Exercise increases insulin signaling in the hippocampus: Physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice†
Disclosure Statement: The authors have nothing to disclose.
Abstract
Increasing evidence indicates that physical exercise induces adaptations at the cellular, molecular, and systemic levels that positively affect the brain. Insulin plays important functional roles within the brain that are mediated by insulin-receptor (IR) signaling. In the hippocampus, insulin improves synaptic plasticity, memory formation, and learning via direct modulation of GABAergic and glutamatergic receptors. Separately, physical exercise and central insulin administration exert relevant roles in cognitive function. We here use CF1 mice to investigate (i) the effects of voluntary exercise on hippocampal insulin signaling and memory performance and (ii) whether central insulin administration alters the effects of exercise on hippocampal insulin signaling and memory performance. Adult mice performed 30 days of voluntary exercise on running wheel and afterward both, sedentary and exercised groups, received intracerebroventricular (icv) injection of saline or insulin (0.5–5 mU). Memory performance was assessed using the inhibitory avoidance and water maze tasks. Hippocampal tissue was measured for [U-14C] glucose oxidation and the immunocontent of insulin receptor/signaling (IR, pTyr, pAktser473). Additionally, the phosphorylation of the glutamate NMDA receptor NR2B subunit and the capacity of glutamate uptake were measured, and immunohistochemistry was used to determine glial reactivity. Exercise significantly increased insulin peripheral sensitivity, spatial learning, and hippocampal IR/pTyrIR/pAktser473 immunocontent. Glucose oxidation, glutamate uptake, and astrocyte number also increased relative to the sedentary group. In both memory tasks, 5 mU icv insulin produced amnesia but only in exercised animals. This amnesia was associated a rapid (15 min) and persistent (24 h) increase in hippocampal pNR2B immunocontent that paralleled the increase in glial reactivity. In conclusion, physical exercise thus increased hippocampal insulin signaling and improved water maze performance. Overstimulation of insulin signaling in exercised animals, however, via icv administration impaired behavioral performance. This effect was likely the result of aberrant phosphorylation of the NR2B subunit. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Increasing evidence indicates that physical exercise induces adaptations at the cellular, molecular, and systemic levels that positively affect the health of the brain (Dietrich et al., 2005; Droste et al., 2006; Stranahan et al., 2009). These effects include an increase in synaptic plasticity and peripheral sensitivity to insulin as well as a slowing of age-related cognitive decline and a delay in the onset of neurodegenerative diseases. Among the possible mechanisms underlying these central benefits of physical exercise, it is the modulation of neurotransmitter systems by trophic factors and hormones, including insulin (Laurin et al., 2001; Cotman and Berchtold, 2002; Duarte et al., 2003; Park et al., 2005; Flores et al., 2006; Balkau et al., 2008).
The brain was long considered an insulin-insensitive organ; however, this assumption is now challenged by recent studies showing that insulin has important functional roles within the brain that are mediated by insulin-receptor signaling (Gispen and Biessels, 2000; Dou et al., 2005; van der Heide et al., 2006). Moreover, insulin and its receptors (IR) have been associated with distinct, region-specific functional roles (Plum et al., 2005). For instance, insulin/IR signaling has been shown to improve learning, memory formation, and synaptic plasticity in the hippocampus and cerebral cortex. In contrast, a resistance to the effects of insulin in the brain is closely related to the etiology of neurodegenerative diseases and impaired cognitive function, suggesting that brain IR may be potential pharmacological targets in the treatment of cognitive disorders (Park, 2001). Some human and animal studies have proposed that intranasal or systemic insulin administration might exert memory-enhancing action (Park, 2001; Hallschmid and Schultes, 2009). Studies delivering insulin directly into the brains of rodents, however, have produced conflicting results regarding memory performance. In general, low to intermediate doses have produced no memory changes while high doses tended to improve memory performance (Schwarzberg et al., 1989; Park et al., 2000; Moosavi et al., 2006, 2007a,b; Babri et al., 2007; McNay et al., 2010). These differences could be related to insulin's modulation of GABAergic and glutamatergic receptors and, in consequence, its modulation of synaptic transmission during learning and memory processes (Wan et al., 1997; Ott et al., 1999; Zhao et al., 2004; Watson and Craft, 2006).
Given the important roles attributed to N-methyl-D-aspartate receptors (NMDARs) in synaptic plasticity and cognitive function (Collingridge, 1987), NMDARs may be a target of insulin/IR signaling and may be involved in changes to learning and memory processes. It has been suggested that insulin transiently modulates the phosphorylation of the NR2A and NR2B subunits and potentiates NMDA responses (Christie et al., 1999). In addition, alterations in the expression and function of NMDARs have been implicated in the behavioral and electrophysiological abnormalities observed in insulin-deficient rats (Di Luca et al., 1999). Neuronal transmission requires that glutamate stimulation of NMDARs be kept at physiological levels because of the delicate threshold between normal and excitotoxic responses caused by this receptor (Danbolt, 2001). Thus, it is plausible that central insulin signaling may participate in fine-tuning the modulation of NMDAR responses via its interaction with functional regulatory subunits.
Drugs that improve insulin sensitivity are currently being tested in human trials for use in treating functional deficits associated with Alzheimer's disease (Cole and Frautschy, 2007; McNay et al., 2010). A number of studies have demonstrated that physical exercise improves cognitive function and peripheral insulin sensitivity, but little is known regarding its possible role in enhancing brain insulin sensitivity. We speculate that physical exercise may increase the brain's sensitivity and response to insulin, thereby resulting in a positive influence on memory performance. Taking these factors into considerations we use mice to investigate: (i) the effects of voluntary exercise on memory performance and hippocampal insulin signaling and (ii) whether insulin delivery into the brain alters the effects of voluntary exercise on memory performance and hippocampal insulin signaling.
METHODS
Animals and Exercise Protocol
Two-month-old CF1 mice were housed in standard cages (48 × 26 cm2) with four animals per cage (van Praag et al., 1999, 2005; Dietrich et al., 2005). To avoid social isolation, the animals were not confined in individual housing (Leasure and Decker, 2009). Animals were kept in a room with controlled temperature (22°C) under a 12 h light/12 h dark cycle and had free access to food and water. Mice were divided into a sedentary group and a voluntary exercise group, which had free access to a running wheel. After 4 weeks of access to the running wheel, each mouse ran an average of about 3.500 m. All experiments followed the guidelines of the Committee on Care and Use of Experimental Animal Resources, UFRGS, Brazil.
Surgical Procedure
Sedentary and exercised animals were anesthetized by an intraperitoneal (i.p.) injection of ketamine (100 mg kg−1 body weight) and xylazine (10 mg kg−1 body weight). A 27-gauge, 7-mm guide cannula was placed 1 mm posterior to the bregma, 1 mm right from the midline and 1 mm above the lateral brain ventricle. Through a 2-mm hole made at the cranial bone, the cannula was implanted 1.5 mm ventral to the superior surface of the skull and fixed with jeweler's acrylic cement (Schmidt et al., 2005). On the third day postsurgery, the mice already displayed normal food intake and water consumption as well as spontaneous locomotion; they were thus considered ready for in vivo experiments.
MEMORY TASKS
Inhibitory Avoidance Task-Aversive Memory
The behavioral apparatus consisted of a 50 × 25 × 25 cm3 acrylic box with a floor composed of parallel-caliber stainless steel bars (1 mm diameter) spaced 1 cm apart (Insight Equipments, SP, Brazil). A platform 2 cm wide and 2.5 cm high was placed against the left wall of the box. To evaluate aversive memory performance, we used a footshock aversive task. Sedentary and exercised mice (n = 12–18 per group) were placed on the platform and their latency to step down on the grid floor with four paws was measured with an automatic device. The latencies in the training session were similar in all groups (data not shown). In training sessions, animals received a 1 s, 0.4 mA footshock when they stepped onto the grid. After the shock, they were immediately injected icv for 5 min (1 μl min−1) with vehicle or insulin (0.5–5 mU) and returned to their home cages. Test sessions were performed 24 h after training to evaluate long-term memory. For test sessions, mice were returned to the platform and the latency to step down was used as a measure of retention (a 180 s cut-off time was used). The footshock was omitted during the testing session.
Morris Water Maze Task-Spatial Memory
The apparatus was a black, circular pool (110 cm diameter) with a water temperature of 21°C ± 1°C. Sedentary and exercised mice (n = 10–12 per group) were trained daily in a four-trial water maze task for three consecutive days; each trial lasted up to 60 s and was followed by 20 s of rest on a hidden black platform. During training, mice learned to escape from the water by finding a hidden, rigid, black platform submerged about 1 cm below the water surface in a fixed location. If the animal failed to find the platform in 60 s, it was manually placed on the platform and allowed to rest for 20 s. Each trial was separated by at least 12 min to avoid hypothermia (the variations of rectal temperature were equal for all groups) and facilitate memory acquisition. Immediately after each daily training session, mice were injected icv for 5 min (1 μl min−1) with vehicle or insulin in a single, 5 mU dose and returned to their home cages. The maze was located in a well-lit, white room with several visual stimuli hanging on the walls to provide spatial cues. Latency to find the platform during each trial was measured as an indicator of learning. A probe test without the platform was performed on the fourth day. The time spent in the target quadrant was measured as an indicator of memory retention.
Glucose and Insulin Serum Levels
To assess peripheral insulin sensitivity, serum glucose and insulin levels (n = 8 per group) were measured after 30 days of treatment. The mice fasted for 12 h and blood was then collected via a small puncture in the tail; blood was collected immediately before (0 min) and 30 min after an i.p. injection of glucose (2 mg/g body weight). Glucose was measured with a glucosimeter (AccuChek Active, Roche Diagnostics®, USA) and serum insulin levels were measured with a commercially available radioimmunoassay kit (BioChem ImmunoSystems®, Italy). The Homeostasis Model Assessment (HOMA2) was used to estimate insulin resistance based on fasting insulin and glucose serum levels [glucose serum (mg dl−1) × insulin serum (μIU ml−1)/405].
Liver Glycogen and Epididymal Fat Pad
Fed mice were sacrificed and liver glycogen was determined using the colorimetric method described by Krisman, (1962). Fat tissue from epididymal regions was dissected and weighted as previously described (Muller et al., 2008).
Hippocampal [U-14C] Glucose Oxidation
Considering the key role of the hippocampus in memory processes and the importance of glucose as an energy substrate in normally functioning neural cells, we evaluated the potential influence of exercise and insulin on glucose oxidation following the protocol by Gilbert and Bergold (2005).
After 30 days, the voluntary exercise group and the sedentary group (n = 8 per group) were decapitated. Hippocampal slices between 20 and 25 mg were incubated in 0.5 ml buffer (KRb + 5.0 mM D-glucose, pH 7.4) containing 0.1 μCi [U-14C] glucose and vehicle or insulin (5 mU). Incubation was performed in flasks after KRb medium was gasified with 95% O2:5% CO2, and flasks were then sealed with rubber caps. Hippocampal slices were incubated at 37°C for 1 h in a Dubnoff metabolic shaker (60 cycles/min) following the method of Dunlop et al. (1975). Incubation was stopped by adding 0.2 ml of 50% TCA through the rubber cap, and 0.1 ml of 1M sodium hydroxide was then injected into the central wells. The flasks were shaken an additional 30 min at 37°C to trap the CO2, and the contents of the central well were then transferred to vials and assayed for CO2 radioactivity in a liquid-scintillation counter (Wallac 1,409).
Synaptic Membrane and Homogenate Preparations of the Hippocampus
In the third day post surgery, mice were injected icv for 5 min (1 μl min−1) with vehicle or insulin in a single, 5 mU dose. After 15 min or 24 h, mice (n = 8 per group) were decapitated and the right hippocampus was immediately dissected and stored at −70°C for synaptic membrane and homogenate preparations.
Synaptic membrane preparations were obtained from the hippocampus as previously described by Dietrich et al. (2005) and stored at −70°C. Membranes were thawed at 37°C for 30 min and washed three times with 5 mM Tris-HCl, pH 7.4 at 27,000g for 15 min. The final pellet was resuspended in PIK buffer (1% NP-40, 150 mM NaCl, 20 mM Tris, pH 7.4, 10% glycerol, 1 mM CaCl2, 1 mM MgCl2, 400 μM sodium vanadate, 0.2 mM PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, and 0.1% phosphatase inhibitor cocktails I and II. Sigma-Aldrich, USA). Total protein content was measured using the method described by Peterson (1977).
Hippocampal homogenates were prepared in PIK buffer and centrifuged. Supernatants were collected and total protein was measured using the method described by Peterson (1977).
Western Blotting
For Western blot analysis, 30 μg of protein from hippocampal homogenates and synaptic membrane preparations were loaded into each well, and IR immunoprecipitates were separated by electrophoresis on a 10% polyacrylamide gel and electrotransfered to PVDF membranes. Nonspecific binding sites were blocked with Tween-Tris buffered saline (TTBS, 100 mM Tris-HCl, pH 7.5) containing 5% albumin for 2 h and then incubated overnight at 4°C with polyclonal antibodies against insulin receptors (IR, Santa Cruz Technology, 1:1,000), pTyrosine (pTyr, Santa Cruz Technology, 1:1,000), pAktser-473 (Cell Signaling Technology, 1:1,000), Akt (Cell Signaling Technology, 1:3,000), pNR2B (Upstate, 1:250) and actin (Sigma, 1:5,000). Membranes were rinsed 3× 10 min with TTBS and incubated with secondary antibodies (1:3,000 dilution, antirabbit, Cell Signaling Technology; 1:5,000 dilution antimouse, Santa Cruz Technology) for 2 h at room temperature. Membranes were then rinsed 4× 10 min with TTBS and incubated with peroxidase-conjugated for 5 min at room temperature. The resulting reaction was displayed on autoradiographic film by chemiluminescence. The films were scanned and band intensity was analyzed using Image J software (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). The pTyr levels in the hippocampus were measured via IR immunoprecipitation. Fifty micrograms of hippocampal protein was incubated with 5 μg primary IR antibody overnight. Protein A-agarose (Invitrogen) was added to the antigen–antibody mixture and incubated while gently stirring for 2 h. The immunoprecipitate was washed three times with the same lysis buffer then resuspended, electrophoresed, and transferred to a nitrocellulose membrane; it was then analyzed by Western blot as previously described.
Total and Na+-Independent [3H] Glutamate Uptake
Sedentary and exercised mice were injected icv with saline or insulin (5 mU) and after 24 h were sacrificed by decapitation. Hippocampal slices were preincubated with HBSS at 37°C for 15 min, followed by the addition of 100 μM [3H] glutamate. Incubation was stopped after 5 min with two ice-cold washes of l mL HBSS. After washing, 0.5N NaOH was immediately added to the slices and they were stored overnight. Na+-independent uptake was measured using the above-described protocol with alterations in the temperature (4°C) and the composition of the medium (N-methyl-D-glucamine instead of sodium chloride). Results (Na+-dependent uptake) were measured as the difference between the total uptake and the Na+-independent uptake. Each incubation was performed in triplicate (Thomazi et al., 2004). Incorporated radioactivity was measured using a liquid scintillation counter (Wallac 1,409).
Immunohistochemistry
For the immunohistochemical study, animals (four mice per group) were killed and left hemispheres were postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4 and then cryoprotected in a 30% sucrose solution in PB at 4°C. Coronal sections (50 μm) were obtained using a Vibratome (Leica, Germany). One-in-8 random series were collected for immunohistochemistry. The sections were incubated for 48 h at 4°C with polyclonal rabbit GFAP antiserum (Dako, UK, 1:500) and NeuN (Chemicon, 1:250) in phosphate buffered saline (PBS)-Tx containing 2% bovine serum albumin (BSA). After being washed several times with PBS, the sections were incubated with 594 alexa-conjugated donkey antirabbit and 488 alexa-conjugated donkey antimouse antibodies for 2 h at room temperature. To determine fluorescence insensitivity, sections were photographed with a confocal microscope (Olympus, Japan) and analyzed using Image J software. Fluorescence insensitivity was expressed as the ratio of GFAP-/NeuN-positive cells. Astrocyte number was estimated by counting the number of GFAP-positive cells using a Nikon Eclipse E-600 microscope (500×, Japan) coupled to a Nikon DXM 1200C CCD camera.
Statistical Analysis
Results were presented as means ±standard error of mean (SEM) with the exception of the step-down latencies in the inhibitory avoidance task, which were expressed as median and interquartile ranges. The data from the water maze task were analyzed using repeated-measures analysis of variance (ANOVA) followed by Tukey's post hoc test. Likewise, the differences between all groups were analyzed using ANOVA and Tukey's post hoc test. The differences between the sedentary and exercised groups were analyzed using Student's t test. The data from the inhibitory avoidance task were analyzed using the Mann-Whitney U test. The differences between groups were considered statistically significant if P < 0.05.
RESULTS
Exercise Improved Spatial Learning: Amnesic Actions of Central Insulin Administration
We confirmed that exercise increases peripheral sensitivity to insulin. At fasting, glucose levels were higher in sedentary animals when compared to exercised ones (Fig. 1A; P < 0.05) while serum insulin levels were unchanged (Fig. 1B). Moreover, the exercise protocol caused an increase in the liver glycogen and decrease in epididymal fat pad (Table 1). In the inhibitory avoidance task, the sedentary and exercised animals presented a similar long-term memory. Furthermore, the icv administration of vehicle or insulin at 0.5 and 2 mU doses had no effect in the sedentary and exercised groups. However, 5 mU icv insulin had an amnesic effect only in the exercised group (Fig. 2A; P < 0.05). Because of this dose response, we decided to evaluate spatial memory using only 5 mU of insulin in subsequent test. In the Morris water maze task, the exercised animals had better learning and retention relative to the sedentary groups. Again, central insulin administration had an amnesic effect only in the exercised group (Figs. 2B,C; P < 0.05). After these initial findings, we then investigated the possible mechanisms underlying the effects of exercise and central insulin administration on memory performance and insulin signaling.
| Body weight (g) | Epididymal fat pad | Liver glycogen (mg%) | |
|---|---|---|---|
| Sedentary n = 10 | 49.7 ± 2.6 | 2.5 ± 0.4 | 1.9 ± 0.2 |
| Exercise n = 10 | 45.8 ± 1.6 | 1.7 ± 0.3* | 2.6 ± 0.2* |
- Epididymal fat pat pad: exercise < sedentary *P < 0.05.
- Liver glycogen: exercise > sedentary *P < 0.05.
We first performed ex vivo experiments to determine whether insulin could affect the ability of the hippocampus to oxidize glucose. There was a significant increase of [U-14C] glucose oxidation in hippocampal slices from exercised mice relative to sedentary mice (Fig. 3; P < 0.05). Insulin did not have an additional affect on glucose oxidation in exercised mice (Fig. 3).

Exercise increases peripheral insulin sensitivity. (A) After 30 days of exercise, mice presented decreased fasting blood glucose levels (0 min) and less increment at 30 min after glucose input (2 mg glucose/g mice) when compared to sedentary animals. (B) Fasting insulin serum levels were not altered by exercise, and a glucose input did not increase insulin serum levels in exercised mice. (C) There was a decrease in the HOMA-IR index as a result of exercise. □ = sedentary; ▴ = exercised (*P < 0.05 exercised vs. sedentary). Values are mean ± SE of eight mice per group.
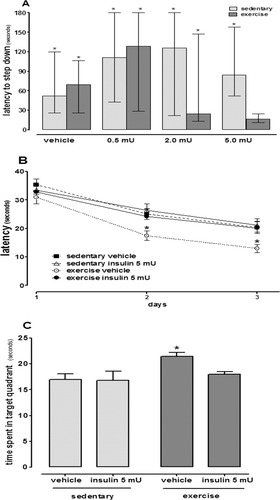
Exercise improves performance in spatial memory and intracerebroventricular (icv) insulin administration had an amnesic effect on both aversive and spatial memory in exercised mice. (A) Sedentary and exercised mice treated with vehicle or insulin at 0.5 or 2 mU icv showed an increased latency to step down onto the platform in the inhibitory avoidance task 24 h after training, demonstrating successful long-term learning. However, 5 mU icv insulin had an amnesic effect on the exercised group (*P < 0.05 training vs. long-term memory). Values are median, interquartile range of 12–18 mice per group. (B and C) Exercise improved spatial learning and memory in the Morris water maze task. Insulin (5 mU, icv) did not alter the performance of the sedentary group; however, this dose also had an amnesic effect on the exercised group during memory acquisition and retention. ▪ = sedentary/vehicle; ▵ = sedentary/insulin 5mU; ○ = exercised/vehicle; • = exercised/insulin 5mU (*P < 0.05 exercised vehicle vs. other groups). Values are mean ± SE of 10–12 mice per group.

Exercise increases hippocampal glucose oxidation. Exercise increased the [U-14C] glucose oxidation by hippocampal slices. Insulin did not affect [U-14C] glucose oxidation in both groups (*P < 0.05 exercised vs. sedentary groups). Values are mean ± SE of eight mice per group.
Exercise Increased Hippocampal Insulin Receptors and Signaling
After 30 days of exercise, the IR immunoprecipitate from hippocampal homogenates showed an increased phosphorylation in tyrosine residues (pTyr-IR) compared to the sedentary group (Fig. 4A, P < 0.05); however, there was no change in total IR immunocontent in the hippocampal homogenate (Fig. 4B). The IR immunocontent in hippocampal synaptic membranes was significantly increased (64%) by exercise when compared to sedentary mice (Fig. 4C; P < 0.05), suggesting that physical exercise may activate molecular machinery that is involved in IR translocation to the synaptic membrane. The phosphorylation of AKT at ser473 (pAKTser473), a downstream protein activated by insulin/IR signaling, was significantly increased by exercise relative to sedentary mice (Fig. 4D, P < 0.05). Overall, these results suggest that insulin signaling in the hippocampus is increased by exercise. As previously demonstrated by Li et al. (2005) and Trejo et al. (2008), we also found an increased number of GFAP-positive cells in the hippocampus of exercised animals when compared to sedentary mice (Fig. 6A, P < 0.05).
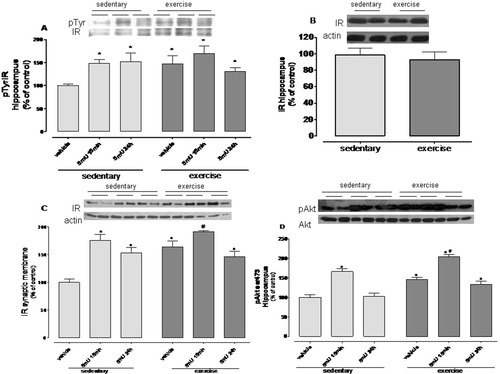
Exercise increases insulin signaling in the hippocampus. (A) Exercise increased the phosphorylation status of Tyr in the immunoprecipitate of insulin receptors (pTyr-IR) in the hippocampus. Insulin increased pTyr-IR only in sedentary groups. (B) Exercise did not increased total IR immunocontent in the hippocampus. (C) Both exercised and sedentary groups showed an increased IR immunocontent in synaptic membranes. (D) Exercise increased the immunocontent of pAkt ser-473 in the hippocampus relative to sedentary mice. Insulin (5 mU, icv) increased pAktser473 at 15 min after administration in both sedentary and exercised groups (*P < 0.05 sedentary vehicle vs. other groups). (#P < 0.05 exercised vehicle vs. exercised insulin at 15 min). Values are mean ± SE of six to eight mice per group.
Implications of icv Insulin Administration on Hippocampal Signaling in Exercised and Sedentary Mice
After 15 min and 24 h of insulin (5 mU) administration to sedentary mice, there was increased phosphorylation of pTyr-IR in hippocampal homogenates as well as increased IR immunocontent in the synaptic membranes of the hippocampus (sedentary vs. sedentary insulin; Fig. 4A, P < 0.05). Insulin administration did not further improve the effects of exercise on pTyr-IR phosphorylation and IR immunocontent in the hippocampus. Exercise significantly increased hippocampal pAKTser473 immunocontent relative to the sedentary group and icv insulin administration further increased the immunocontent of this protein after 15 min in both the sedentary and exercised groups (Fig. 4D P < 0.05).
Central Insulin Administration in Exercised Mice Increases NR2B Phosphorylation and Causes Glial Reactivity
We postulated that alterations in NMDA receptor (NMDAR) activity may be involved in the aversive and spatial memory deficits observed in exercised mice receiving icv insulin; this hypothesis was based on previous evidence showing that these receptors are involved in learning and memory processes (Collingridge, 1987). We thus measured the phosphorylation of NR2B (pNR2B), a functional subunit of the NMDAR. Physical exercise did not affect NR2B phosphorylation in hippocampal homogenates; however, when insulin was combined with physical exercise, there was a rapid (15 min; 73%) and persistent (24 h; 39%) increase in the immunocontent of pNR2B (Figs. 5A,B, P < 0.05).
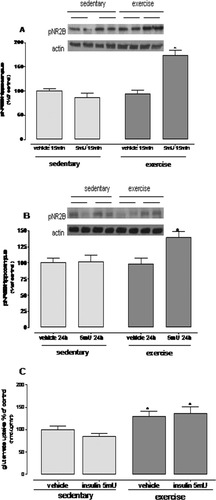
Exercise and/or 5mU icv insulin increases NMDAR activation and increases glutamate uptake. Insulin (5 mU, icv) increased the phosphorylation state of the NR2B subunit in the exercised group after 15 min (A) and 24 h (B) of administration. Neither insulin nor exercise alone altered the phosphorylation state. (C) Exercise increased glutamate uptake in hippocampal slices independently of insulin (*P < 0.05 exercised vs. sedentary groups). Values are mean ± SE of 8–10 mice per group.
Impaired control of glutamate levels in the synaptic cleft is closely associated with excitotoxic events via NMDAR. We thus investigated glutamate uptake capacity as a potential factor influencing NMDAR NR2B activation. Hippocampal slices obtained from exercised groups displayed an increased glutamate uptake capacity (30%) relative to sedentary mice (Fig. 5C; P < 0.05). However, insulin had no effect on uptake capacity. Thirty days of exercise increased the number of GFAP-positive cells (astrocyte proliferation) with no apparent activation of glial cells (Figs. 6A,B, P < 0.05). In the exercised group, however, insulin administration (5 mU) caused a marked activation of glial cells at 24 h with no change in the number of GFAP-positive cells (Figs. 6A,B, P < 0.05).
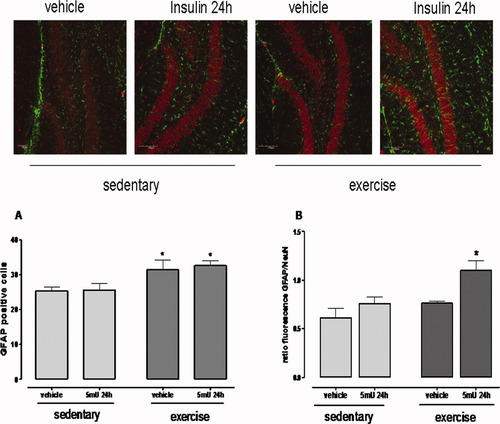
Exercise and 5 mU icv insulin increase glial reactivity in the hippocampus. (A) Exercise increased the number of GFAP-positive cells in the hippocampus independently of insulin (*P < 0.05 exercised vs. sedentary groups). (B) Exercise and 5 mU icv insulin increased the fluorescence intensity of GFAP in the hippocampus at 24 h (*P < 0.05 exercised insulin 24 h vs. other groups). Values are mean ± SE of four mice per group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Although physical exercise is known to increase peripheral insulin sensitivity, little is known regarding its effects on brain insulin sensitivity. In this study, we showed that exercise enhanced performance in spatial learning memory and increased insulin signaling in the mouse hippocampus. These results may be related to the observed increase in IR translocation/activation (pTyr-IR) and the increased responsiveness of downstream proteins to insulin signaling (e.g., pAKTser473). The presence of IR in the hippocampus and cerebral cortex suggests its functional involvement in learning and memory processes (Zhao et al., 1999). Indeed, insulin has been shown to enhance memory in both humans and experimental animals (Zhao et al., 2004). Similarly, physical exercise positively affects hippocampal plasticity and memory function (Cotman et al., 2007).
It is now recognized that brain insulin administration controls peripheral glucose homeostasis in a dose-dependent manner. In addition, peripheral or central glucose injections improve cognitive processes in both rodents and humans. These behavioral findings support the hypothesis that glucose acts directly on the brain to facilitate memory processing. However, there is still intense debate regarding insulin's ability to regulate brain glucose metabolism (Messier, 2004). In our study, icv insulin (5 mU) administration did not alter serum glucose levels measured at 30 min, 2 h, and 24 h in either sedentary or exercised mice (data not shown), ruling out the possibility that insulin-induced changes in glucose availability could account for the differences in memory performance. Dietrich et al. (2008) showed that spontaneous physical exercise increased mitochondrial activity in synaptic terminals in mouse hippocampus. Here, the increase in glucose oxidation might reflect the high energy demands of synaptic terminals, demands that parallel the increased mitochondrial activity induced by exercise. Insulin did not affect glucose oxidation in the ex vivo hippocampus of sedentary and exercised mice, supporting the previous theory that insulin plays a neuromodulatory role in the brain rather than controlling glucose metabolism.
The fact that IR is mainly detected in neuronal cells may indicate a cell-type specific modulation. Indeed, emerging data suggest that the brain is an insulin target and that insulin receptor signaling within the brain is involved in diverse functions including neuronal survival (Valenciano et al., 2006), synaptic plasticity (Lee et al., 2005; Stranahan et al., 2008) and learning and memory (Zhao et al., 1999; Dou et al., 2005). Here, we showed that exercise increased IR signaling (as indicated by increased levels of pTyr-IR and pAktser473 immunocontent) and increased IR levels in hippocampal synaptic membrane preparations without affecting the total levels of IR. However, insulin administration did not further increase IR immunocontent in exercised animals, suggesting that physical exercise may be a nonpharmacological, therapeutic intervention for neurological disorders associated with brain insulin resistance. The increased translocation and activation of IR was thus achieved independently of insulin administration. It has been suggested that insulin/IR signaling is activated in the early stages of memory formation and may play a role in sorting memories for long-term storage (Zhao et al., 2004).
Although several studies have suggested that insulin may improve memory performance in rats, there are few studies employing mice protocols. In the present study, we combined a lifestyle intervention known to positively affect peripheral insulin sensitivity and brain function with icv insulin intervention, trying to improve learning and memory performance. While we found that exercise improved performance in the water maze as previously described (Molteni et al., 2004; Parachikova et al., 2008), neither exercise nor icv insulin alone were able to modulate aversive memory in the inhibitory avoidance task. Surprisingly, we found that 5 mU of insulin had amnesic effects only in exercised animals. This effect was time-dependent, once after 15 days of exercise; insulin did not impaired performance on inhibitory avoidance task (data not shown).
Impairments in memory performance after insulin treatment have previously been described. Schwarzberg et al. (1989) reported that icv insulin administration prior to a retention trial impaired performance in a passive-avoidance task, while Kopf and Baratti (1999) showed that an intraperitoneal injection of insulin delivered to mice immediately after training impaired retention memory in an inhibitory avoidance task. In contrast to these findings, Park et al. (2000) showed that delivery of icv insulin to rats at 4 mU improved memory consolidation in a passive avoidance task. Even considering these aspects, we believe that some methodological differences (for example, route of administration, dose, memory task employed and rodent strain) could account for discrepancies among these studies [for a review see (Park, 2001)]. As previously proposed by Park (2001), the conflicting results observed in memory tasks after insulin administration may be caused by specific aspects of memory modulation such as acquisition, retrieval, or consolidation. In addition, different concentrations of insulin may modulate memory or other memory-influencing processes in opposite directions, resulting in a range of physiological effects. Here, we showed that icv insulin administration (5 mU) in exercised animals impaired long-term memory. Because the effects of insulin were examined after training, we propose that insulin affects the consolidation and retrieval of memory.
Insulin can potentiate NMDA activity and synaptic transmission in the hippocampal CA1 region (Liu et al., 1995). Because the activity of the NMDAR is dependent on its phosphorylation status, it has been proposed that insulin may directly alter NMDAR phosphorylation through multiple signal transduction pathways. The modulation of NMDA activity by insulin involves the phosphorylation of the functional NR2A and NR2B subunits (Christie et al., 1999). In the present study, the administration of icv insulin to exercised mice resulted in a rapid and persistent activation of the NMDAR NR2B subunit. We propose that this hyperactivation may be related to excitotoxic events as previously shown by Schumann et al. (2008), who found that NR2B hyperactivation is associated with brain damage. Our conjecture is based on the increased glial activation observed in insulin exercised mice. Physiologically, glial reactivity may have either neurotoxic or neuroprotective effects (Ren and Dubner, 2008). Therefore, the administration of insulin when insulin signaling is already increased, as it is after exercise, may be detrimental to brain functioning. Our results thus highlight the fine balance in brain insulin signaling and shed light into the neuromodulatory effects of insulin on the glutamatergic system. In accordance with this conjecture (Ramsden et al., 2003) showed that exercise animals have increased neuronal vulnerability to excitotoxicity induced by kainate when compared to sedentary ones. Thus, exercised animals seem to be more sensitive to a neurotoxic insult associated with overexcitation of glutamatergic system.
CONCLUSION
In conclusion, our main findings were that physical exercise increased hippocampal insulin receptor/signaling (IR/pTyrIR/pAktser473), glucose utilization and glutamate uptake. These mechanisms might underlie the enhanced spatial memory performance in exercised mice. In contrast, icv insulin administration (5 mU) had an amnesic effect in exercised mice likely caused by hyperactivation of NMDAR subunit NR2B; this effect paralleled increased glial activation.




