A Telephone and Mail Outreach Program Successfully Increases Uptake of Hepatocellular Carcinoma Surveillance
Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide. Society guidelines recommend surveillance with abdominal ultrasound with or without serum alpha-fetoprotein every 6 months for adults at increased risk of developing HCC. However, adherence is often suboptimal. We assessed the feasibility of a coordinated telephone outreach program for unscreened patients with cirrhosis within the Veteran’s Affairs (VA) health care system. Using a patient care dashboard of advanced chronic liver disease in the VA Greater Los Angeles Healthcare System, we identified veterans with a diagnosis of cirrhosis, a platelet count ≤ 150,000/uL, and no documented HCC surveillance in the previous 8 months. Eligible veterans received a telephone call from a patient navigator to describe the risks and benefits of HCC surveillance. Orders for an abdominal ultrasound and alpha-fetoprotein were placed for veterans who agreed to surveillance. Veterans who were not reached by telephone received an informational letter by mail to encourage participation. Of the 129 veterans who met the eligibility criteria, most were male (96.9%). The most common etiology for cirrhosis was hepatitis C (64.3%), and most of the patients had compensated cirrhosis (68.2%). The patient navigators reached 32.5% of patients by phone. Patients in each group were similar across clinical and demographic characteristics. Patients who were called were more likely to undergo surveillance (adjusted odds ratio = 2.56, 95% confidence interval: 1.03-6.33). Most of the patients (72.1%) completed abdominal imaging when reached by phone. Conclusion: Targeted outreach increased uptake of HCC surveillance among patients with cirrhosis in a large, integrated, VA health care system.
Abbreviations
-
- AFP
-
- serum alpha-fetoprotein
-
- ALD
-
- advanced liver disease
-
- aOR
-
- adjusted odds ratio
-
- CT
-
- computed tomography
-
- EMR
-
- electronic medical record
-
- GI
-
- gastroenterology
-
- HCC
-
- hepatocellular carcinoma
-
- PCP
-
- primary care provider
-
- QI
-
- quality improvement
-
- US
-
- ultrasound
-
- VAGLAHS
-
- VA Greater Los Angeles Healthcare System
-
- VA
-
- Veterans Affairs
-
- VHA
-
- Veterans Health Administration
Liver cancer is the sixth most commonly diagnosed cancer and fourth leading cause of cancer-related death worldwide.(1) Hepatocellular carcinoma (HCC) is by far the most common histologic cell type, accounting for as much as 90% of all cases in the United States.(2) The incidence of HCC increased by 115% between 2000 and 2012 in the United States and is projected to continue to rise.(3) This is in part due to an aging population of patients with cirrhosis, untreated cohorts of patients with chronic hepatitis C virus (HCV),(4, 5) and the rising incidence of nonalcoholic fatty liver disease, in whom risk of HCC development is thought to be driven by metabolic risk factors such as obesity and diabetes mellitus.(6, 7) In an effort to detect early lesions that may be amenable to curative therapies, international society guidelines recommend surveillance for HCC with biannual ultrasound for certain high-risk patients with chronic hepatitis B and all patients with cirrhosis.(8-10) The American Association for the Study of Liver Diseases recommends using biannual alpha-fetoprotein (AFP) levels along with abdominal ultrasound.(11) In addition, the guidelines recommend that surveillance be conducted in health care settings with standardized surveillance tests, recall procedures, and quality control measures.(11) Despite these guidelines, the adherence to these recommendations remains low, and targeted efforts to address low rates are sparse.(12, 13)
The U.S. Veterans Health Administration (VHA) cares for one of the nation’s largest population of patients with HCV and advanced liver disease (ALD).(14) Adherence to HCC surveillance for patients with cirrhosis in the VHA is estimated at only 42%.(15) The reasons for this low rate are unclear. Access to gastroenterology and hepatology in the VHA is associated with higher reported rates of surveillance, but rates are still low despite most centers now offering these specialists.(16) Patients in non-veteran settings report that factors such as scheduling, cost of the test, uncertainty of where to have surveillance performed, and transportation reduce surveillance rates.(17) Provider factors include not being up to date with surveillance guidelines, difficulty communicating the importance of surveillance to patients, and prioritizing other issues in the clinic.(18, 19) The process therefore requires a multilevel and multistep approach for appropriate completion. For this process to be effective, providers need to identify high-risk patients and order the appropriate surveillance. In addition, health care systems must facilitate timely scheduling of surveillance exams, and patients must recognize surveillance completion as a priority.(20, 21) In an effort to overcome some of these barriers, interventions such as mailed patient outreach and primary care reminders have been shown to improve HCC surveillance rates.(21, 22) A recent randomized control trial by Singal et al. demonstrated that mailed outreach increased HCC surveillance uptake compared with standard of care in a non–Veterans Affairs (VA) health care system, supporting the idea that providing systematic outreach programs does improve quality improvement measures.(21)
In this study, we conducted a pilot and feasibility quality-improvement study of coordinated telephone and mail-outreach program for HCC surveillance in a VA facility in Los Angeles. We focused on high-risk patients, defined as patients with cirrhosis who had fallen out of the appropriate HCC surveillance protocol at study initiation, with the intention of improving HCC surveillance and identifying patient-specific barriers that are unique to its diverse population.
Methods
Data Source
We abstracted data from a patient care dashboard of advanced liver disease in the VA Greater Los Angeles Healthcare System (VAGLAHS). The Veterans Integrated Service Networks (VISN) 22 ALD dashboard draws data from the VA’s corporate data warehouse to allow for tracking of characteristics of patients with ALD, to centralize and advance care. Access to the ALD dashboard is available within the VA network to VA providers, to allow patient tracking, monitoring, and linkage to care. We identified patients using the International Classification of Diseases, 9th and 10th revisions (Supporting Table S1).(23) The VAGLAHS Institutional Review Board deemed our work exempt, consistent with ongoing hospital QI efforts.
Study Population
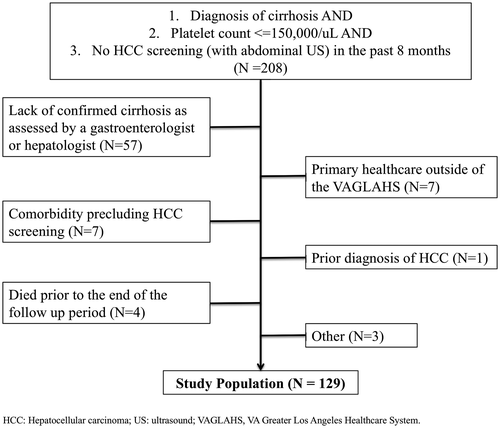
Eligible patients had (1) a diagnosis of cirrhosis (identified using the ALD dashboard and confirmed by hepatology and gastroenterology provider notes), (2) platelet count ≤ 150,000/uL, and (3) no electronic medical record (EMR) documentation of abdominal imaging for HCC surveillance in the preceding 8 months (based on time frames established by the preprogrammed ALD dashboard). Next, we performed a chart review of the EMR for these patients and excluded patients without a confirmed cirrhosis diagnosis based on clinical documentation from a gastroenterologist or hepatologist before study initiation, to minimize anxiety for patients who had not been formally evaluated and informed of a diagnosis of cirrhosis. Patients with primary health care outside the VA or with no address or phone number in the EMR were excluded. We excluded patients with significant comorbid conditions, including patients with Child-Pugh C cirrhosis who were not eligible for liver transplantation, and patients with end-stage malignancy and/or on hospice care, given the limited benefit of HCC surveillance.(24)
Intervention
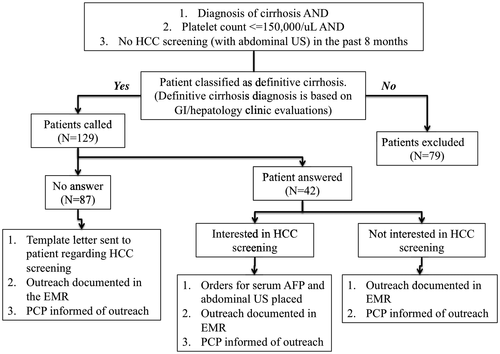
The pilot and feasibility QI intervention was performed between January 2019 and June 2019 (Fig. 1). Eligible veterans received a telephone call once from a patient navigator (E.A., A.W., J.L., and A.B.) to describe the risks and benefits of HCC surveillance and to assess barriers to surveillance, based on previous work that had evaluated patient-reported barriers to HCC surveillance (Supporting Table S2).(17) Most of the phone calls were conducted after hours or at times on weekends or weekdays. For veterans reached by telephone who agreed to surveillance, the patient navigator placed orders for both an abdominal ultrasound (US) and serum AFP. If patients already had an order placed by their primary care provider (PCP) before our patient navigator outreach, they were called and reminded to complete the US and AFP. During the telephone encounter, patients were given the option to call the Radiology department for scheduling or wait to be contacted. They were scheduled for an abdominal US after either (1) calling on their own or (2) being contacted by the Radiology department. Reminder phone calls were not attempted in this initial intervention. Veterans who could not be reached by telephone outreach received an informational letter (in English) by mail with an invitation to participate in surveillance (Supporting Fig. S1). Letters were sent from a centralized mailing center in Sacramento, California, through the EMR. All letters contained the contact information of a dedicated gastroenterology and hepatology case manager, who would then contact a dedicated hepatologist for order placement or patient contact (J.B.). To prevent orders from being placed without confirming a patient’s interest in the screening program and to minimize excessive workflow for the Radiology department, orders were only placed after the patient was reached by phone or through the informational letter. Patients who contacted the case manager had an abdominal US and AFP ordered. Following completion of the ordered studies, patients with normal imaging and laboratory results received a letter informing them of the results and recommending repeat surveillance in 6 months. Patients with an abnormal result were contacted by phone and referred to a hepatology clinic for further evaluation. Both primary care and radiology leadership participated in the design of this intervention. All letters were evaluated by our local informatics group, to ensure that the language was appropriate and at a sixth-grade reading level (Supporting Fig. S1). During the intervention, PCPs received an EMR notification for each patient contacted (all EMR notes were also forwarded to a patient navigator, J.B.) (Supporting Fig. S2). Unrelated to our efforts, VAGLAHS implemented PCP clinical reminders for HCC surveillance during the intervention period, which affected all patients in our intervention equally. If patients had not been evaluated in a hepatology or gastroenterology clinic within the year after review of the EMR, a consult request was placed for clinic follow-up.
Measures
The primary outcome was receipt of HCC “surveillance,” defined as completion of abdominal US with or without AFP, during the 6-month interval following patient contact. If a patient had a contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) ordered in the interim by another provider, completion of the CT or MRI was considered sufficient to have met the primary outcome of HCC surveillance with or without an AFP. Receiving serum AFP alone did not qualify as receipt of adequate surveillance. We reviewed all patients’ EMRs for uploaded and scanned documents, to identify patients who had completed their abdominal US +/- AFP outside of the VA. Process measures included whether patients were reached by telephone and whether they were sent a letter. Given that letters were mailed from a centralized office in Sacramento, our efforts could not identify which patients did not have a correct address (no returns were sent to us). Other variables, including patient demographics (age, gender, race, ethnicity, and zip code), health care access (number of PCP visits and number of gastroenterology [GI]/hepatology subspecialty visits), and clinical data (etiology of cirrhosis and complications of portal hypertension), were obtained by standardized manual data abstraction from the EMR. Zip codes were geocoded and linked to publicly available geographic and sociodemographic data to calculate patient distance (miles) from the VA.(25)
Statistical Analysis
Data are described using frequencies, means, and SDs as appropriate. Student t tests (continuous variables) and analysis of variance (categorical variables) were used to analyze associations between covariates and the outcome of interest. Univariate and multivariate logistic regression models were used to determine predictors of surveillance uptake. Our final logistic regression model included all observed variables, other than etiology of cirrhosis. Moderation effects were evaluated using interaction terms in both univariate and multivariate models. Variables for interaction terms were selected a priori based on their significance in univariate models as well as clinical relevance (distance from VA, health care access, received telephone call, presence of encephalopathy). A P value of less than 0.05 was considered statistically significant. All calculations were performed using STATA 14.2 (College Station, TX).
Results
Characteristics of Study Population
Of the 208 patients who were assessed for eligibility,129 met the inclusion and exclusion criteria (Fig. 2). Patients were predominantly male (96.9%) with a mean age of 65.9 years (SD = 9.88; Table 1). Most patients were non-Hispanic white (39.5%), followed by 30.2% Hispanic or Latino, and 14.7% non-Hispanic black. The most common etiology for cirrhosis was hepatitis C (64.3%), followed by alcohol-related cirrhosis (20.1%). Thirty-two percent of patients had decompensated cirrhosis, with ascites (85%) as the most common complication. Although patients had an average of 2.1 visits with their PCP in the year before study initiation, only 27.1% of patients saw a gastroenterology or hepatology provider during this timeframe.
| Variables | Total (Mean, SD) OR (n, %) | No Surveillance (n = 26) | Surveillance (n = 79) | P Value |
|---|---|---|---|---|
| Age, in years | 65.9 (9.88) | 68.2 (5.98) | 65.2 (11.6) | 0.29 |
| Male gender | 125 (96.9%) | 24 (92.3%) | 77 (97.5%) | 0.22 |
| Race/Ethnicity | 0.23 | |||
| Non-Hispanic white | 51 (39.5%) | 12 (46.2%) | 30 (38.0%) | |
| Non-Hispanic black | 19 (14.7%) | 2 (7.7%) | 13 (16.5%) | |
| Hispanic or Latino | 39 (30.2%) | 5 (19.2%) | 27 (34.2%) | |
| Asian | 2 (1.6%) | 1 (3.9%) | 0 (0%) | |
| Other/Unknown | 18 (14.0%) | 6 (23.1%) | 9 (11.4%) | |
| Etiology of Cirrhosis | 0.72 | |||
| Hepatitis C | 83 (64.3%) | 17 (65.4%) | 53 (67.1%) | |
| ALD | 26 (20.1%) | 4 (15.4%) | 15 (19.0%) | |
| Nonalcoholic Steatohepatitis | 13 (10.1%) | 2 (11.5%) | 6 (7.6%) | |
| Other/Unknown | 7 (6.0%) | 2 (7.7%) | 5 (6.3%) | |
| Decompensated Cirrhosis and Complications | 41 (31.8%) | 9 (34.6%) | 27 (34.2%) | 0.47 |
| Ascites | 35 (27%) | 9 (34.6%) | 22 (27.9%) | 0.82 |
| Hepatic encephalopathy | 16 (12.4%) | 7 (26.9%) | 8 (10.1%) | 0.33 |
| Variceal hemorrhage | 18 (14.0%) | 2 (7.7%) | 14 (17.7%) | 0.12 |
| Number of PCP visits in the previous year | 2.1 (1.7) | 2.1 (1.9) | 2.3 (1.8) | 0.28 |
| Number of GI/hepatology visits in the previous year | 0.53 (1.1) | 0.73 (1.46) | 0.57 (1.02) | 0.57 |
| Successfully reached by patient navigator | 42 (32.5%) | 5 (19.2%) | 29 (36.7%) | 0.21 |
| Distance from the VAGLAHS, in miles | 45.6 (45.9) | 41.6 (44.1) | 43.0 (44.7) | 0.42 |
Note:
- “Surveillance” is defined as abdominal imaging with AFP or abdominal imaging alone. “No surveillance” is defined as neither abdominal imaging nor serum AFP within the follow-up period.
Of the eligible patients who were contacted by telephone, only 32.5% spoke with a patient navigator by phone (Table 1). All patients who were reached by telephone were appreciative of the outreach efforts, with the exception of one patient who was not interested in liver cancer screening. Patients who were not reached by telephone (67.5%) either did not answer their phone or had a nonworking telephone number and were contacted through a template letter. We opted not to leave voicemails to protect our patients’ privacy.
Surveillance Uptake
In the 6 months following the intervention, 61.2% of patients underwent abdominal imaging and 69.0% of patients underwent serum AFP testing (Table 2). Two patients had contrast-enhanced CT scans completed that had been placed by other providers who were not part of our intervention. These were included to have met our primary outcome of abdominal imaging completion and therefore HCC surveillance. Of the patients who spoke with a patient navigator regarding HCC surveillance, 72.1% completed abdominal imaging and 81.8% completed serum AFP testing. One patient was found to have an abdominal mass on imaging and was subsequently diagnosed with HCC.
| n (%) | |
|---|---|
| Abdominal imaging completed after being ordered by the outreach team | 72.1 |
| Serum AFP completed after being ordered by the outreach team | 81.8 |
| Abdominal imaging completed in the 6 months following outreach | 61.2 |
| Serum AFP completed in the 6 months following outreach | 69.0 |
Factors Associated With Successful Surveillance
There were no significant differences in clinical or demographic characteristics between patients who were not screened and those who were screened with abdominal US ± AFP (Table 1). Although patients who received a telephone call were more likely to receive surveillance than those that did not (36.7 vs. 19.2%, P = 0.21), this difference was not statistically significant. None of the tested covariates significantly predicted surveillance uptake in univariate logistic regression. However, in multivariate analyses, patients successfully reached by telephone were more likely to uptake surveillance when compared with patients who were not successfully reached (adjusted odds ratio [aOR]: 2.56; 95% confidence interval [CI]: 1.03-6.33; Table 3). The presence of hepatic encephalopathy was inversely associated with receiving successful surveillance; however, this was not statistically significant (aOR = 0.24; 95% CI: 0.05-1.02). From our test of interactions, we found that differences in surveillance rates between patients with and without hepatic encephalopathy varied depending on their number of GI visits per year, particularly after two visits. The association between successfully reaching a patient by phone and achieving surveillance still persisted after including this interaction in our model (aOR = 2.83; 95% CI: 1.12-7.20; Supporting Table S3).
| Variables | OR of Surveillance | aOR of Surveillance |
|---|---|---|
| Age, in years | 0.98 (0.94-1.02) | 0.96 (0.93-1.01) |
| Male gender | 0.62 (0.08-4.57) | 0.95 (0.11-8.46) |
| Race/Ethnicity | ||
| Non-Hispanic white | Ref | Ref |
| Non-Hispanic black | 1.52 (0.50-4.63) | 1.23 (0.36-4.18) |
| Hispanic or Latino | 1.58 (0.65-3.79) | 1.75 (0.66-4.62) |
| Asian | — | — |
| Other/Unknown | 0.7 (0.24-2.06) | 0.80 (0.24-2.58) |
| Decompensated Cirrhosis | 1.34 (0.62-2.89) | 1.26 (0.34-4.64) |
| Ascites | 1.10 (0.49-2.45) | |
| Hepatic encephalopathy | 0.59 (0.21-1.69) | 0.24 (0.05-1.02) |
| Variceal hemorrhage | 2.48 (0.77-8.01) | 3.89 (0.79-19.2) |
| Number of PCP visits in the previous year | 1.13 (0.91-1.41) | 1.08 (0.84-1.39) |
| Number of GI/hepatology visits in the previous year | 1.11 (0.78-1.57) | 1.16 (0.74-1.80) |
| Successfully reached by patient navigator | 1.65 (0.76-3.60) | 2.56 (1.03-6.33) |
| Distance from the VAGLAHS, in miles | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) |
Note:
- “Surveillance” is defined as abdominal imaging with AFP or abdominal imaging alone. “No surveillance” is defined as neither abdominal imaging nor serum AFP within the follow-up period.
- Abbreviation: OR, odds ratio.
Discussion
Within a large and ethnically diverse VA health care system, our pilot intervention using a combined telephone and mailed outreach was an effective and successful strategy to increase HCC surveillance uptake among at-risk patients.
Despite the increased completion of appropriate HCC surveillance after outreach with telephone calls or letters, the overall rates of appropriate surveillance remained low, with only 61.2% of patients undergoing abdominal imaging. Of note, this study focused on high-risk patients who had fallen out of the appropriate HCC surveillance protocol at the initiation of the study and who had previously been evaluated by a liver subspecialist.
There were no major demographic differences between patients who did and did not ultimately receive surveillance, including age, patient gender, race/ethnicity, decompensated cirrhosis, distance from the hospital, and number of primary care visits in the previous year.
Interestingly, distance from the VA did not affect our surveillance rates, which is consistent with some previous studies.(26, 27) Ultimately, our study did not capture data on the locations where patients received their laboratory work and abdominal imaging, including those completed outside the VA, which may have affected our results. Although distance did not affect surveillance completion in our study, technological innovations such as telehealth, telemedicine, and mobile health may help close the quality gaps, given the projected increase in cirrhosis care in the future.(28)
The number of gastroenterology or hepatology care visits in the previous year was similar among those screened and those unscreened after outreach. This differs from previously reported work, which suggested that being seen by a gastroenterologist or hepatologist is associated with a higher likelihood of HCC surveillance.(29) Our study included only patients previously evaluated by a gastroenterology or hepatology specialist, thereby excluding anyone without a confirmed diagnosis by a subspecialty care clinic. Although patients diagnosed only through primary care or identified by the ALD dashboards were not targeted in our intervention, these patients are likely to have true cirrhosis, and future work is needed to incorporate these patients in subspecialty care.
Our data suggest that when adjusting for other factors, patients who were successfully reached by telephone outreach were more likely to undergo appropriate HCC surveillance than those who were not. This indicates that contact with a patient navigator may assist with decreasing barriers to care that we have not yet identified. Our data further supports the randomized control trial conducted outside of the VA health care system, demonstrating that telephone outreach improves HCC surveillance.(21) Similar interventions have been effective for patients who were recommended to undergo breast, cervical, and colorectal cancer surveillance.(30, 31) Our test of interactions found that the negative association between hepatic encephalopathy and surveillance rates worsens with each subsequent GI visit. More GI visits in this setting likely reflects greater illness severity, and may suggest that targeting patients with hepatic encephalopathy should be considered in future QI efforts.
Limitations
Although our study is strengthened by the large and diverse patient population that our VA health care system serves, it has several limitations. First, this study was conducted at a single VHA site, at the VAGLAHS. Second, the patient population in the VA is predominantly insured, male veterans who speak English as their primary language; therefore, our results may not be generalizable to other health systems. Third, although our study was meticulous in understanding the work flow of who placed the abdominal orders and for what intent, our design did not completely allow us to differentiate whether imaging was done for the “intent” of surveillance or performed incidentally. Data on test indication may be needed for developing systems that ensure reliable surveillance and will be considered in future subsequent QI iterations. Fourth, our outreach was conducted at the same time PCP clinical reminders for HCC surveillance were implemented, therefore potentially inflating the success of our outreach efforts. Although not formally measured in our study, PCP participation was noted to have increased through the use of the EMR notification system (or telephone or mailed letter). Although it is unclear to what degree the PCP reminders and EMR contact efforts had on the improved surveillance rates seen in our study, future work at our VA will aim to better understand this. Finally, it is possible that imaging or laboratory studies were collected outside the VA, but these were not always readily available on chart review and therefore may not have been included in our final analysis.
Future Directions
This pilot QI study demonstrated the feasibility of using HCC surveillance as an outreach effort, which can provide the basis and framework for future outreach work. We hope that the data collected and analyzed as part of this initiative will inform future efforts to improve HCC surveillance. Specific future work will focus on identifying key barriers to appropriate HCC surveillance completion, including patient-level, provider-level, and system-level factors, which were likely not captured by the data collected for the study. Further inquiry, perhaps with qualitative methods, is needed. However, given the effectiveness of our pilot study, we will continue to invest resources in adopting intensive interventions with a multipronged approach. Future interventions will make stronger efforts toward contacting patients with reminder phone calls during different hours of the day and on subsequent days in an attempt to achieve direct patient contact as well as address questions and concerns. Although English was the main language for our veterans, we will also improve our outreach efforts by providing informational letters in different languages. In addition to targeting high-risk patients, we hope to expand these HCC surveillance practices to all patients with cirrhosis. Finally, future surveillance interventions should focus on sustainability of the intervention through lasting resources at the health system level, including working with our radiology colleagues, to ensure the ease of placement orders and patient scheduling. Although this project was carried out using volunteer outreach without cost to the institution, further studies may require more resources to ensure consistent surveillance at a system-wide level.
In summary, HCC surveillance is associated with early HCC detection and improved overall survival in patients with cirrhosis; however, adherence often falls short.(12, 32) We demonstrated a successful outreach initiative to reach patients with cirrhosis who have not participated in surveillance. Further work is needed to determine which interventions most efficiently generate the greatest population-level response for appropriate HCC surveillance and how best to implement sustainable and effective strategies.
Acknowledgment
We thank Dr. Karine Rozenberg for her assistance with the VA health care system ALD dashboard and all contributing members of the ALD dashboard.