvents.31 50 51 In aromatic solvents, the reaction occurred indeed in consistently high yields (Table 1, entries 6 – 10). In the relatively π-acidic solvent C6F6, both yield and stereoselectivity were among the best (Table 1, entry 10). Although differences were very minor, this increase could originate from synergistic anion-π interactions on π stacks of NDI and solvent. In π-basic solvents (Table 1, entries 7 – 9), solvents and NDIs could form inactivating donor-acceptor complexes, which could account for minor decreases in yield and stereoselectivity (Table 1, entries 7 – 9).
| Entry | Cat [mol%] | Solvent | η [%][b] | dr [c] | ee [%][d] |
|---|---|---|---|---|---|
| 1 | 6 (10) | CD2Cl2 | 92 | 0.9:1 | 16 |
| 2 | 6 (10) | CDCl3 | 97 | 1.2:1 | 18 |
| 3 | 6 (10) | CH[e] | 72 | 0.8:1 | 16 |
| 4 | 6 (10) | (D8)THF | 63 | 1.2:1 | 26 |
| 5 | 6 (10) | CD3CN | 51 | 0.5:1 | 14 |
| 6 | 6 (10) | (D7)DMF | Trace | – | – |
| 7 | 6 (10) | Benzene | 82 | 0.9:1 | 18 |
| 8 | 6 (10) | Toluene | 84 | 0.8:1 | 24 |
| 9 | 6 (10) | Mesitylene | 79 | 0.8:1 | 22 |
| 10 | 6 (10) | C6F6 | 87 | 0.9:1 | 24 |
| 11 | 6 (5) | Toluene | 79 | 1.0:1 | 35 |
| 12 | 6 (3) | Toluene | 81 | 1.3:1 | 24 |
| 13[f] | 6 (5) | Toluene | 72 | 0.9:1 | 34 |
| 14[g] | 6 (5) | Toluene | 29 | 0.9:1 | 34 |
| 15[h] | 6 (5) | Toluene | 79 | 1.0:1 | 37 |
| 16 | 4 (5) | Toluene | 91 | 1.4:1 | 19 |
| 17 | 5 (5) | Toluene | 82 | 1.5:1 | 11 |
| 18 | 7 (5) | Toluene | 92 | 1.4:1 | 27 |
| 19 | 8 (5) | Toluene | 75 | 1.5:1 | 16 |
| 20 | 9 (5) | Toluene | 72 | 5.3:1 | −8 |
| 21 | 10 (5) + 11 (5) | Toluene | 28 | 1.3:1 | 19 |
| 22 | 12 (5) | Toluene | 99 | 1:3.8 | −19 |
| 23 | 13 (5) | Toluene | 98 | 3.0:1 | 35 |
- [a] Reactions were performed at room temperature with 0.5 m substrate 1, 2.0 m of substrate 2, the indicated amount of catalyst (in mol%) and monitored by 1H-NMR spectroscopy with dibromomethane as internal standard. [b] NMR yield based on product formation after five days. [c] Diastereoselectivity determined with 1H-NMR spectroscopy of the reaction mixture. [d] Enantiomeric excess of 3. [e] CH = cyclohexane. [f] The reaction was run at 5 °C. [g] The concentration of the reaction was decreased to 0.25 m. [h] 0.5 m Substrate 1 and 1.0 m substrate 2 were used.
Considering both results as well as solubility of all catalysts, toluene was used as the optimal solvent for further investigation. Intriguingly, reducing the loading of catalyst 6 to 5 mol% increased the enantioselectivity to 35% ee and removed all diastereoselectivity to 1:1 dr (Table 1, entry 11). Diastereoselectivity was recovered by decreasing the catalyst loading to 3 mol% (Table 1, entry 12). However, this also resulted in deceleration of the reaction and decreased enantioselectivity. Neither decreasing the reaction temperature nor dilution would positively affect the outcome of the cascade transformation (Table 1, entries 13 and 14).
The reactivity of the catalysts was not much influenced by the variation of the alkyl- and arylsulfide substituents at the edge of NDI surface in 4 and 5, respectively (Table 1, entries 16 and 17). This was reasonable because the π acidity of the NDI surface was not significantly changed by these weak donors.52 53 However, the diastereoselectivity of the reaction was improved to 1.5:1 after introducing two phenylsulfide substituents in the NDI core of 5, which implied that steric hindrance around the active NDI surface plays a non-negligible role (Table 1, entry 17).
Modification of the tertiary amine part of the catalyst was studied next (Table 1, entries 18 – 20). The cinchona-alkaloid fusion catalyst 7 was thought to be quite interesting in this transformation for two reasons: 1) cinchona derivatives have been reported to catalyze this reaction with moderate to good diastereoselectivity and enantioselectivity;37 2) we have previously shown that anion-π cinchona fusion catalysts exhibit excellent reactivity and stereoselectivity in cascade transformations.31 However, anion-π cinchona fusion catalyst 7 failed to change the outcome of this conjugate addition-protonation cascade reaction significantly (Table 1, entry 18).
Catalyst 8 bearing two tertiary amines on both sides of the π surface was examined as well. Compared to the original catalyst 4 with one tertiary amine and one amide, the diamine catalyst 8 gave similar diastereoselectivity but decreased enantioselectivity (Table 1, entry 19).
Tertiary amine 10 and amide 11 were prepared as negative controls to probe for contributions from anion-π interactions. Used together, the combination of 10 and 11 could structurally mimic NDI catalysts as precisely as possible except for the π-acidic surface. Compared to NDI catalyst 6, the reaction catalyzed by 10 and 11 was much slower, and only 28% yield of product was obtained after five days (Table 1, entry 21). In terms of the diastereoselectivity, all the anion-π catalysts except 6 without substituents in the NDI core gave a slightly better dr compared to controls 10 and 11. For the best anion-π catalysts, enantioselectivities nearly doubled compared to the 19% ee of controls 10 and 11.
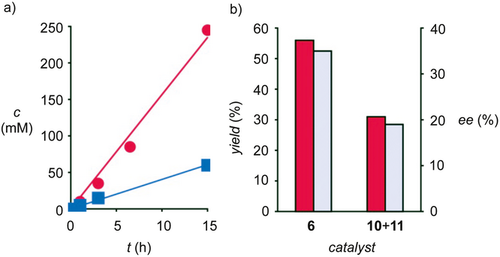
Consistent with previous observations, contributions from anion-π interactions to the reaction were evidenced by a rate enhancement of v/v0 = 3.9 (Figure 3,a). This rate enhancement corresponded to a transition-state stabilization of ΔEa = −3.3 kJ mol−1. Such a transition-state stabilization was within expectations for isolate contributions from anion-π interactions of intermediate strength. For instance, enolate-π interactions up to 13.5 kJ mol−1 have been determined quantitatively in covalent model systems of systematically increasing π acidity.54 Corroborative support for operational anion-π interactions was secured by interrupting the reaction after 40 h, showing much better yield and enantioselectivity in the presence of anion-π catalyst 6 compared to controls 10 and 11 (Figure 3,b). Such increasing enantioselectivity with increasing transition-state recognition is one of the hallmarks of catalysis33 and supports significant contributions of anion-π interactions also to the stereoselective formation of nonadjacent stereocenters by conjugate addition-protonation cascade reactions.
The positive cinchona-alkaloid control 12 has been reported to catalyze this cascade transformation in good yield and stereoselectivity.37 For comparison, this control catalyst was prepared and examined. The reported 1:4.0 dr was with 1:3.8 dr successfully reproduced (Table 1, entry 22). The major diastereomer obtained under optimized conditions with all anion-π NDI catalysts was opposite to the major diastereomer obtained with conventional catalysts (Table 1, entries 11, 12, and 16 – 20). This inversion of stereoselectivity was found also for the anion-π cinchona fusion catalyst 7 with 1.4:1 dr (Table 1, entry 18). Compared to the original control 12, this fusion catalyst 7 not only contains an additional π surface for contributions from anion-π interactions but also an inverted absolute configuration of the exocyclic stereogenic carbon and a methoxy substituent on the quinoline. The observed inversion of diastereoselectivity should thus not be overinterpreted. For comparison, the more precise addition of a π-acidic surface to controls 10 and 11 influenced the enantioselectivity of the resulting anion-π catalysts much more than their diastereoselectivity. Indeed, with anion-π catalysts, this preferred diastereomer was obtained under standard conditions with 35% ee for the best performing 6 or 27% ee for the anion-π cinchona fusion catalyst 7 (Table 1, entries 11 and 18). Like negative control catalysts 10 and 11 with 19% ee, the positive cinchona control 12 produced the same diastereomer with a much lower −19% ee and a stereoselectivity for the other enantiomer (Table 1, entries 22 and 23).
With anion-π fullerene catalyst 9, the diastereoselectivity could be significantly improved to 5.3:1 dr (Table 1, entry 20). This diastereoselectivity was significant because it exceeded not only the 1.5:1 dr obtained at best with anion-π NDI catalysts (Table 1, entry 19) but also the record 1:4.0 dr reported37 and 1:3.8 found for conventional catalysts clearly (Table 1, entry 22). The enantioselectivity of anion-π fullerene catalyst 9, however, was with −8% ee existing but not competitive (Table 1, entry 20).
The comparison of the anion-π fullerene catalyst 9 with the fullerene-free but otherwise identical control 13 turned out to be most intriguing. Namely, upon removal of the fullerene, diastereoselectivity decreased by almost 50% from 5.3:1 dr to 3.0:1 dr, whereas enantioselectivity inverted and increased from −8% ee to an outstanding 35% ee with regard to previous results on this reaction (Table 1, entry 23 vs 20). The presence of the fullerene thus clearly influenced catalyst performance. Increasing diastereoselectivity to a new record of 5.3:1 dr was consistent with the possible stabilization of the anionic transition state TS1 of the conjugate enolate addition and the resulting azaallenyl anion for protonation in TS2 by anion-π interactions on the fullerene surface (Figure 1). This result thus supported42 the promise of the large and polarizable fullerene surface to control selectivity, here diastereoselectivity. The high enantioselectivity found without fullerene implied that control 13 acts as an intriguing, most compact chiral bifunctional catalyst with hydrogen-bond donors stabilizing enolate/ester and cyano/azaallenyl acceptors in pseudo-macrocyclic TS3 and TS4 (Figure 1). This operational bifunctionality of control 13 was supported by the much less pronounced 19% ee found with control 11 that lacks the hydrogen-bond donors next to the tertiary amine base (Table 1, entries 21 and 23). Increased diastereoselectivity and poor, inverted enantioselectivity upon addition of the fullerene to control 13 supported that the bulky, flat π surface in 9 hinders hydrogen bonding to the amide donor in the more enantioselective pseudo-macrocyclic TS3 and TS4 and instead offers face-selective stabilization by anion-π interactions to this large and polarizable π surface in the more diastereoselective TS1 and TS2 (Figure 1). Competition from less favorable transition states with high but inverted enantioselectivity and reduced face-selectivity analog to pseudo-macrocyclic TS3 and TS4 might explain the surprisingly poor enantioselectivity with anion-π fullerene catalyst 9 and implies how to further improve both enantioselectivity as well as diastereoselectivity in the future.
Conclusions
In summary, this report expands anion-π catalysis to include the first example for the direct formation of the more challenging nonadjacent stereocenters on π-acidic surfaces. Consistent with the contribution from anion-π catalysis to other transformations,31 41 42 rate, diastereoselectivity and enantioselectivity of the selected cascade process to nonadjacent stereocenters increase in the presence of a π-acidic surface in the catalyst. These consistent trends provide experimental support for operational anion-π interactions. The diastereoselectivity obtained by anion-π catalysis on large and polarizable, face-selective but achiral fullerene surfaces exceeds the performance of conventional catalysts for the installation on nonadjacent stereocenters. This result supports the emerging power of anion-π catalysis to control chemo- and stereoselectivity, particularly with regard to the selective acceleration of disfavored pathways on large, polarizable π surfaces. In clear contrast, the enantioselectivity obtained with anion-π catalysts for the installation on nonadjacent stereocenters was naturally best on smaller, confined π surfaces with strong quadrupole moments. They exceed the performance of conventional catalysts under identical conditions except for comparable results found with a new, most compact bifunctional control catalyst that might deserve further attention in the future. With regard to anion-π catalysis, our current interests mainly focus on synergistic anion-π interactions and the integration of this unorthodox interaction into more complex systems on the one hand and ring-forming cascade reactions of increasing length on the other hand. Studies related to these areas are ongoing and will be reported in due course.
Experimental Section
Acknowledgements
We thank the NMR and the MS 2.0 platforms for service, and the University of Geneva, the Swiss National Centre of Competence in Research (NCCR) Chemical Biology, the NCCR Molecular Systems Engineering and the Swiss NSF for financial support.
Author Contribution Statement
X. Z. and L. L. contributed equally to this work. X. Z., L. L., N. S., and S. M. conceived this work, designed the experiments, discussed the results, and wrote the manuscript. X. Z., L. L., J. L.-A., and C. W. synthesized the catalysts, X. Z. and L. L. measured catalysis.




