The effects of sleep on the neural correlates of pattern separation
Abstract
Effective memory representations must be specific to prevent interference between episodes that may overlap in terms of place, time, or items present. Pattern separation, a computational process performed by the hippocampus, overcomes this interference by establishing nonoverlapping memory representations. Although it is widely accepted that declarative memories are consolidated during sleep, the effects of sleep on pattern separation have yet to be elucidated. We used whole-brain, high-resolution functional neuroimaging to investigate the effects of sleep on a task that places high demands on pattern separation. Sleep had a selective effect on memory specificity and not general recognition memory. Activity in brain regions related to memory retrieval and cognitive control demonstrated an interaction between sleep and delay. Surprisingly, there was no effect of sleep on hippocampal activity using a group-level analysis. To further understand the role of the hippocampus on our task, we performed a representational similarity analysis, which showed that hippocampal activation was biased toward pattern separation relative to cortical activation and that this bias increased following a delay (regardless of sleep). Cortical activation, conversely, was biased toward pattern completion and this bias was preferentially enhanced by sleep.
1 INTRODUCTION
Declarative memories for unique episodes involve memory for what happened when and where. Accordingly, effective memory representations must be specific to prevent interference between episodes that may overlap. The ability to store and retrieve declarative memories depends heavily on the medial temporal lobe (MTL), and particularly on the hippocampus (Squire, Stark, & Clark, 2004). The hippocampus is thought to employ the computational process of pattern separation to accomplish the task of forming unique memory representations (Norman & O'Reilly, 2003; Schacter, Norman, & Koutstaal, 1998; Treves & Rolls, 1994). Pattern separation involves creating distinct, minimally overlapping memory representations thus allowing the successful retrieval of specific details of previously encountered stimuli and discriminating between similar representations with minimal interference. Researchers have proposed that the structure of the hippocampus uniquely biases it to perform pattern separation (Norman & O'Reilly, 2003; Schacter et al., 1998; Yassa & Stark, 2011; Yassa et al., 2011).
Predictions of computational models regarding pattern separation have been widely studied using animal models (Clelland et al., 2009; Leutgeb, Leutgeb, Moser, & Moser, 2007) and functional magnetic resonance imaging (fMRI) methods in humans (Bakker, Kirwan, Miller, & Stark, 2008; Kirwan & Stark, 2007; Yassa & Stark, 2011). Consistent with computational models (Norman & O'Reilly, 2003) these studies indicate that the dentate gyrus (DG) and CA3 subregions of the hippocampus are the main sites for the computations necessary for pattern separation to occur while the CA1 subregion and MTL cortex are biased toward the complementary process of pattern completion. Additionally, computational models suggest that the hippocampus plays back representations to the cortex during sleep to facilitate consolidation (Norman, Newman, & Perotte, 2005). Behaviorally, sleep has been associated with overcoming interference that occurs during normal daytime activities (Gaab, Paetzold, Becker, Walker, & Schlaug, 2004) and when interference is introduced explicitly during memory testing (Ellenbogen, Hulbert, Stickgold, Dinges, & Thompson-Schill, 2006)
Sleep has a strengthening effect on memory and learning, likely through enhanced consolidation. Indeed, sleep improves memory whether the interval of sleep is eight hours or just six minutes long (Diekelmann & Born, 2010), and has been found to improve consolidation of declarative memories (Ellenbogen et al., 2006; Jenkins & Dallenbach, 1924; Maquet et al., 2000) and nondeclarative memories (Walker & Stickgold, 2004). However, whether sleep has an enhancing effect depends on the type of task utilized in the study (Daurat, Terrier, Foret, & Tiberge, 2007; Drosopoulos, Wagner, & Born, 2005). In spite of extensive literature on sleep-dependent consolidation and the burgeoning literature on pattern separation in humans, there is little data bearing on how sleep affects performance on a task that taxes pattern separation. Our first aim, therefore, was to test whether or not sleep improves behavioral performance on such a task.
Although much has been done using high-resolution fMRI to study the role of the MTL and hippocampal subregions in pattern separation processing (Bakker et al., 2008; Doxey & Kirwan, 2015; Suthana et al., 2015; Yassa et al., 2010), previous studies have not obtained high-resolution fMRI of the whole brain to study pattern separation in the context of the entire brain. Obtaining high-resolution functional images is problematic and typically requires decreased acquisition volume (i.e., field of view) or longer acquisition times. Multi-band fMRI (Feinberg et al., 2010; Moeller et al., 2010; Yassa et al., 2011) overcomes these challenges by acquiring multiple slices simultaneously instead of acquiring the traditional one slice at a time. This greatly reduces acquisition time and allows for acquiring whole-brain, high-resolution scans. We used multi-band imaging to investigate the neural activation associated with pattern separation processing simultaneously in hippocampal subregions and in the cortex. In particular, we were interested in how sleep affected activity patterns in the hippocampus relative to sleep-related activation differences in cortical areas associated with memory encoding and retrieval. We hypothesized that activation in the hippocampus would favor pattern separation processing while activation in the cortex would be more consistent with pattern completion. Following a 12-hr delay, we further hypothesized that this difference would be further exaggerated, particularly following sleep-dependent consolidation.
2 METHOD
2.1 Participants and behavioral testing
We tested 52 participants using a variant of a mnemonic discrimination task that taxes pattern separation processes (Doxey & Kirwan, 2015; Holden, Toner, Pirogovsky, Kirwan, & Gilbert, 2013; Kirwan & Stark, 2007; Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa et al., 2011). Data from six participants were excluded from MRI analyses due to equipment failure or failure to follow instructions. In total, data from 46 participants were used in subsequent analyses (28 female; mean age = 22.9; SD = 2.4; range 18–28). The local Institutional Review Board approved all research protocols and participants gave written informed consent prior to participation.
Participants performed separate study and testing blocks (see Figure 1). Participants first studied images of random every-day objects outside the MRI scanner. Stimuli were presented using E-Prime (v. 2.0) for 2,000 ms each with an inter-stimulus interval of 500 ms. Participants were asked to respond, via button press, according to whether the object viewed is typically found indoors or outdoors. Participants were additionally instructed to pay close attention to the pictures because their memory would be tested in detail later. This phase consisted of 3 blocks of 131 stimuli each, for a total of 393 stimuli.
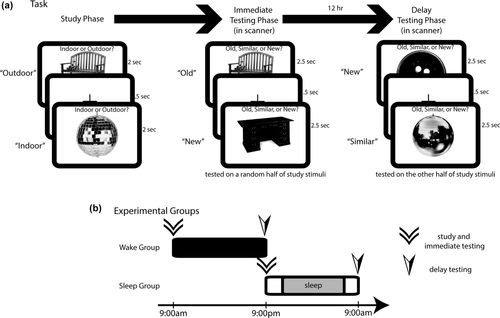
Depiction of the study protocol. (a) Participants first completed a study portion in which they were shown random pictures of everyday objects and asked to make a judgment as to whether the object was typically found indoors or outdoors. They were then immediately tested on half of the studied stimuli, and tested again 12 hr later on the other half of studied stimuli. Here, participants indicated whether the tested stimulus was a repeat (“old”), lure (“similar”), or novel (“new”). (b) Participants were divided into two groups—wake and sleep. Those in the wake group performed study and immediate testing phases in the morning and were asked to go about their normal daily activities (without napping) before returning in the evening for the delay test. Those in the sleep group performed study and immediate testing phases in the evening and were asked to get a normal night's sleep before returning the following morning
Following the study phase, participants engaged in an immediate testing phase in the MRI scanner (approximately 10 min delay between completion of the study phase and beginning the immediate test phase). Participants returned to the scanner 12 hr later for a delay testing phase (see below). The stimuli for the two testing phases consisted of exact repeats of stimuli displayed in the study phase (Repeats), images that were visually and semantically similar to those presented in the study phase (Lures), and novel images (Foils). Half the stimuli from the study phase were randomly assigned to be tested as repeated/lure stimuli in each of the immediate and delayed test conditions. While being scanned with fMRI, participants were asked to respond to Repeats as “old,” Lures as “similar,” and Foils as “new” via button press using an MRI-compatible button box. Each testing phase consisted of 3 blocks with 109 stimuli per block. There were a total of 58 Repeat, 108 Lure, and 161 Foil items in the immediate phase, while the delay phase consisted of 52 Repeat, 112 Lure, and 163 Foil items. Stimuli were presented in a pseudorandom order, and the blocks within each phase were randomized.
Half the participants were randomly assigned to the sleep group (n = 24) and the remaining half (n = 23) were assigned to the wake group (see Figure 1b). Those in the sleep group began the study phase at 9:00 p.m. and were asked, on completion of the immediate test, to return the following morning at 9:00 a.m. for the delay test. Participants in the sleep group were instructed not to alter their sleep habits but to obtain a “normal night's sleep” between the immediate and delay test phases. We did not collect sleep histories for participants; however, all participants were randomly assigned to either the sleep or wake group to counteract any systematic differences in sleep history across groups. The wake group completed the study and immediate phases beginning at 9:00 a.m. and was asked to go about their normal activities (without napping) during the day and to return the following night at 9:00 p.m. for final testing. Each participant was given an ActiGraph accelerometer to monitor sleep/activity and asked to wear it during the entire 12-hr delay. The sleep group reported a mean sleep time of 427 minutes (SD = 42 min). The wake group was asked not to nap during the day, and only one participant reported having taken a nap. Results were not affected by removing this participant, so these data were not excluded from the analysis. Sleep duration and sex were included as covariates in our analyses.
2.2 MRI acquisition and processing
2.2.1 Scan parameters
All MRI scans were performed on a Siemens 3T Tim Trio scanner using a 32-channel head coil at the BYU MRI Research Facility. Standard-resolution structural images were acquired using a T1-weighted MPRAGE sequence with the following parameters: TR = 20 ms; TE = 4.92 ms; slices = 192; flip angle = 25°; FOV = 256 × 256 mm; matrix size = 256 × 256; slice thickness = 1 mm; voxel size = 1.0 × 1.0 × 1.0 mm. We also acquired high-resolution T2-weighted structural images to visualize hippocampal subfields. These scans had high in-plane resolution which was oriented perpendicular to the long axis of the hippocampus to give the best view for segmenting hippocampal subfields. These scans used the following parameters: TR = 6,000 ms; TE = 64 ms; slices = 35; flip angle = 129°; FOV = 200 × 200 mm; matrix size = 512 × 512; slice thickness = 2 mm; voxel size = 0.39 × 0.39 × 2.0 mm, FOV = 256 mm. While participants performed the task, we tracked blood oxygen level dependent (BOLD) activity using a multi-band EPI sequence with the following parameters: multi-band factor = 8; TR = 875 ms (374 TRs); TE = 43.6 ms; slices = 72 interleaved; voxel size = 1.8 × 1.8 × 1.8 mm; FOV = 180 × 180 mm; flip angle = 55°; total acquisition time = 5:30 min. Slices were acquired parallel to the long axis of the hippocampus and the volume was positioned to cover the entire cortex. The first five volumes acquired were discarded to allow for T1 equilibration.
2.2.2 MRI data preprocessing and first level analysis
Imaging data were analyzed using the Analysis of Functional Neuroimages (AFNI) suite of programs (Cox, 1996). Standard-resolution structural images were coregistered to the functional scans. Functional data scans were corrected for incidental head motion and aligned across scanner runs. TRs in which there was a significant motion event (> .6 mm translation and/or > .3° rotation) and those that immediately preceded or followed the motion event were excluded from further analysis. Spatial normalization for group analyses was done using the Advanced Normalization Tools (ANTs) (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2009), which uses diffeomorphic mapping to calculate a transformation from individual structural scans to a model template based on voxel intensities.
Behavioral vectors were created that coded for response types of interest in the first level regression analysis. Vectors coded for trials where participants correctly identified a Foil as “new” (correct rejection or CR), correctly identified a Repeat as “old” (Hit), correctly identified a Lure as “similar” (Lure Correct Rejection or LureCR), and incorrectly identified a Lure as “old” (Lure False Alarm or LureFA). All other possible responses to stimuli were included in the first level regression model but not included in further analyses. Half the CR trials were randomly assigned to serve as the functional baseline in fMRI analyses. A similar baseline has been used before when performing fMRI investigations of pattern separation processes (Bakker et al., 2008; Motley & Kirwan, 2012). The fMRI model also included vectors that coded for scan run, scanner drift, and motion, which included three rotational vectors (pitch, yaw, and roll) and three translational vectors (x, y, and z). The resulting beta coefficients were blurred using a 4 mm FWHM Gaussian blur, were normalized to the ANTs template, and then were entered into group-level analyses as described below. All analyses utilizing anatomical regions of interest were conducted in subject-specific space (i.e., were not normalized to the ANTs template). Voxel-wise analyses were corrected for multiple comparisons using the AFNI 3dClustSim program, which uses Monte Carlo simulations to calculate the appropriate clusters of voxels that are large enough to be statistically significant (Forman et al., 1995; Xiong, Gao, Lancaster, & Fox, 1995). Using a voxel-wise threshold p-value of < .005 the calculated minimum cluster threshold was 42 voxels for the whole-brain analysis.
Hippocampal subfields were identified on the high-resolution T2-weighted scans based on the atlas of Duvernoy (1998) similar to our previous research (Kirwan, Shrager, & Squire, 2009; Kirwan & Stark, 2007; Bakker et al., 2008). Subregion masks were then coregistered to the functional data and resampled to match the functional data resolution.
2.2.3 Representational similarity analysis
To understand the differential effects of sleep on activity patterns in the hippocampus, we created a hippocampal mask by segmenting our ANTs normalized template via the Joint Label Fusion toolkit, referencing OASIS-TRT-20 priors (A. Klein & J. Tourville, 2012; Klein et al., 2017; Tustison et al., 2014; Wang et al., 2013), thereby producing a set of 103 cortical and subcortical masks in template space, labeled according to the Desikan-Killiany-Tourville protocol. Further, cortical gray-matter masks sans the MTL (GMxmtl) were constructed by stitching together the various cortical labels via c3d (Yushkevich et al., 2006), while the label corresponding to the left and right MTL (1,015 and 2,015, respectively) were used as gray-matter MTL masks (GMmtl). Finally, anterior (head) and posterior (body and tail) hippocampal masks were constructed, where all hippocampal tissue anterior of Y-coordinate = −20 (in MNI ICBM 152 space) was considered “head” and with the rest of the tissue considered “body and tail,” roughly corresponding to the posterior aspect of the uncus in the coronal view. These masks were back-transformed into subject space using individual ANTs transformations, and were then resampled to the resolution of the functional scans. Representational similarity was calculated for Foils-Lures, Foils-Repeats, and Lures-Repeats comparison pairs by calculating the voxel-by-voxel correlations of activation for hippocampal voxels associated with each pair. Correlation coefficients were z-transformed and averaged across all hippocampal voxels to obtain a representational similarity score for each participant.
3 RESULTS
3.1 Behavioral results
We calculated pattern separation scores, or corrected Lure correct rejections (LureCR), as the proportion of “similar” responses to lure items and correcting for a “similar” response bias using the following formula: p(“similar” lure) – p(“similar” novel). We also calculated recognition memory scores, or corrected Hits, as the proportion of “old” responses to repeat items and correcting for “old” response bias using the following formula: p(“old” repeat) – p(“old” novel). Finally, we calculated pattern separation errors, or corrected Lure false alarms (LureFA), as: p(“old” lure) – p(“old” novel) and corrected “new,” termed CR, response scores via p(“new” novel) – p(“new” repeat).
The distribution of behavioral responses during the immediate phase is depicted in Figure 2a, and behavioral performance during the delay phase is depicted in Figure 2b. Because our groups were not matched for the time of day in which they were tested, we tested for time-of-day effects by comparing the distribution of responses in the immediate test condition between the two groups (sleep and wake) on corrected Hit, LureCR, and CR responses. No effect of group was detected using a 2 (group) × 3 (response) between-group MANOVA (F(3,43) = .073, p = .974, η2p = .005). Additionally, we compared mean response times between groups in the immediate and delayed phases. The mean response times (RT) of the sleep and wake groups did not differ in either the immediate or delayed phase (one-way ANOVA F(3,356) = .228, p = 0.877, η2 = .008; post hoc Bonferroni corrected t tests: immediate p = 1, CI [–73, 64]; delay p = 1, CI [–51, 82]). Comparison of mean RT between phases by response type yielded the same result (all p > .5). Taken together, these results indicate an absence of time-of-day effects in our data.
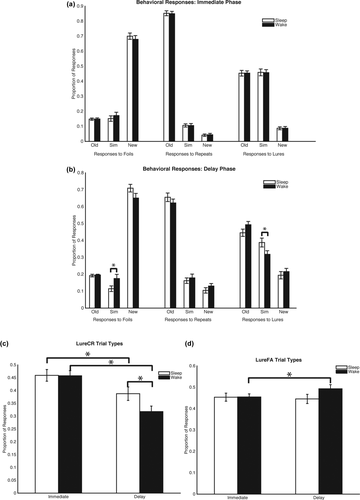
Behavioral responses. (a) Depiction of the proportion of responses to foils, repeats, and lures during the immediate phase and (b) during the delayed phase. Significant differences between groups were only found in the delay phase in proportion of “similar” responses to foils and lures. Participants in the sleep group had better overall performance as they were less likely to respond to a foil as being similar, but more likely to label a lure as similar. (c) LureCR responses changed over time, but were also different between groups. (d) LureFA responses only changed over time in the wake group. * p < .05
To test overall performance, a 2 (group) × 2 (phase) × response (Hit, LureCR, CR) mixed repeated measures MANOVA revealed significant main effects of phase (F(1,45) = 176.91, p < .0001, η2p = .797) and response (F(2,44) = 463.47, p < .0001, η2p = .96), as well as phase × response interaction (F(2,44) = 80.65, p < .0001, η2p = .79). The group × phase × response interaction was not significant (F(2,44)= 2.35, p < .11, η2p = .097). LureFA was not included in this analysis due to its lack of independence.
A 2 (group) × 2 (phase) mixed repeated measures ANOVA was conducted separately for LureFA and LureCR response types to determine how group and phase impacted both behaviors, respectively. A main effect of phase (F(1,45) = 40.74, p < .0001, η2p = .48) and a group × phase interaction (F(1,45) = 16.75, p < .001, η2p = .27) was detected for LureCR, but only a main effect of phase was detected for LureFA (F(1,45) = 4.41, p = .04, η2p = .09; Figures 2c,d). Post hoc one-way ANOVA analyses of pattern separation performance between groups indicated no differences on the immediate test (F(1,45) = .034, p = .86, η2p = .001), but significant differences on the delay test (F(1,45) = 9.142, p < .01, η2p = .17; see Figure 3a). Finally, analyses of pattern separation scores between the immediate and delay phases indicated no significant difference for the sleep group (pair-wise t(23) = 1.73, p = .10, CI [−.006, .07]), but a significant decrease for the wake group (pair-wise t(22) = 6.97, p < .0001, CI [.11, .20]; Figure 3a; See Table 1 for full post-hoc analyses). Taken together, these results indicate preserved mnemonic discrimination ability in the sleep group relative to the decreased performance over a 12-hr delay in the wake group.
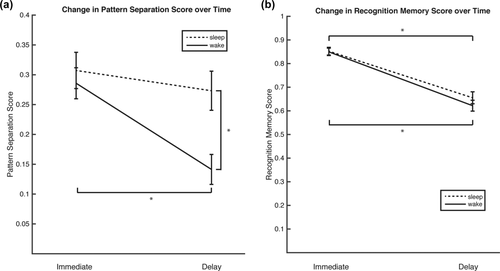
Changes in pattern separation and memory scores between immediate and delay testing phases. (a) Pattern separation scores are relatively unchanged between immediate and delay testing phases if sleep is part of the 12 hr delay. Scores do significantly decrease between phases if the 12 hr delay is during daytime activities. Between groups, immediate scores are statistically similar, but delay scores are significantly different. (b) Recognition memory scores are affected similarly in both groups. Whether a participant slept or not, there is a similarly significant drop in performance after 12 hr. * p < .05
| Response | F(1,45) | p |
|---|---|---|
| Foils | ||
| Old | 0.07 | .788 |
| Similar | 5.45 | .024* |
| New | 3.30 | .076 |
| Repeats | ||
| Old | 1.82 | .184 |
| Similar | 0.84 | .363 |
| New | 1.46 | .233 |
| Lures | ||
| Old | 4.10 | .049* |
| Similar | 11.40 | .002* |
| New | 1.08 | .304 |
- *p<.05 Analyzing these results with the behavioral response graphs indicates that participants who sleep during the delay are less likely to call a foil “similar,” less likely to call a lure “old” and more likely to call a lure “similar” compared to the wake group.
For recognition memory performance, a mixed repeated measures ANOVA analysis of revealed a significant main effect of phase (F(1,45) = 310.43, p < .0001, η2p = .87), driven by decreased performance in the delay condition in the sleep group (pair-wise t(23) = 11.27, p < .0001, CI [.20, .29]), and the wake group (t(22) = 13.78, p < .0001, CI [.24, .32]). There was no main effect of group (F(1,45) = .38, p = .54, η2p = .008) or phase × group interaction (F(1,45) = 1.55, p = .22, η2p = .03; see Figure 3b). Sleep, therefore, seems to have a selective effect on memory specificity only, and not recognition memory in general.
3.2 Imaging results
3.2.1 Hippocampal subregions
We first investigated fMRI activation in anatomically-defined subregions of the hippocampus by masking the parameter estimate (beta) masks with the hippocampal subfield masks. As hippocampal subfields were generally small and had small total numbers of voxels and as we did not have strong predictions about laterality effects, we collapsed across left and right hemispheres for all analyses. Mean parameter estimates for each condition and subregion are presented in Table 2. Given that the behavioral results indicated differences in performance to the Lure stimuli between the sleep and wake groups, we focused on LureCR and LureFA trials in this analysis. We conducted separate 2 (stimulus type: LureFA vs. LureCR) × 2 (phase) × 2 (group) repeated-measures ANOVAs for each subregion (CA3/DG, CA1, subiculum), which revealed significant phase × stimulus type interactions in the CA1 (F(1,44) = 7.65, p < .01, η2p= .148) and subiculum (F(1,44) = 4.50, p < .05, η2p= .093), but no phase × stimulus type × group interactions (Fs < 1). In both regions, there was an increase in fMRI activation for LureFAs across a 12-hr delay while there was a decrease in activation for LureCRs collapsing across both groups (Figure 4). While there was a qualitatively similar pattern of activation in the CA3/DG (see Figure 4), this interaction failed to reach significance (F(1,44) = 2.54, p = .12, η2p= .055). In a further exploratory analysis, we examined the correlation between the change in fMRI activation for LureFA and LureCR trials across the 12-hr delay and total sleep time in the sleep group for each of the hippocampal subregions. There were no significant correlations between sleep time and activation changes for LureCRs or LureFAs in any subregion.
| Mean sleep immediate (SEM) | Mean wake immediate (SEM) | Mean sleep delay (SEM) | Mean wake delay (SEM) | |
|---|---|---|---|---|
| CA3/DG | ||||
| Hit | −.077 (.06) | .036 (.05) | −.013 (.05) | −.055 (.06) |
| CR | −.002 (.04) | −.083 (.05) | .038 (.04) | −.011 (.04) |
| LureFA | −.067 (.07) | −.086 (.06) | .009 (.07) | −.082 (.06) |
| LureCR | −.050 (.05) | −.057 (.06) | −.110 (.05) | −.087 (.07) |
| CA1 | ||||
| Hit | −.036 (.04) | −.030 (.04) | −.115 (.04) | −.138 (.04) |
| CR | −.051 (.04) | −.028 (.04) | −.029 (.02) | −.068 (.03) |
| LureFA | −.155 (.04) | −.194 (.05) | −.093 (.04) | −.134 (.05) |
| LureCR | −.130 (.05) | −.140 (.05) | −.179 (.06) | −.198 (.06) |
| Subiculum | ||||
| Hit | .037 (.05) | .034 (.08) | −.040 (.06) | −.132 (.05) |
| CR | −.100 (.05) | −.057 (.04) | .001 (.04) | −.120 (.04) |
| LureFA | −.134 (.07) | −.211 (.07) | −.039 (.06) | −.108 (.07) |
| LureCR | −.110 (.05) | −.087 (.07) | −.083 (.07) | −.150 (.07) |
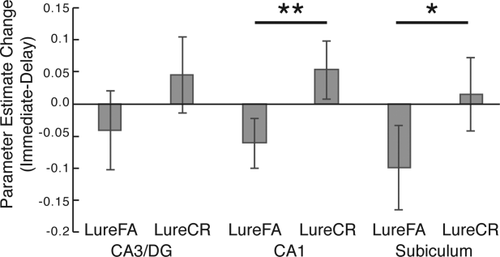
fMRI activation changes over a 12-hr delay in anatomically defined hippocampal subfields. There was a significant change in activation with an increase for LureCR and decrease for LureFA in the CA1 and subiculum. **p < .01; *p < .05
3.2.2 Whole-brain analysis
We next investigated the effects of sleep and delay on neural activity by conducting a repeated-measure ANOVA on the whole-brain fMRI data using group (sleep, wake) and phase (immediate, delayed) as fixed factors. To avoid a voxel selection bias, we identified regions that demonstrated a significant group × phase interaction and then interrogated these regions for differential effects of stimulus type. We identified seven regions of interest (ROIs) where there was a significant group × phase interaction: three noncontiguous ROIs in right dorsolateral prefrontal cortex (DLPFC), and one each in left inferior parietal lobule (near the angular gyrus), left medial frontal cortex (near the supplemental motor area or SMA), left cuneus, and left thalamus (see Table 3 for cluster information). We further interrogated these ROIs for interactions with stimulus type (i.e., for group × phase × stimulus type interactions). In ROIs with significant three-way interactions, we conducted follow-up tests to examine the effects of sleep on fMRI activation within specific stimulus types (Table 4). The three-way interaction was significant in the right middle frontal ROIs, the left inferior parietal lobule, and the left medial frontal cortex. We restricted follow-up analyses to Hits, LureFAs and LureCRs as half the CR trials were randomly assigned to be the baseline condition in our fMRI deconvolution model and thus restricting our ability to detect differences in processing truly novel stimuli. The phase × group interaction was significant for Hits, LureFAs, and LureCRs in each of the ROIs tested, though observed effect sizes tended to be larger for the LureFA and LureCR conditions than for Hits. These results indicate that the interaction between group and phase was strongest for Lure and Repeat stimuli. Figure 5 depicts the location and common patterns of activation for the right middle frontal and left medial frontal ROIs. Comparing brain activity in these regions between groups at the delay phase, we found significantly different activity between groups for Hit, LureFA, and LureCR trials in the middle frontal gyrus and medial frontal cortex ROIs but not left inferior parietal lobule ROI. In each of these regions, there was a relative decrease in fMRI activation for the sleep group compared to the wake group in the delay phase. These results indicate that the behavioral effect of decreased pattern separation scores in the wake group compared to the sleep group during the delay phase may be related to decreases in activation in frontal regions during subsequent recall.
| Phase*Group*Response Intx. | |||||||
|---|---|---|---|---|---|---|---|
| Volume (mm3) | X | Y | Z | F(3,132) | p | Partial η2 | |
| R. DLPFC 1 | 671 | 34 | 45 | 23 | 4.972 | .003 | .102 |
| L. Inferior parietal lobule | 653 | −63 | −43 | 25 | 2.858 | .04 | .061 |
| L. Medial frontal cortex | 391 | −7 | 5 | 58 | 7.892 | < .001 | .152 |
| L. cuneus | 379 | −4 | −99 | −5 | 2.177 | .094 | .047 |
| R. DLPFC 2 | 362 | 27 | 43 | 16 | 4.683 | .004 | .096 |
| R. DLPRC 3 | 332 | 36 | 29 | 32 | 4.313 | .006 | .089 |
| L. Thalamus | 262 | −20 | −13 | 11 | 0.373 | .773 | .008 |
- *Follow-up analyses first examined whether the regions also demonstrated a significant three-way interaction between phase, group, and response type (Hit, LureFA, and LureCR).
| Phase*Group (Hit) | Phase*Group (LureFA) | Phase*Group (LureCR) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F(1,44) | p | Partial η2 | F(1,44) | p | Partial η2 | F(1,44) | p | Partial η2 | |
| R. DLPFC 1 | 10.833 | .002 | .198 | 16.163 | <.001 | .269 | 21.167 | <.001 | .325 |
| L. Inferior Parietal Lobule | 6.240 | .016 | .124 | 19.177 | <.001 | .303 | 13.353 | .001 | .233 |
| L. Medial Frontal Cortex | 16.359 | <.001 | .271 | 19.499 | <.001 | .307 | 29.589 | <.001 | .402 |
| R. DLPFC 2 | 9.604 | .003 | .179 | 20.422 | <.001 | .317 | 18.652 | <.001 | .298 |
| R. DLPFC 3 | 19.195 | < .001 | .304 | 14.724 | <.001 | .251 | 11.687 | .001 | .21 |
- *Only those clusters that also demonstrated a significant three-way interaction between phase, group, and response type were subjected to follow-up analysis.
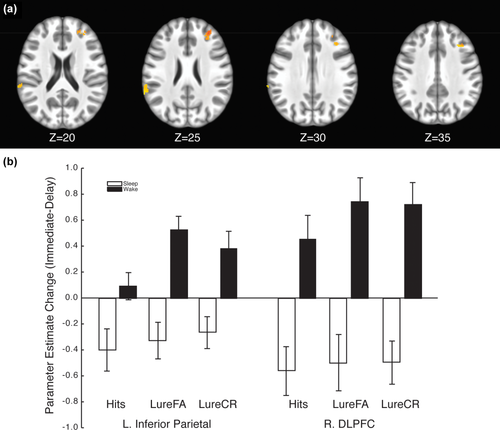
A whole-brain voxel-wise analysis revealed a significant interaction between delay and group in several regions including the right DLPFC and inferior parietal lobule (a). fMRI activation decreased across a 12-hr delay for the sleep group but increased for the wake group (b) in both regions [Color figure can be viewed at wileyonlinelibrary.com]
We further investigated the effects of sleep time on hippocampal activity by performing a repeated measure ANOVA within the sleep group, using phase and stimulus as fixed factors and sleep time as a random factor. Given our a priori hypothesis about hippocampal activity changes due to sleep, we restricted our analyses to the hippocampus and MTL cortex and used a small-volume correction for multiple comparisons. We did not observe any significant clusters in the hippocampus or MTL cortex where activity for phase or stimulus type was influenced by the duration of sleep time. Accordingly, we focused our analysis on the sleep versus wake group comparisons as described above.
3.2.3 Representational similarity analysis
The purpose of the representational similarity analysis (RSA) was to determine if there were patterns of neural activation to which the traditional univariate analyses described above were insensitive. To address this, we created anatomical masks for CA3/DG, CA1, Subiculum, total Hippocampus, MTL cortex, and all gray matter exclusive of the hippocampus and MTL cortex. We focused on the similarity between Hits versus LureFAs and Hits versus LureCRs reasoning that regions that are biased toward pattern separation would have large differences between the RSA scores for hits compared to these two lure conditions (with higher similarity between Hits and LureFAs than between Hits and LureCRs) while regions that are biased toward pattern completion would have smaller differences as repeated stimuli and lure stimuli would evoke similar patterns of activation regardless of the behavioral response to the lures.
We first asked whether RSA scores for the Hit-LureFA comparison differed from the Hit-LureCR comparison in the hippocampus for the immediate test (collapsing across sleep/wake groups). A repeated-measures ANOVA revealed a main effect of comparison (F(1,45) = 19.94, p<.001, η2p= .307) but no main effect of hippocampal subregion (CA3/DG, CA1, and Subiculum; F(2,90) = 0.21, p = .81, η2p= .005). The subregion × comparison interaction failed to reach significance (F(2,90) = 3.06, p = .052, η2p= .064). Follow-up t tests revealed significant differences in each of the subregions (p's < .01). Interestingly (and contrary to our predictions), RSA scores were higher for the Hit-LureCR comparison than for the Hit-LureFA comparison, indicating that the pattern of activation was more similar for Hits and LureCRs than for Hits and LureFAs, in spite of the fact that Hits and LureFAs share a common behavioral response (i.e., “old”). This similarity of response patterns may be consistent with a correct mnemonic decision instead. A further comparison of RSA scores between the anterior (head) and posterior (body/tail) of the hippocampus revealed no differences between RSA scores when dividing the hippocampus this way. Accordingly, as there was not a differentiation across hippocampal subregions or across anterior/posterior hippocampus, subsequent analyses utilized a whole-hippocampus mask.
We next asked if the RSA scores for the Hit-LureFA comparison differed more from the Hit-LureCR comparison in the hippocampus than in the cortex. Again, we restricted our analysis to the immediate test condition and collapsed across sleep/wake groups. A repeated-measures ANOVA revealed a main effect of region (F(1,45) = 110.16, p < .001, η2p=.710), no main effect of comparison (F(1,45) = 2.93, p = .094, η2p=.061), and a significant region × comparison interaction (F(1,45) = 16.43, p < .001, η2p = .267). As observed in the hippocampal subregion analysis, follow-up t tests revealed that RSA scores differed between comparisons in the hippocampus, with higher RSA scores for the Hit-LureCR comparison (m = .55, SD = .098) than for the Hit-LureFA comparison (m = .50, SD = .11; t(45) = 3.98, p < .001). RSA scores for the two comparisons did not differ in the cortex (t(45) = 0.15, p = .885). RSA scores were higher in the cortex than the hippocampus for both the Hit-LureFA (t(45) = 12.28, p < .001) and Hit-LureCR (t(45) = 6.46, p < .001) comparisons, consistent with a greater bias toward pattern completion in the cortex than in the hippocampus.
Finally, we asked what effect delay and sleep had on RSA scores in the hippocampus and cortex. A repeated-measures ANOVA revealed a main effect of region, with higher RSA scores in the cortex than the hippocampus (F(1,45) = 330.72, p < .001, η2p = .883), a main effect of delay with higher RSA scores following a 12-hr delay (F(1,44) = 5.20, p < .05, η2p = .106), a region × delay interaction, with greater RSA increases occurring in the cortex than the hippocampus across the 12-hr delay (F(1,44) = 18.62, p < .001, η2p = .297), and a region × comparison interaction, similar to the interaction for the immediate test data reported above (F(1,44) = 22.85, p < .001, η2p = .342). No other main effects or interactions were significant. Together, these results indicate that the bias of the cortex toward pattern completion increases across a 12-hr delay, but that change is not significantly affected by sleep.
4 DISCUSSION
We examined the effect of sleep on both behavioral and neural responses in a task that places high demands on pattern separation processes. Behaviorally, we observed a decrease in mnemonic discrimination (i.e., pattern separation scores) following a wake-filled delay but preserved performance when the delay contained sleep. There was no effect of sleep on recognition memory performance. Using high-resolution whole-brain fMRI, we were able to examine activation in hippocampal subregions and in the broader cortex simultaneously. We observed an interaction between sleep, delay, and stimulus condition in a network of brain regions related to cognitive control during recognition memory (discussed below). Further, we observed that hippocampal activation distinguished between targets and lures to a greater extent than cortical regions, consistent with a bias in the hippocampus toward mnemonic discrimination and a bias in the cortex toward pattern completion. Representational similarity was more affected by sleep in the cortex, consistent with an increase toward pattern completion or generalization in the cortex following sleep.
4.1 Behavioral performance
Previous research has indicated that sleep preserves declarative memories from interference when subjects are retested over a 12 hr interval (Ellenbogen et al., 2006). REM sleep, in particular, improves memory discrimination performance retroactively despite the introduction of high interference (McDevitt, Duggan, & Mednick, 2015). These findings are consistent with our observation that sleep seems to have a compensatory effect on interference. The paradigm used here did not introduce explicit interference as in previous studies. Instead, the task involved interference caused by daily activities (including college classes and other activities requiring extensive cognitive resources) and by interference inherent in a difficult mnemonic discrimination task.
Another specific effect sleep has on learning is that it is associated with improved consolidation of explicit memories that are more difficult to encode or that are only weakly encoded (Diekelmann & Born, 2010). In our behavioral task, obtaining a high pattern separation score is much more challenging and computationally intensive compared to obtaining a high recognition memory score. An interesting and novel finding from our results is that sleep does not appear to affect recognition memory in general whilst it does benefit mnemonic discrimination that is dependent on pattern separation. These benefits to mnemonic discrimination align with previous studies using similar research paradigms. Previously, other sleep have shown sleep to have a restorative effect on mnemonic function and sleep deprivation to significantly reduce mnemonic function (Saletin, Goldstein-Piekarski, Greer, Stark, Stark, & Walker, 2016). Together, it is reasonable to conclude that sleep has a greater effect on pattern separation processing compared to other processes underlying declarative memory.
4.2 Hippocampal subregional analysis
We hypothesized that hippocampal activation would reflect a bias in activation consistent with pattern separation. Previous high-resolution fMRI investigations of mnemonic discrimination in the hippocampus have shown a bias toward pattern separation in CA3/DG (Bakker et al., 2008) and that the CA3/DG responds in a graded fashion to changes in mnemonic similarity while the CA1 responds in a more linear fashion (Lacy et al., 2011). In our study, we did not observe strong pattern separation effects of stimulus type in the hippocampus in the immediate test, possibly due to our use of an explicit version of the mnemonic similarity test as opposed to an implicit version of the task as used in previous studies (Bakker et al., 2008; Lacy et al., 2011). Explicit, top-down retrieval instruction has been shown to modulate activation in the hippocampus in a mnemonic similarity task (e.g., Motley & Kirwan, 2012).
We observed an increase in hippocampal activation for the LureCR trials and a decrease for LureFA trials following a 12-hr delay. Although this pattern of activation change was present in the CA3/DG, the change in activation was significant in the CA1 and subiculum only. As correct responses to lure stimuli (i.e., LureCRs) are thought to tax pattern separation processes (Kirwan & Stark, 2007), this increase in LureCR related activity is consistent with an increase in pattern separation demands over a 12-hr delay. Contrary to our hypotheses, however, this change in activation was not modulated by sleep, as both sleep and wake groups displayed this change in activation.
4.3 Voxel-wise group fMRI analysis
Consistent with our behavioral results, our whole-brain imaging results indicate that activity in a network of brain regions (including the right DLPFC and left inferior parietal lobule) have decreased activation following a delay that contains sleep while there is increased activation when the delay does not contain sleep. This change in activation, particularly the increase following a waking delay, was greater (i.e., larger effect sizes) when the task placed higher demands on pattern separation (lure trials) than when it did not (target trials).
The inferior parietal lobule has often been implicated in memory retrieval and may be specifically involved in attentional control during recognition memory retrieval. For example, the inferior parietal lobule has been implicated in recall through visual cues (Fletcher, Shallice, Frith, Frackowiak, & Dolan, 1998; Konishi, Wheeler, Donaldson, & Buckner, 2000; Nee, Wager, & Jonides, 2007). A PET study in which visual categories of studied patterns were used as a condition resulted in increased blood flow to the inferior temporal gyrus and angular gyrus (inferior parietal lobule) compared to a working memory condition (Herath, Kinomura, & Roland, 2001). According to some models of recognition memory (e.g., Cabeza, 2008; Ciaramelli, Grady, & Moscovitch, 2008), the ventral parietal cortex is involved in externally driven attention while the dorsal parietal cortex is involved in internally driven attention. According to this hypothesis, ventral parietal processing will be engaged during memory retrieval as subjects search for relevant memory representations and the correct targets engage attention in a bottom-up fashion (Cabeza, 2008). In a recent study, Rosen, Stern, Michalka, Devaney, and Somers (2015) demonstrated that the angular gyrus (inferior parietal) was one of several cognitive control regions that were preferentially engaged when participants performed a change detection task when guided by long-term memory representations. The authors argue for the existence of a network of regions that are preferentially engaged in guiding attention based on long-term memory representations. In the current context, we observed increased inferior parietal activation in a memory retrieval task following a 12-hr delay but only when that delay did not contain sleep. Further, this increase in activation was larger for trials where participants were required to make recognition memory judgments about visually and semantically similar lures rather than exact repeats.
DLPFC activation is commonly associated with working memory (Manoach et al., 1997) and cognitive control (Ridderinkhof, Van Den Wildenberg, Segalowitz, & Carter, 2004). The DLPFC has been shown to be involved in memory retrieval (Dudukovic & Kuhl, 2017), and in particular it has been implicated in retrieval success, as demonstrated in a large meta-analysis by Spaniol and colleagues (Spaniol et al., 2009). In a recent study, O'Neil, Watson, Dhillon, Lobaugh, and Lee (2015) showed that the DLPFC, perirhinal cortex, and posterior parietal lobe are preferentially recruited under conditions of high visual interference, similar to the mnemonic similarity task used in the present study. Our results extend this finding by showing that the DLPFC is even more critical following a 12-hr waking delay as compared to a delay that contains sleep.
Reas and Brewer (2013) showed that DLPFC, dorsal ACC (in a region similarly located to our medial frontal cortical activation), and inferior parietal lobule all respond differentially to memory strength and memory search in recognition memory. Reas and Brewer demonstrated that activation in dorsal ACC increased during memory search and was further modulated by memory strength (with greatest activation in the “low” strength condition). Activation in DLPFC and inferior parietal regions was modulated by memory strength, albeit in opposite directions (greater activation for “low” memory strength in DLPFC and greater activation for “high” memory strength in inferior parietal). While we did not explicitly measure memory strength, accuracy and reaction time data are consistent with low memory strength in the delay conditions of the current study. According to this interpretation, our results of greater activation when memory strength is the lowest are consistent with those of Reas and Brewer (2013) in the DLPFC and dorsal ACC (though not in the inferior parietal lobule).
4.4 Representational similarity
Given our failure to observe differences in hippocampal activation using traditional univariate analyses, we used RSA to capture patterns of fMRI activation to which traditional univariate methods may be insensitive. We hypothesized that the comparison of the pattern of responses to Hits and LureFAs would be more similar than the comparison of Hits and LureCRs given that Hits and LureFAs both have the same behavioral response (“old”) and presumably arise from similar neural processes. Contrary to this prediction, RSA scores in hippocampal subregions were higher for the Hit-LureCR comparison, possibly reflecting correct mnemonic decision processes.
We further hypothesized that RSA scores in the hippocampus would differ from those in the cortex. We found that RSA scores in the hippocampus differentiated between LureFA and LureCR trials while those in the cortex did not. This is broadly consistent with the computational prediction that the cortex is biased toward pattern completion while the hippocampus is biased toward pattern separation (Norman & O'Reilly, 2003). We found that this bias toward pattern completion was increased with sleep, consistent with our predictions. We suggest that this shift in bias is due to sleep-dependent consolidation resulting in stronger cortical representations of the to-be-remembered information.
4.5 Limitations and future directions
As noted above, one limitation of using noninvasive functional neuroimaging to assess pattern separation processes is that as a cellular computation, pattern separation processing can truly be measured only at the cellular level (Aimone, Deng, & Gage, 2011; Leutgeb et al., 2007; McClelland, McNaughton, & O'Reilly, 1995; Norman & O'Reilly, 2003; Treves & Rolls, 1994). While high-resolution fMRI gives superb spatial resolution over the whole brain, it cannot measure cellular processes directly. Previous studies, however, indicate that activity in hippocampal subregions is associated with differential processing of lure stimuli relative to repeated and novel stimuli (Bakker et al., 2008; Leal, Tighe, Jones, & Yassa, 2014). These studies found increased activity in CA3/DG when participants correctly identify a lure as “similar.” Therefore, though fMRI cannot directly measure pattern separation, behavioral performance can be used to make inferences about regions where pattern separation processing takes place.
This study provides initial insight as to the effects of sleep on neural activity on a task that places high demands on pattern separation. Because we did not test specific sleep states, future studies may wish to examine the effects of REM/non-REM sleep on behavioral performance and on neural activity. Another direction for the future might involve a more longitudinal approach. While it is interesting that we show a single night's sleep had such a dramatic effect on behavioral performance, future studies would need to investigate whether this translates into a long-term effect or not and if the sleep-related changes that we observe in the fMRI activation reflect long-term consolidation effects. Further, we did not assess sleep history for our participants. While our random group assignment should have precluded any systematic differences in sleep history between groups, there remains the possibility that one group was more rested on average than the other at the beginning of the study. Sleep seems to enhance performance on our task by assisting with overcoming interference. A question remains as to whether we would see similar group differences with a delay of 24 hr or longer. If studying a list of items, like in the current study, immediately before sleep has a significant long-term effect, this would provide important implications about optimal study habits in educational settings.
5 Conclusion
Our results indicate that sleep is important for performing well on memory tasks that place a high demand on memory specificity. While we observed an overall increase in hippocampal activity for correct responses to similar lure stimuli after a 12-hr delay, this increase was not unique to the sleep group. Rather, it appears that cortical mechanisms support the behavioral benefit observed in the sleep group after a delay. Representational similarity analyses suggest that the hippocampus is biased to pattern separation while the cortex is biased toward pattern completion and that sleep increases this difference in bias. These results provide interesting insight as to the role of the hippocampus in pattern separation processing and how a single bout of sleep does not necessarily affect these processes.
ACKNOWLEDGMENT
The authors gratefully acknowledge Kayla Alder, Malia Anderson, Briana Cook, Corinne DeVault, Mandi Ellgen, Athena Howell, Bryce Owen, Seth Spencer, and Brigham Wright for their assistance in data collection and analysis.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.




