Ritonavir and Lopinavir Suppress RCE1 and CAAX Rab Proteins Sensitizing the Liver to Organelle Stress and Injury
Abstract
Organelle stress and Liver injuries often occur in human immunodeficiency virus (HIV) infected patients under anti-HIV therapies, yet few molecular off-targets of anti-HIV drugs have been identified in the liver. Here, we found through total RNA sequencing that the transcription of a host protease Ras converting CAAX endopeptidase 1 (RCE1) was altered in HepG2 cells treated with anti-HIV protease inhibitors, ritonavir and lopinavir. Levels of RCE1 protein were inhibited in HepG2 and primary mouse hepatocytes and in the liver of mice treated with the anti-HIV drugs, which were accompanied with inhibition of two potential substrates of RCE1, small GTP binding protein Rab13 and Rab18, which are with a common CAAX motif and known to regulate the ER-Golgi traffic or lipogenesis. Neither Rce1 transcription nor RCE1 protein level was inhibited by Brefeldin A, which is known to interfere with the ER-Golgi traffic causing Golgi stress. Knocking down Rce1 with RNA interference increased ritonavir and lopinavir-induced cell death as well as expression of Golgi stress response markers, TFE3, HSP47 and GCP60, in both primary mouse hepatocytes and mouse liver, and deteriorated alcohol-induced alanine aminotransferase (ALT) and fatty liver injury in mice. In addition, overexpressing Rab13 or Rab18 in primary human hepatocytes reduced partially the anti-HIV drugs and alcohol-induced Golgi fragmentation, Golgi stress response, and cell death injury. Conclusion: We identified a mechanism linking a host protease and its substrates, small guanosine triphosphate–binding proteins, to the anti-HIV drug-induced Golgi dysfunction, organelle stress response, and fatty liver injury.
Abbreviations
-
- AAV
-
- adeno-associated virus
-
- AIDS
-
- acquired immune deficiency syndrome
-
- ALB
-
- albumin
-
- ALT
-
- alanine aminotransferase
-
- ART
-
- anti-retroviral treatment
-
- Br
-
- brefeldin A
-
- CMV
-
- cytomegalovirus
-
- Ct
-
- control
-
- ER
-
- endoplasmic reticulum
-
- Et
-
- alcohol
-
- GTP
-
- guanosine triphosphate
-
- HIV
-
- human immunodeficiency virus
-
- LPV
-
- lopinavir
-
- mRNA
-
- messenger RNA
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- PBS
-
- phosphate-buffered saline
-
- PHH
-
- primary human hepatocyte
-
- PI
-
- protease inhibitor
-
- PMH
-
- primary mouse hepatocyte
-
- RCE1
-
- Ras converting CAAX endopeptidase 1
-
- RL
-
- ritonavir-boosted lopinavir
-
- RLE
-
- RL combined with alcohol
-
- RNA-Seq
-
- RNA sequencing
-
- RT-PCR
-
- real-time polymerase chain reaction
-
- RTV
-
- ritonavir
-
- scRNA
-
- scrambled RNA
-
- shRNA
-
- short hairpin RNA
-
- siRNA
-
- small interfering RNA
-
- UPR
-
- unfolded protein response
Anti-retroviral treatment (ART) improves the quality of life for millions of human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) patients. ART also allows HIV-infected individuals to survive into older age. It is estimated that greater than 50% of HIV-infected Americans will be 50 years or older by 2020,(1, 2) and most of them will need ART for their extended life. Such unprecedent longer time of anti-HIV medication will likely increase side effects of the drugs. There are numerous reports indicating that some current antivirals singly or in combination increase the risk of comorbidities.(3-5) Although some side effects of anti-HIV drugs are manageable, some can be very serious and fatal. For instance, hepatic injuries have emerged as the major non-AIDS-related cause of death among patients with HIV/AIDS.(6-10) Under ART, increased rates of nonalcoholic fatty liver disease (NAFLD) have been reported in HIV mono-infected subjects, mostly with persistently elevated liver enzymes.(9) Before introduction of ART, patients with AIDS could develop dyslipidemia characterized by isolated elevation of triglycerides and decrease in total cholesterol.(3-5) With the advent of ART, especially with the use of combined anti-HIV protease inhibitors (PIs) or alcohol consumption, this situation changed to a severe lipid profile that is more prone to developing NAFLD.(6, 11, 12) Given that the overall NAFLD prevalence among the adult population is projected to be greater than 30% by 2030,(13) the prevalence of NAFLD in patients with HIV under ART could reach as high as 80%. It is of paramount significance to explore pathogenic mechanisms underlying the hepatoxicity of anti-HIV drugs to provide a basis for better management of patients with AIDS suffering from liver injury.
Anti-HIV drugs of current regimens such as ritonavir (RTV), lopinavir (LPV), indinavir, or atazanavir are reported to induce organelle stress, cell death, and fatty liver.(14-17) Two organelles, endoplasmic reticulum (ER) and Golgi apparatus, are particularly involved in the pathogenesis of the anti-HIV drug-induced fatty liver disease. The ER stress triggers protective unfolded protein response (UPR), which involves three ER stress sensors, IRE1, PERK and ATF6, to restore initially ER homeostasis and minimize injuries. However, prolonged UPR such as under long-term ART induces fat accumulation and cell death, which are well documented to lead to development of hepatic steatosis.(18) The Golgi is part of the cellular endomembrane system, which receives secretory and membrane proteins from the ER and delivers them to various destinations through membrane-bound vesicles. Like the ER, the Golgi can be stressed under prolonged anti-HIV drug use and initiates Golgi stress response, which involves a few regulating factors/pathways: TFE3, CREB3-ARF4 pathway, and HSP47.(19, 20) TFE3 regulates transcriptional activation of genes encoding vesicular trafficking components or Golgi resident enzymes. The CREB3-ARF4 pathway involves both ER and Golgi, in which ARF4 is a member of the small GTPase family that regulates Golgi-to-ER vesicular trafficking. HSP47 is an ER chaperone that protects cells against the Golgi stress and cell death. We reported previously that treating mice with a standard regimen for HIV-infected patients (i.e., RTV-boosted LPV [RL]) induced moderate dilatation of the ER as well as dispersed Golgi apparatus, which was accompanied with fatty liver injury.(15, 21) Furthermore, we found that the three canonical UPR branches, IRE, PERK and ATF6, were differentially expressed in hepatocytes in response to RL.(21) The ATF6 branch as well as its downstream factors were inactivated, whereas the other two branches of UPR were up-regulated. These observations are of great interest, as the activation of ATF6 is known to require ER-to-Golgi trafficking for proteolytic processing that involves both the ER and the Golgi.(22) We further reported that co-localization of ATF6 and the Golgi was lower in the liver cells treated with the antivirals than in the cells treated with the specific ER stress–inducing agent tunicamycin.(21) In parallel to the reduced co-localization, marked Golgi fragmentation, fat accumulation, and cell death were observed. Moreover, the severity of the Golgi fragmentation in response to other anti-HIV drugs including amprenavir, nelfinavir and darunavir, was well correlated with downstream ER stress, cell death, and fatty liver injury.(21) All of these pieces of evidence suggest that Golgi dysfunction and disturbed ER-Golgi trafficking contributes to the anti-HIV drug–induced ER stress and liver injuries. In this study, we hypothesized that there are off-target(s) of the anti-HIV drugs that either regulate or constitute the ER-Golgi trafficking machinery, and we identified through total RNA-sequencing a host protease as one specific off-target. We found that the protease inhibition is associated with inhibition of its substrates, Rab proteins with CAAX motif, which belong to a family of small guanosine triphosphate (GTP)–binding proteins known to coordinate the ER-Golgi traffic or Golgi stress response.
Methods
Liver Cell Cultures and Treatments
Three types of liver cells were used: HepG2, primary mouse hepatocytes (PMHs), and primary human hepatocytes (PHHs). Sources, catalog numbers, and culturing conditions for HepG2 and PMHs are described in the Supporting Information. PMHs were isolated by the USC Liver Cell Culture Core and cultured as reported previously.(15) In some experiments, the 5C long-term culturing of PHHs or PMHs was conducted according to Xiang et al.(23) The liver cells were treated in fetal bovine serum–free medium for 6 hours with 20 nM of Brefeldin A, 85 mM of alcohol, 25 μg/mL of RTV, 25 μg/mL of LPV, RL, or other alcohol and drug combinations. DMSO (< 0.05%, wt/vol) or ethanol (<0.05%, vol/vol) in Dulbecco's modified Eagle's medium was used as vehicle control. After the treatments, a portion of the treated cells were washed with ice-cold phosphate-buffered saline (PBS) and prepared for double staining with Syntax green and Hoechst blue for cell death or immunohistochemistry for confocal microscopy of organelle (e.g., Golgi apparatus) morphology described previously.(21) Other portions of the cells were detached with 0.05% trypsin–ethylene diamine tetraacetic acid solution for RNA isolation for total RNA-sequencing analysis and real-time polymerase chain reaction (RT-PCR) or for protein extraction for western blotting. In vitro experiments were repeated in triplicate.
Animal Models
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The animals were grouped into six groups: groups 1, 2, 3, and 4 were given with isocaloric control diet, and groups 5 and 6 were given with 5% alcohol liquid diet (Dyets, Inc., Bethlehem, PA) for 10 days. During the 10-day period and from the fifth day, groups 2, 3, and 4 with the control diet were injected intraperitoneally with RTV (25 μg/g), LPV (25 μg/g), and RL (25 μg/g each), respectively, for 5 days, and group 6 with the alcohol diet were injected with RL (25 μg/g each) for 5 days. On the 11th day, groups 5 and 6 were gavaged with 30% alcohol in PBS (5 mg/g) and groups 1, 2, 3, and 4 were gavaged with equal volume of isocaloric maltose solution. The mice were euthanized for serum and liver tissues 8 hours after the gavage. All animals were treated in accordance with the Guide for Care and Use of Laboratory Animals, and the study was approved by the local animal care committee. Analyses for serum alanine aminotransferase (ALT) and liver triglycerides, liver histology by hematoxylin and eosin staining, and liver steatosis by Oil Red O staining were described previously.(15, 21, 24)
Total RNA Sequencing
Total RNA was isolated from the HepG2 cells that were pooled from the same duplicated treatments using the SMARTer Stranded Total RNA-Seq Kit v2-Pico Input Mammalian Kit (Clontech; Takara, Tokyo, Japan). The RNA sequencing (RNA-Seq) featuring Illumina's next-generation systems was conducted by the Molecular Genomics Core of USC. Subsequent total RNA-Seq data analysis was performed with the Partek Flow software, version 5.0 (Partek Inc., St. Louis, MO). A z-score of 1 is 1 SD above the mean. A score of 2 is 2 SDs above the mean. The Ingenuity Pathway Analysis (IPA) provided by Qiagen (Venmo, Netherlands) was applied to identify drug targets and predict downstream/upstream drug effects. The Core Analysis tabs were filtered with organelle stress responses that were specific to the anti-HIV drugs and alcohol.
Immunoblotting and RT-PCR
Methods for protein and RNA extractions, immunoblotting, and RT-PCR were described previously.(21) All names and sources of the primary antibodies are listed in Supporting Table S1. For PCR analysis, about 30 mg of tissue was grinded in Qiazaol and extracted with the DirectZol RNA extraction kit (Zymo Research, Irvine, CA). All primers for Golgi and ER stress markers are listed in Supporting Table S2. Quantifications of the protein bands on the western blots were conducted with ImageJ (National Institutes of Health).
RNA Interference
Stealth small interfering RNAs (siRNAs) generated from the next-generation RNA interference chemistry were purchased from Thermo Fisher Scientific (Waltham, MA). Assay IDs for Ras converting CAAX endopeptidase 1 (RCE1) and ZMPSTE24 were HSS145393 and HSS115697, respectively. Briefly, siRNAs were diluted at 30 pmol/200 μL OptiMEM and mixed with 3 μL of Lipofectamine 3000 at room temperature for 15 minutes, to which 0.1 million of HepG2 cells in 1 mL of antibiotics-free complete medium was added. The transfected cells were incubated in 12-well plates coated with collagen I for 48 hours. siRNA negative control (ID# 4390844) and positive control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) siRNA (ID# AM4605) were used for optimizing the knocking-down conditions. The gene knockdown was checked by RT-PCR or western blotting. The subsequent drug/alcohol treatments of the transfected cells were the same as described previously. Short hairpin RNA (shRNA) expression vector (pAV-RCE1v1-shRNA; SH898883) for knocking down RCE1 in PMHs and adenovirus with cytomegalovirus (CMV) promoter-driven expression of RAB13 (VH806960) or Rab18 (VH800874) in PHHs were purchased from ViGene Biosciences (Rockville, MD). The shRNA sequences are listed in Supporting Table S3. For systemic in vivo shRNA delivery in mice, the shRNA sequences were cloned into an adeno-associated virus (AAV) vector under the control of a mouse albumin (ALB) promoter for liver expression (AAV-ALB-RCE1-shRNA). AAV8-ALB-shRNA or AAV8–ALB–scrambled RNA (scRNA) (control) was produced through the custom cloning and viral packaging service from ViGene Biosciences. Each mouse was injected intravenously with 3 × 109 AAV in 100 μL PBS and were examined for subsequent drug and/or alcohol treatments 2 days after the injection.
Statistics
Data are presented as means ± SEM unless otherwise indicated. Statistical analyses were performed with GraphPad Prism 6 software using the one-way analysis of variance (ANOVA) for comparison of multiple groups and two-way ANOVA for comparison of trends between different treatments. The P values of 0.05 or less are considered significant.
Results
Identification of Genes Responsive to Anti-HIV Protease Inhibitors and Alcohol by Total RNA-Seq
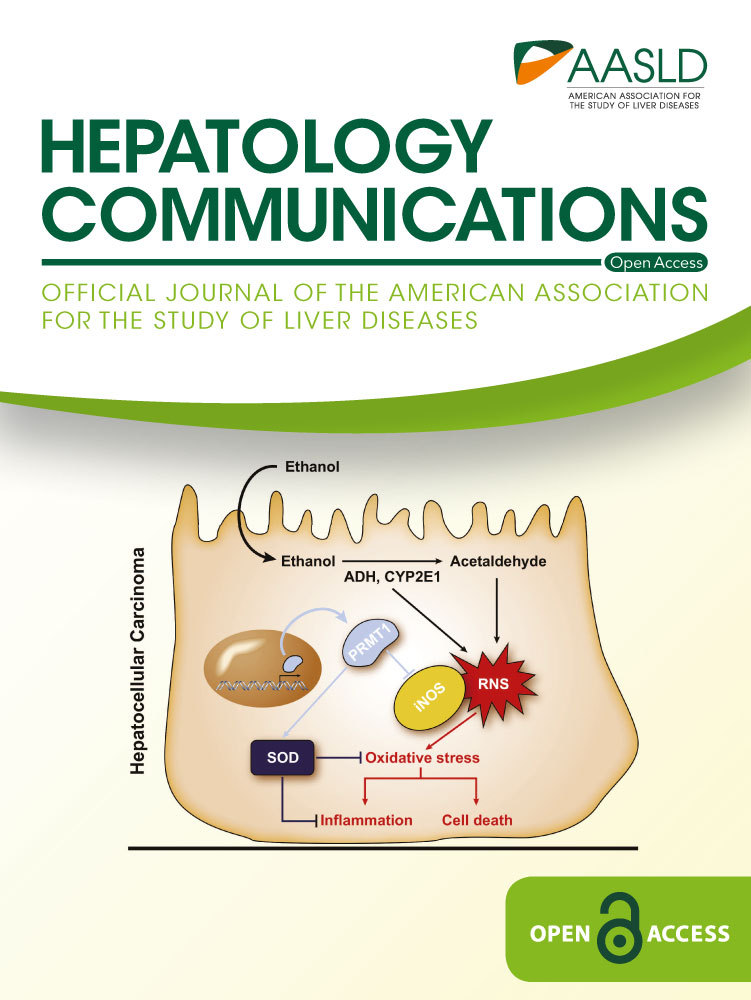
It was reported previously that anti-HIV PIs (e.g., RTV and LPV) induced Golgi fragmentation, ER stress and hepatic injury, which could be deteriorated by alcohol.(15, 21) We assumed that there are specific off-target(s) of the anti-HIV drugs that are relevant to Golgi or organelle stress. To find the specific off-target(s), we conducted total RNA-seq on RNA samples from HepG2 cells treated with brefeldin A (positive control for Golgi dysfunction), alcohol, RL, or RL combined with alcohol (RLE). The output of RNA-seq was analyzed using the Qiagen software for IPA, which predicts downstream effects and identifies new drug targets. First, genes/factors that are not relevant to organelle (e.g., ER, Golgi, mitochondria, lysosome) stress and with z-scores of less than 1.5 were filtered out, which narrowed the number of the genes of interest down to 132 (Fig. 1A, the heatmap). Eighty-seven genes were up-regulated and 45 were down-regulated in response to the anti-HIV drug treatment. Second, genes/factors that were drug-responsive but were not further affected by alcohol exposure were filtered out, which narrowed down the genes of interest to 10. These genes are CHD2 (encodes the chromodomain DNA helicase binding protein 2 involved in chromatin remodeling), FOSL2 (FOS like 2, AP-1 transcription factor subunit), GP73 (hepatic Golgi protein 73), MAFK (one of the small Maf transcription factor proteins with basic region and leucine zipper [bZIP]), MKRN2 (E3 ubiquitin-protein ligase makorin-2), MT-ATP6 (mitochondrially encoded ATP synthase membrane subunit 6), OSGIN2 (oxidative stress induced growth inhibitor family member 2), RCE1, SIAT4A (Golgi-related sialyltransferase 4A), and ZFPL1 (zinc finger protein like 1) (Fig. 1, highlighted heatmaps).
The transcriptional expression of the 10 genes was further examined in PMHs treated with brefeldin, alcohol, RL, or RLE. The expression patterns of CHD2, FOSL2, GP73, MAFK, MT-ATP6, OSGIN2, or SIAT4A revealed by RT-PCR were not consistent with those in HepG2 revealed by the RNA-seq (data not show). The expression patterns of three genes, MKRN2, RCE1 and ZFPL1, were consistent with those by the RNA-seq (Fig. 1B). However, only the expression patterns of RCE1 were robustly and significantly consistent in both PMHs and HepG2.
Association of Inhibited RCE1 Protein With Alcohol and Anti-HIV Drug-Induced Golgi Stress and Injury
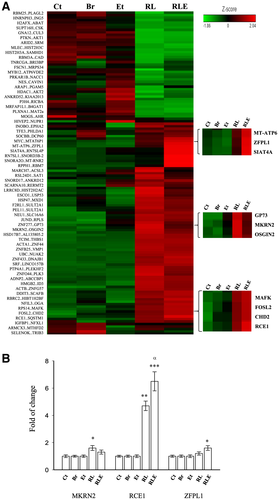
In HepG2 cells, treatment of RTV, LPV, or alcohol slightly reduced the protein levels of RCE1 compared with vehicle control (Fig. 2A and Supporting Table S4). Remarkable reductions of RCE1 were detected in RL or in the combination of RL and alcohol. Brefeldin, which inhibits protein trafficking from the Golgi to the ER,(25) did not inhibit RCE1, indicative of the specific inhibition of RCE1 protein by the anti-HIV drugs and alcohol. Messenger RNA (mRNA) of Rce1 was significantly increased by RL and was nearly doubled in the combination treatment with the drugs and alcohol (Fig. 2B). The feedback increase of Rce1 mRNA was associated with increased Golgi stress markers: TFE3, Hsp47, and GCP60. In contrast, no significant changes of Rce1 mRNA were detected in response to brefeldin, despite that it induced increase of the Golgi stress markers by more than 2-fold. To confirm that the drugs and alcohol-induced RCE1 reduction is accompanied with Golgi dysfunction and cell injury, Golgi morphology and cell death were examined. As expected, either alcohol or the drugs induced Golgi fragmentation in HepG2, and the Golgi fragmentation was much severe in the liver cells treated with the drugs combined with alcohol (Fig. 2C). In addition, near 30% cell death was detected in the HepG2 treated with the anti-HIV drugs and alcohol, which was comparable to the cell death induced by the positive-control brefeldin (Fig. 2D).

The drug and alcohol-induced reduction of RCE1, increase of Golgi stress (e.g., TFE3), Golgi fragmentation, and cell death were also detected in PMHs (Fig. 2E-G and Supporting Table S4).
In addition, in the liver of mice fed the anti-HIV drugs or alcohol alone, minor changes in the protein levels of RCE1 and fatty liver injury indicated by ALT and Oil Red O staining were detected (Fig. 3 and Supporting Table S5). Consistent with the in vitro results, reduction of RCE1 and increase of Golgi stress marker TFE3 were observed in the liver of mice treated with combinations of the drugs and alcohol, which were accompanied with greater than 10-fold increase of ALT and accumulation of fat. In contrast, brefeldin induced Golgi stress, increased ALT, and caused moderate fat accumulation without reducing RCE1 protein.
Effects of Knocking Down Rce 1 on Golgi Stress and Hepatic Injury
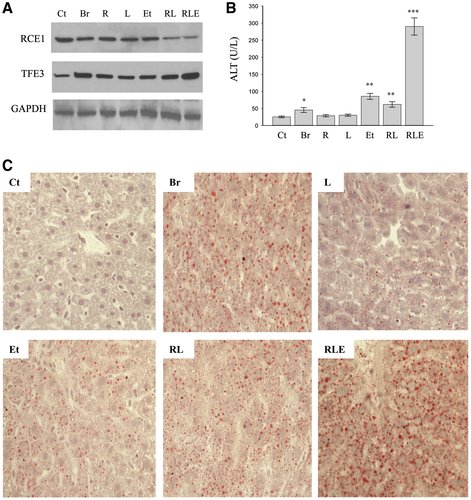
Knocking down Rce1 with RNA interference was conducted to determine further the role of Rce1 in the drug and alcohol-induced Golgi stress and cell injury. Expression of RCE1 protein in HepG2 was reduced by 70% by Rce1 siRNA. The reduction of RCE1 in HepG2 did not lead to apparent morphological differences from the control cells treated with scRNA in the absence of the drugs or alcohol. However, the RCE1 knocking down sensitized the drugs and alcohol-induced Golgi stress response (Fig. 4A). The Golgi stress markers, TFE3 (protein expression) and GCP60 (mRNA expression), were increased by 4-fold and 5-fold, respectively, in the cells with the RCE1 being knocked down and treated with the drugs and alcohol (Fig. 4A-C). In contrast, the effect of knocking down RCE1 on brefeldin-induced Golgi stress was not significant. In addition, the anti-HIV PIs were reported to inhibit STE24 overexpressed in yeast.(26) We confirmed that knocking down of STE24 had significant effects on the drug-induced Golgi stress response (Fig. 4D-F). Consequently, knocking down RCE1, or STE24 to a less extent, sensitized the cells to the drugs and alcohol-induced death injury (Fig. 4G).
shRNA for RCE1 was chosen to suppress RCE1 in the liver of mice to further address the role of the identified host proteases in the anti-HIV drugs and alcohol-induced hepatic Golgi stress and injury. Interestingly, compared to mice treated with scRNA and shRNA, knocking down RCE1 in the liver did not result in significant changes in TFE3 or GCP60 expression, liver histology, or ALT (Fig. 5). However, when the mice were challenged with the anti-HIV drugs, the Golgi stress response induced by TFE3 and GCP60 was significantly higher in the liver with RCE1 knocking down than without the knocking down (Fig. 5A,B). The drug-induced hepatic foamy and ballooning degenerations were more severe in the liver of mice treated with RCE1 siRNAs, which were accompanied with increased liver triglycerides and ALT (Fig. 5C-E), indicative of exacerbated damage to the liver by the anti-HIV drugs.
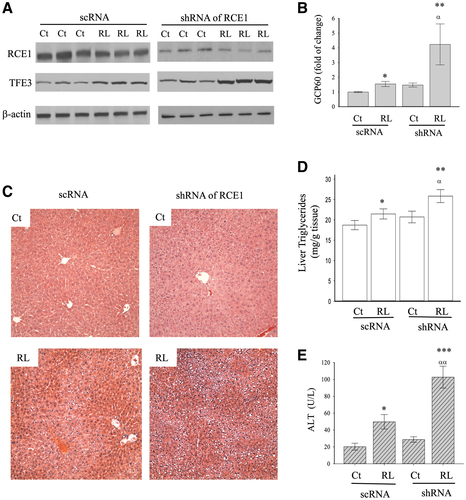
Association of CAAX Rab Proteins With RCE1 Expression and Golgi Stress in Liver Cells
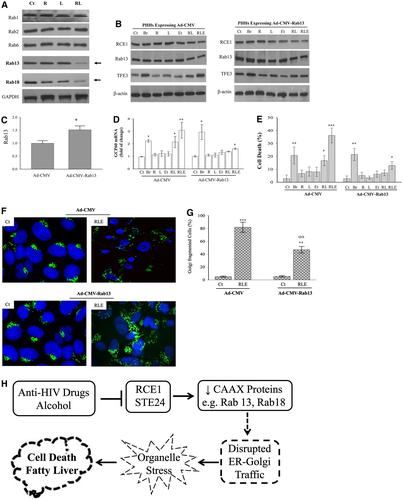
From previous results, we know that disturbed ER-Golgi trafficking underlies the anti-HIV drug and alcohol-induced liver injury.(21) The ER-Golgi traffic involves many effectors such as vesicle tethers, SNAREs, and membrane and motor proteins, which are coordinated by the Rab GTPases.(27, 28) We assumed that there is a biochemical connection between RCE1 and the Rab proteins and examined protein expression of the Rab GTPases in the liver of mice treated with the anti-HIV drugs. Immunoblotting was conducted for 20 Rab proteins that are likely to regulate the ER-Golgi trafficking in mammalians: Rab1, Rab2, Rab6, Rab8, Rab10, Rab11, Rab12, Rab13, Rab14, Rab18, Rab19, Rab24, Rab31, Rab32, Rab33, Rab34, Rab36, Rab39, Rab42, and Rab43 (Fig. 6A and Supporting Figs. S1 and S2). Two Rab proteins, Rab13 and Rab18, were significantly suppressed in the presence of the anti-HIV drugs. Intriguingly, both Rab13 and Rab18 have a common CAAX motif, and their maturation is known to require proteolytic cleavage of the motif by RCE1 or the evolutionarily unrelated protease STE24.(29, 30) Because prelamin A is the substrate of STE24,(30) Rab13 and/or Rab18 are potential substrates of RCE1. In support of this assumption, Rab13 overexpression in PHHs infected with adenovirus capable of CMV promoter-driven expression of Rab13 (Ad-CMV-Rab13) rescued the human liver cells from the drug-induced RCE1 inhibition, Golgi stress, and injury. Rab13 was increased by more than 40% in PHHs compared with the mock control (Fig. 6B,C and Supporting Table S6). The Rab13 overexpression in PHHs decreased the drugs and alcohol-induced GCP60 mRNA by 47%, as well as reduced the cell death by 67% in the presence of RCE1 inhibition (Fig. 6D,E). In contrast, brefeldin-induced Golgi stress and cell injury were not affected by the Rab13 overexpression in PHHs. Moreover, the drug-induced Golgi fragmentation was reduced in the human hepatocytes overexpressing Rab13 (Fig. 6F,G). Similar rescuable Golgi stress response and cell death were observed in the human hepatocytes overexpressing Rab18 (Supporting Fig. S3).
Discussion
Investigations on the molecular mechanisms of hepatotoxicity by certain anti-HIV drugs have been going on for two decades since their side effects on the liver were first reported in 1997.(31) However, few specific off-targets of the anti-HIV drugs have been identified. Our research has been focusing on metabolic and/or organelle stress mechanisms, because the stress responses generally generate danger signals co-stimulating/compromising the immune system, leading to liver injury, and the molecular mechanisms of the stress responses have been well established to underlie lipogenesis and promote fatty liver injury,(18, 19, 32) the latter of which could lead us to reveal specific molecular off-targets of the anti-HIV drugs in the liver. In this investigation, following previous evidence that the antivirals disrupted ER-Golgi trafficking, causing organelle stress responses, cell death and fatty liver, we used large-scale screening with the total RNA-seq for genes/pathways pertinent to the cellular organelles and stress responses and revealed a couple of candidates. We believe that RCE1 is one of the specific off-targets of the anti-HIV drugs in the liver, based on our experimental findings. First, the feedback transcriptional up-regulation of Rce1 RNA and the RTV-induced and LPV-induced inhibition of RCE1 protein occurred in both HepG2 and PMHs, as well as in vivo mouse livers that accumulate fat, which were associated with Golgi fragmentation and stress responses. Second, brefeldin is known to inhibit protein transport from the Golgi apparatus to the ER indirectly, by preventing the association of COP-I coat to the Golgi membrane leading to the Golgi stress. Although both brefeldin and the anti-HIV drugs induced Golgi fragmentation and Golgi stress response in the liver cells, expression of Rce1 RNA or protein was not responsive to the brefeldin challenge, suggesting that the inhibition of RCE1 by the anti-HIV drugs is specific. Third, our loss of gene-function analysis with RNA interference supports the role of RCE1 in mediating the side effects of the anti-HIV drugs. Knocking down Rce1 with siRNA or shRNA potentiated the anti-HIV drug-induced injuries in both liver cells and mouse livers, which were accompanied by increased expression of Golgi stress marker TFE3 or GCP60, liver triglycerides, and ALT values.
Our findings that the anti-HIV PIs inhibited RCE1 or STE24 are of mechanistic significance in the anti-HIV drug-caused hepatotoxicity. RCE1 and STE24 are the only two endopeptidases that are known to function in the maintenance and processing of CAAX-type prenylated proteins.(29, 30) It was reported that the enzymatic activity of ZMPSTE24 purified from yeast expressing human Ste24 was inhibited by the anti-HIV PIs in a manner that is indicative of a competitive mechanism.(26) STE24 was already identified to process prelamin A to a mature nuclear form.(30) Mutation of Ste24 leads to premature-aging disease, lipodystrophy, steatohepatitis, and skeletal abnormalities,(30, 33, 34) which are often seen in patients with AIDS under anti-HIV therapies. For RCE1, our results support its association with Rab13 or Rab18, as levels of the two Rab proteins were reduced in the mouse liver treated with the anti-HIV drugs. Because both Rab13 and Rab18 have the CAAX motif and their maturity requires proteolytic processing, Rab13 and Rab18 are likely the direct substrates of RCE1. The connection between RCE1 and Rab proteins is interesting. It provides a mechanic link between an inhibited protease and the disrupted ER-Golgi trafficking and subsequent cellular organelle stress responses. The ER-Golgi trafficking involves vesicle budding, uncoating, docking, and fusion that are often through recruitments of effectors such as vesicle tethers, SNAREs, membrane, and motor proteins.(27, 35) These effectors are generally regulated by the Rab proteins that belong to a large family of small GTP-binding proteins, which accomplish their functions by switching between an inactive GTP-bound and an active GTP-bound form.(27, 28) Rab13 regulates ER-Golgi trafficking,(36) and other Rab proteins such as Rab18 are involved in cellular processes including formations of inflammasome and autophagosome, lipid droplets turnover, and ER calcium homeostasis,(37, 38) all of which could promote fatty liver injuries that are increasingly observed in patients with AIDS under anti-HIV treatments. Although direct biochemical evidence for the enzyme-substrate relation between RCE1 and Rab13 or Rab18 awaits further investigation, the observation that overexpression of Rab13 or Rab18 in PHHs not only mitigated the Golgi stress response but also partially rescued the liver cell from death injury supports the critical role of the RCE1-Rab pathway in the anti-HIV drug-induced hepatotoxicity.
The HIV PIs were originally designed to inhibit the HIV aspartyl protease, which is required for the essential peptide bond hydrolysis during the life cycle of HIV. RCE1 or STE24 is a glutamyl protease classified as a member of the zinc metalloproteinase family.(29, 30) The question is: How could the aspartyl PIs inhibit the host glutamyl proteases? There are three possibilities. First, recent crystallization and analysis of the enzyme protein of glutamyl protease STE24 indicated that many ordered water molecules locate inside the internal cavities of the enzyme, which provides structural stability and promotes tetrahedral coordination of the active site zinc atoms.(39) This arrangement of the waters in the active site differ somewhat from other metalloproteases, which could accommodate some structural flexibility, which permits off-target access by certain anti-HIV PIs. Second, our previous results demonstrated that LPV-induced Golgi fragmentation and injury in the liver cells were less severe than RTV, and darunavir (newly developed)–induced Golgi stress and hepatic damages were less than LPV, suggesting that certain molecular structures of the inhibitors are more prone to exert the drug's side effects. In fact, the core region of LPV that contains a hydroxyethylene dipeptide isostere is near the same as that of RTV, except that RTV has a longer side isopropyl thiazolyl group that is used to inhibit cytochrome P450 3A4 and boost the circulating concentration of other HIV PIs.(40, 41) On the other hand, darunavir has a benzyl group that may hinder off-target access to the host protease. However, because the current regimens often contain RTV-boosted HIV inhibitors including darunavir,(42) it appears inevitable to avoid the liver injury side effects in patients with AIDS under ART without developing newer anti-HIV drugs. Third, although it independently induces the Golgi stress and liver injury that was reported previously,(21, 43) alcohol alone appeared to contribute little to the inhibitory effects on RCE1 or STE24. Our observations that the drugs combined with alcohol potentiated the inhibitory effects on RCE1 and deteriorated liver injuries are likely due to alcohol's boosting effects on the bioavailability of the anti-HIV drugs. We previously reported that the plasma concentrations could be increased by 5-fold in mice treated with alcohol and RTV and by more than 10-fold in mice treated with alcohol, RTV, and LPV.(15)
In summary, we have found that anti-HIV PIs inhibited RCE1 and its potential substrates, Rab proteins with CAAX motif, which interfered with the ER-Golgi trafficking, leading to organelle stress responses and fatty liver disease (Fig. 6H). This study may provide a molecular basis for designing anti-HIV drugs with less side effects in the liver.
Acknowledgments
The authors thank the USC Liver Research Center for technical assistance.
[Corrections added on April 30, 2020, after first online publication: “Acknowledgments” section has been included.]