Hepatocellular Protein Arginine Methyltransferase 1 Suppresses Alcohol-Induced Hepatocellular Carcinoma Formation by Inhibition of Inducible Nitric Oxide Synthase
Abstract
Alcohol is a well-established risk factor for hepatocellular carcinoma (HCC), but the mechanisms by which alcohol promotes liver cancer are not well understood. Studies suggest that ethanol may enhance tumor progression by increasing hepatocyte proliferation and through alcohol-induced liver inflammation. Protein arginine methyltransferase 1 (PRMT1) is the main enzyme responsible for cellular arginine methylation. Asymmetric dimethyl arginine, produced by PRMT1, is a potent inhibitor of nitric oxide synthases. PRMT1 is implicated in the development of several types of tumors and cardiovascular disease. Our previous work has shown that PRMT1 in the liver regulates hepatocyte proliferation and oxidative stress and protects from alcohol-induced liver injury. However, its role in HCC development remains controversial. In this study, we found that hepatocyte-specific PRMT1-knockout mice develop an increased number of tumors in an N-nitrosodiethylamine (DEN) alcohol model of liver tumorigenesis in mice. This effect was specific to the alcohol-related component because wild-type and knockout mice developed similar tumor numbers in the DEN model without the addition of alcohol. We found that in the presence of alcohol, the increase in tumor number was associated with increased proliferation in liver and tumor, increased WNT/β-catenin signaling, and increased inflammation. We hypothesized that increased inflammation was due to increased oxidative and nitrosative stress in knockout mice. By blocking excess nitric oxide production using an inducible nitric oxide synthase inhibitor, we reduced hepatocyte death and inflammation in the liver and prevented the increase in WNT/β-catenin signaling, proliferation, and tumor number in livers of knockout mice. Conclusion: PRMT1 is an important protection factor from alcohol-induced liver injury, inflammation, and HCC development.
Abbreviations
-
- 4-HNE
-
- 4-hydroxynonenal
-
- AAV
-
- adeno-associated virus
-
- ADMA
-
- asymmetric dimethyl arginine
-
- ALD
-
- alcoholic liver disease
-
- ALT
-
- alanine aminotransferase
-
- Axin2
-
- axin 2
-
- CD
-
- cluster of differentiation
-
- CRE
-
- causes recombination
-
- DDAH
-
- dimethylarginine dimethylaminohydrolase
-
- DEN
-
- N-nitrosodiethylamine
-
- DN
-
- dominant negative
-
- EGFR
-
- epidermal growth factor receptor
-
- F4/80
-
- adhesion G protein-coupled receptor E1
-
- FOXO
-
- forkhead box sub-group O
-
- gc
-
- genome copies
-
- GSNOR
-
- S-nitrosoglutathione reductase
-
- HCC
-
- hepatocellular carcinoma
-
- HNF4α
-
- hepatocyte nuclear factor 4 alpha
-
- iNOS
-
- inducible nitric oxide synthase
-
- KO
-
- knockout
-
- Lef1
-
- lymphoid enhancer binding factor 1
-
- Mcp1
-
- chemokine (C-C motif) ligand 2
-
- mRNA
-
- messenger RNA
-
- MYC
-
- MYC proto-oncogene bHLH transcription factor
-
- NOS
-
- nitric oxide synthase
-
- PBS
-
- phosphate-buffered saline
-
- PBST
-
- phosphate-buffered saline–Tween 20
-
- PRMT1
-
- protein arginine methyltransferase 1
-
- ROS
-
- reactive oxygen species
-
- SNO
-
- S-nitroso-cysteine
-
- SOD
-
- superoxide dismutase
-
- TAM
-
- tumor-associated macrophage
-
- TBG
-
- thyroxine binding globulin
-
- Tnf
-
- tumor necrosis factor
-
- TUNEL
-
- terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
-
- WT
-
- wild type
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide.(1, 2) Unlike other cancers with known risk factors, molecular mechanisms of HCC development are not completely understood. Most patients with HCC have a history of chronic liver disease and cirrhosis,(3) but cirrhosis associated with hepatitis B and C and chronic alcohol drinking account for most cases.(4)
About 40% of individuals who chronically consume alcohol develop fatty liver, which can advance to alcoholic hepatitis and/or cirrhosis. Although there have been conflicting findings, currently it is accepted that alcohol-associated cirrhosis is a medium-high risk factor for HCC.(1) Alcohol interacts with other causes of liver disease, including hepatitis B and C, and conditions, such as diabetes and obesity, to increase the risk for developing HCC. HCC risk in patients with alcoholic liver disease (ALD)-associated cirrhosis increases with age and with quantity and duration of alcohol consumption.(5)
HCC development is a multistep process that involves genetic and epigenetic modifications in the liver that lead to malignant transformation of hepatocytes.(6) A number of studies have been performed to define the changes induced by alcohol consumption that can lead to cancer development. In mouse models, chronic ethanol consumption increases HCC risk by stimulating hepatocyte proliferation through activation of the Wnt/β-catenin signaling pathway(7, 8) and by increasing neutrophil and macrophage activation, thus contributing to chronic tissue inflammation and promoting tumor development.(8-10)
Protein arginine methylation is a common posttranslational modification that plays a role in multiple pathways, including cell-cycle control, RNA processing, and DNA replication. Protein arginine methyltransferase 1 (PRMT1) is responsible for about 85% of total cellular arginine methylation(11) and catalyzes arginine monomethylation and dimethylation using S-adenosyl methionine (SAM) as a methyl donor. Arginine methylation impacts gene transcription and splicing as well as upstream signal transduction.(12) PRMT1 methylates histone H4 at arginine 3, generating H4R3me2a, a transcriptional activation mark, thus contributing to the histone code. As a transcriptional coactivator, PRMT1 is recruited to promoters by a number of different transcription factors.(13-15) Abnormal function of PRMT1 is closely associated with several types of cancer and cardiovascular diseases.
PRMT1 has many targets that are important for growth control. The list of PRMT1 targets includes both promoters of cell proliferation (MYC proto-oncogene bHLH transcription factor [MYC], epidermal growth factor receptor [EGFR], SAM68) and suppressors of cell growth or inducers of apoptosis (forkhead box sub-group O [FOXO], hepatocyte nuclear factor 4 alpha [HNF4α], tumor protein 53 [p53]). Numerous publications support the role of PRMT1 in promoting cell proliferation and survival.(16-18) Others suggest that PRMT1 can suppress proliferation through activation of its target HNF4α.(14, 19) This contradiction suggests that PRMT1 function in growth control may be context dependent.
Previously, we demonstrated that PRMT1 protects from alcohol-induced liver injury.(18) In this study, we aimed to define the role of PRMT1 in HCC development, using an N-nitrosodiethylamine (DEN) alcohol-induced liver tumorigenesis model in mice. In this model, we found that PRMT1 knockout resulted in increased inflammation and liver injury through increased oxidative and nitrosative stress. These hepatocyte-specific PRMT1-knockout mice also had an increased number of tumors in the presence of alcohol. This increase was associated with increased proliferation in both the liver and in tumor tissues, an increase in WNT/β-catenin signaling, and increased inflammation. This was associated with increased NO production as expected because PRMT1 is required for production of the NO synthase inhibitor, asymmetric dimethylarginine (ADMA). We further showed that blocking excess nitric oxide production using an inducible nitric oxide synthase (iNOS) inhibitor corrected the inflammatory and growth abnormalities of the PRMT1-knockout mice as this was able to reduce hepatocyte death and inflammation in the liver and prevent the increase in WNT/β-catenin signaling, proliferation, and tumor growth in livers of knockout mice.
Materials and Methods
Mice
Prmt1-floxed mice have been described.(18) All mice were housed in a temperature-controlled specific pathogen-free environment with 12-hour light–dark cycles and fed regular mouse chow and water ad libitum. All animal handling procedures were approved by the Institutional Animal Care and Use Committees at the University of Kansas Medical Center (Kansas City, KS).
To induce tumor development, mice were injected with 10 mg/kg DEN at 2 weeks of age. Alternatively, mice received five injections of 50 mg/kg DEN weekly starting at 4 weeks of age.
Mice were fed Lieber-DeCarli alcohol liquid diet (4.8% alcohol), with alcohol concentration increasing in a stepwise manner 0%, 1.6%, 3.2%, to 4.8% during the first week of feeding. Control mice received control liquid diet without alcohol. Mice received weekly injections of 10 mg/kg of the iNOS inhibitor 1400 W or saline control.
Antibodies Used
Anti-PRMT1 (F339) and anti-β-catenin (N terminal, cat. #9581) antibodies were from Cell Signaling. We purchased 4-hydroxynonenal (4-HNE) antibodies from Alpha Diagnostics. Anti- S-nitroso-cysteine (anti-SNO) came from Sigma. Anti-iNOS (sc-651) and anti-3-nitrotyrosine monoclonal antibodies were from Santa Cruz. Rabbit anti-adhesion G protein-coupled receptor E1 (anti-F4/80), anti-mannose receptor C type 1 (anti-Mrc1), and anti-cluster of differentiation (cd)11b antibodies were from Abcam.
Cell Culture
Huh7 and HepG2 cells were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 50 U/mL penicillin, and 50 mg/mL streptomycin. Cells were transfected using Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer's protocol.
Vectors
pCMV6-PRMT1, pCMV6-DDAH1, pCMV6-DDAH2 vectors were from Origene. AAV8.TBG.PI.Null, AAV8.TBG.PI.Cre were from Vector BioLabs (Malvern, PA) and were used at 1-2 × 1011 genome copies (gc)/mouse.
Human Specimens
De-identified human specimens were obtained from the Liver Center Tissue Bank at the University of Kansas Medical Center. All studies using human tissue samples were approved by the Human Subjects Committee of the University of Kansas Medical Center.
Immunohistochemistry
Immunostaining on formalin-fixed sections was performed by deparaffinization and rehydration, followed by antigen retrieval by heating in a pressure cooker (121°C) for 5 minutes in 10 mM sodium citrate, pH 6.0, as described.(19) Peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 10 minutes. Sections were rinsed 3 times in phosphate-buffered saline (PBS)/PBS-Tween 20 (PBST; 0.1% Tween-20) and incubated in Dako Protein Block (Dako, Carpinteria, CA) at room temperature for 1 hour. After removal of the blocking solution, slides were incubated overnight with an antibody and diluted 1:300 in Dako Protein Block at 4°C. Antigen was detected using the SignalStain Boost immunohistochemistry (IHC) detection reagent (catalog no. 8114; Cell Signaling Technology, Beverly, MA), developed with diaminobenzidene (Dako), counterstained with hematoxylin (Sigma-Aldrich), and mounted. Signal intensity was analyzed using Aperio ImageScope 12.1.
Immunofluorescence
Immunostaining on formalin-fixed sections was performed by deparaffinization and rehydration, followed by antigen retrieval by heating in a pressure cooker (121°C) for 5 minutes in 10 mM sodium citrate, pH 6.0. Sections were rinsed 3 times in PBS/PBST (0.1% Tween-20) and incubated in Dako Protein Block (Dako) at room temperature for 1 hour. After removal of the blocking solution, slides were incubated overnight with an antibody and diluted 1:300 in Dako Protein Block at 4°C. After washing with PBST, coverslips were incubated with Alexa Fluor-conjugated secondary antibody (1:500) in 0.1 µg/mL 4′,6-diamidino-2-phenylindole for 1 hour in the dark at room temperature. Coverslips were washed and mounted with FluorSave Reagent (Calbiochem. La Jolla, CA). Slides were observed in a Nikon Eclipse 800 upright epifluorescence microscope (Nikon Instruments, Melville, NY). Images were acquired using a Nikon CoolSNAP camera.
Terminal Deoxynucleotidyl Transferase–Mediated Deoxyuridine Triphosphate Nick-End Labeling
Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) was performed using the DeadEND Colorimetric TUNEL System (Promega) according to the manufacturer's instructions.
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay for ADMA detection was performed using a commercial kit (Novus) according to the manufacturer's instructions.
NO Measurement
NO concentration in the liver was measured as total nitrite and nitrate concentration using the Nitric Oxide detection kit (Enzo Life Sciences).
Statistics
Results are expressed as mean ± SD. The Student t test, paired t test, Pearson's correlation, or one-way analysis of variance with Bonferroni post hoc test was used for statistical analyses. P < 0.05 was considered significant.
Results
PRMT1 Regulates Tumor Cell Proliferation
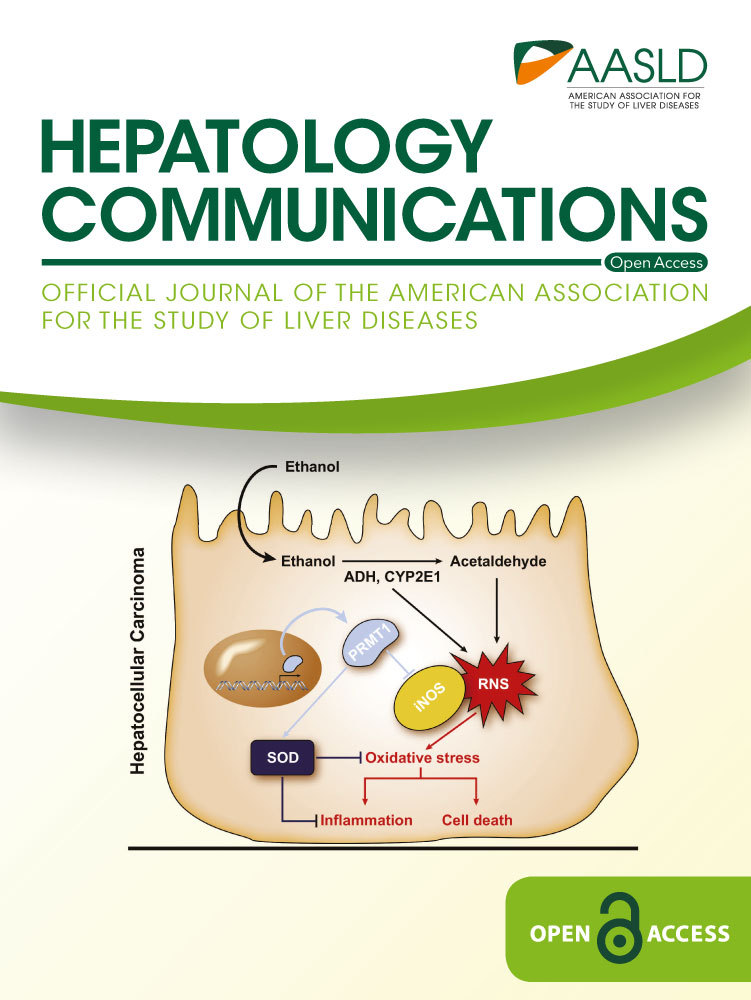
PRMT1 is known to promote proliferation of many cancer cell lines in vitro and in vivo,(16, 17, 20-22) including hepatoma cell lines. We confirmed that PRMT1 inhibition using a small molecule inhibitor, protein arginine methyltransferase inhibitor, reduced hepatoma cell proliferation under normal conditions. Overexpression of wild-type PRMT but not its activity-deficient (dominant negative [DN]) mutant promoted hepatoma cell proliferation (Fig. 1A). Moreover, PRMT1 overexpression in isolated mouse hepatocytes resulted in up-regulation of several proliferation-associated genes, including cyclin B1 and cyclin D1. PRMT1 overexpression increased expression of the β-catenin target genes axin 2 (Axin2) and lymphoid enhancer binding factor 1 (Lef1) in the presence and absence of LiCl, used to induce the WNT pathway (Fig. 1B). In contrast to wild-type PRMT1, the DN form of PRMT1 failed to up-regulate expression of these genes, suggesting that PRMT1 enzymatic activity is necessary for its proproliferative functions.
Next, we examined the role of PRMT1 in HCC growth in vivo. We used PRMT1-floxed mice that were injected with 10 mg/kg DEN at 2 weeks of age. At 6 months, these animals were injected with adeno-associated virus (AAV)-thyroxine binding globulin (TBG)-control vector or AAV-TBG-causes recombination (CRE) vector to induce PRMT1 knockout in hepatocytes. Livers were harvested 2 months later and analyzed for tumors and gene expression in the tumors and nontumor liver tissues (Fig. 1C). We confirmed that AAV-CRE injection resulted in PRMT1 knockout both in tumor cells and adjacent tissue hepatocytes of these mice (Supporting Fig. S1). Next, we analyzed gene expression of proliferation-related genes in these samples. We found that PRMT1 knockout resulted in reduced expression of proliferation-related genes in tumors (Fig. 1D), suggesting that PRMT1 promotes tumor growth.
However, PRMT1 knockout also resulted in about 50% decrease in hepatic HNF4α levels (Fig. 1E). HNF4α is an important tumor suppressor for HCC.(23-25) These data suggest that PRMT1 potentially can also protect from tumor development. We hypothesized that the progrowth and antigrowth effects of PRMT1 might differ under different conditions, and thus its impact on tumor growth could depend on environmental conditions, such as diet or alcohol consumption. Because our previous work showed that alcohol causes PRMT1 dephosphorylation and alters its effects on gene expression, we next determined whether PRMT1 might play a specific role in alcohol-associated hepatocarcinogenesis.
PRMT1 Protects From Alcohol-Associated HCC in Mice
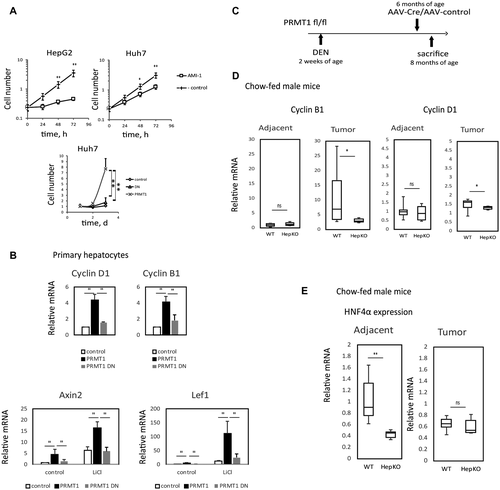
We tested the role of PRMT1 in HCC development in mice on standard chow diet, Lieber-DeCarli alcohol liquid diet (4.8% ethanol, i.e., 27% calories from alcohol, 36% from fat), or control liquid diet (36% calories from fat), as shown in Fig. 2A. Because female mice do not develop DEN-induced tumors on chow diet at 8 months of age, we included female mice only in the alcohol-fed group. Representative images of livers of these mice are shown in Fig. 2B. PRMT1 knockout resulted in an increase in liver to body weight ratio in all groups (Fig. 2C). However, it resulted in an increase in surface tumor number only in both female and male mice of the alcohol group while other diet groups showed no differences in tumor number (Fig. 2D). A similar difference was observed in the average number of tumors within liver sections (Supporting Fig. S2). These data suggest that PRMT1 is protective against HCC development in the presence of alcohol.
PRMT1 Knockout Results in Higher Tumor Proliferation in the Presence of Alcohol
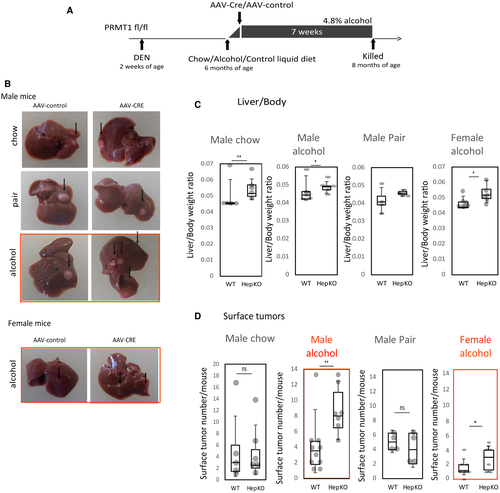
To determine the mechanism of PRMT1-dependent effects on HCC development in alcohol-fed mice, we examined the histology and gene expression of wild-type and knockout mice. Histology was evaluated by a blinded pathologist. Histologically, we found no difference between the wild-type and knockout mice on the chow diet. However, in the alcohol groups, hepatocellular PRMT1 knockout resulted in increased steatosis (Fig. 3A,B). Only a few wild-type mice sections had hepatocyte ballooning (one out of five mice), while the majority of knockout mice had hepatocyte ballooning present (four out of six mice). These data were in accordance with the phenotype of hepatocyte-selective PRMT1-knockout mice fed alcohol, as described.(18)
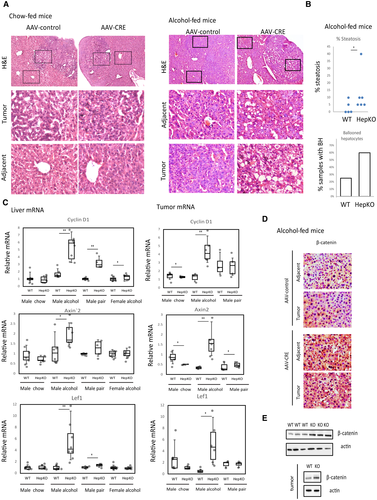
PRMT1 knockout in alcohol-fed mice also resulted in an increase in messenger RNA (mRNA) expression for cyclin D1 and the WNT/β-catenin target genes Axin2 and Lef1 in the tumors and liver tissue (Fig. 3C). Additionally, we confirmed β-catenin accumulation by IHC and western blot analysis in the livers and tumors of knockout mice compared to wild-type mice fed alcohol (Fig. 3D).
Gene expression of both cyclin D1 and Axin2 positively correlated with tumor number in these groups of mice (Fig. S2), suggesting that the increase in proliferation and β-catenin signaling contributes to the increase in tumor number in knockout animals.
Hepatic PRMT1 Protects From Inflammation in the Liver of Alcohol-Fed Mice
There was a significant increase in Wnt1 and Wnt2 expression in livers and tumors of knockout mice fed alcohol (Fig. 4A). However, because PRMT1 directly promotes WNT/β-catenin signaling in hepatocytes (Fig. 1B), the increase in WNT/β-catenin signaling in PRMT1-knockout mice fed alcohol cannot be easily explained by hepatocyte intrinsic regulation and could be due to more indirect effects involving other cell types.
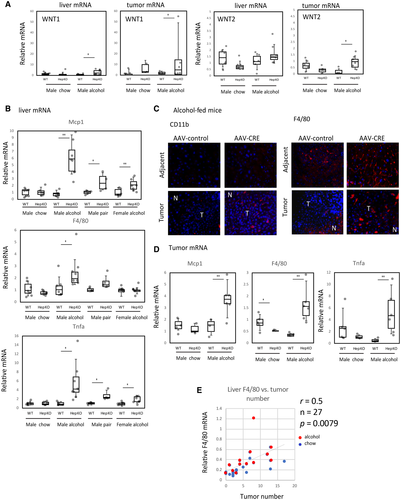
Liver macrophages are one of the major sources of WNT ligands in the liver under various stress conditions.(26) In response to liver damage, monocyte-derived macrophages are recruited into the liver and contribute to liver regeneration by stimulating proliferation of hepatocytes.(27) We examined chemokine (C-C motif) ligand 2 (Ccl2; also known as Mcp1), F4/80, and tumor necrosis factor (Tnf) mRNA levels in the livers of wild-type and knockout mice to assess monocyte recruitment, macrophage numbers, and liver inflammation. We found that PRMT1 knockout resulted in elevated levels of all three genes in total liver mRNA from alcohol-fed mice but not chow-fed mice (Fig. 4B). We confirmed this finding by staining of CD11b-positive and F4/80-positive cells in the livers of alcohol-fed mice. We found higher numbers of F4/80-positive cells in the nontumor liver of knockout mice compared to wild-type mice, and in tumors there were increased numbers of both CD11b-positive and F4/80-positive cells in knockout mice (Fig. 4C). Additionally, we found increased levels of F4/80, Mcp1, and Tnf mRNA in the tumors of knockout mice fed alcohol but not the control chow diet (Fig. 4D), suggesting that tumor-associated macrophage (TAM) recruitment is increased in knockout mice and can contribute to increased tumor proliferation and tumor number. Additionally, we found that PRMT1 knockout mice have an increased number of Mrc1-positive TAMs in their tumors (Supporting Fig. S3).
Finally, we found a significant correlation between F4/80 expression and tumor number in these mice (Fig. 4E), suggesting that inflammation contributes to tumor growth in our model of HCC, similarly to published HCC models.(8, 28)
PRMT1 Deficiency Results in an Increase in Oxidative Stress in the Livers of Alcohol-Fed Mice
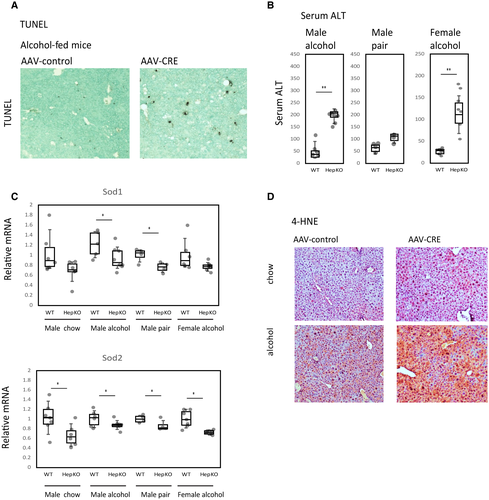
We hypothesized that macrophages accumulate in the knockout-mice livers in response to increased liver injury induced by alcohol in these mice. We previously demonstrated that PRMT1 protects from alcohol-induced liver injury and inflammation through regulation of oxidative stress response gene expression.(18) Here, we showed that PRMT1 knockout in an alcohol-associated HCC model also resulted in an increase in number of TUNEL-positive cells in the liver (Fig. 5A) and an increase in serum alanine aminotransferase (ALT) levels (5-fold for male mice and 4-fold in female mice) (Fig. 5B). Alcohol sensitivity correlated with decreased levels of superoxide dismutase 1 (Sod1) and Sod2 mRNA in the livers of knockout mice (Fig. 5C). As a result, knockout mice fed alcohol showed elevated levels of 4-HNE, suggesting an increase in oxidative stress in the liver (Fig. 5D).
PRMT1 Regulates Nitric Oxide Production in the Liver
Alcohol is known to cause nitrosative stress by increased production of reactive oxygen species (ROS) and NO, which together give rise to reactive nitrogen species (RNS), particularly peroxynitrite. RNS and ROS act together to cause cellular stress and cell death.(29, 30) In addition to its role in the oxidative stress response, PRMT1 is one of the major regulators of NO production in the cell(11, 18) through its ability to produce ADMA, a potent inhibitor of NO synthases (NOS).(31, 32) We tested the role of the ADMA-NO pathway in PRMT1 regulation of HCC development in the presence of alcohol and found that alcohol-fed PRMT1-knockout mice had lower levels of ADMA in the serum compared to their wild-type littermates (Fig. 6A).
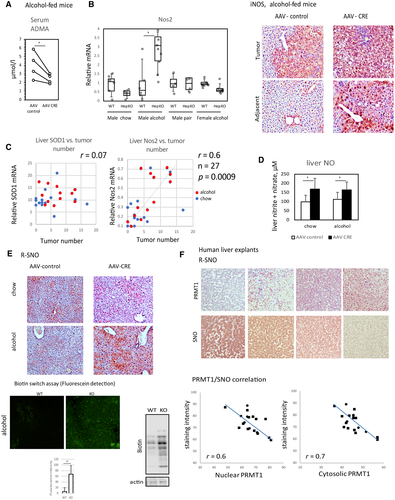
NOS activity is a tightly regulated process, and NOS activation often results in feedback inhibition of NOS expression. However, in the case of iNOS, activation can result in positive feedforward accumulation of iNOS, resulting in a pathogenic effect.(33) We measured Nos1, Nos2 (iNOS), and Nos3 levels in the livers of mice from the eight groups and found that while Nos1 and Nos3 were unchanged or down-regulated in knockout mice (Supporting Fig. S4), iNOS expression was induced in the male PRMT1-knockout mice fed alcohol, the group with the highest median tumor number (Fig. 6B). We stained liver sections from these mice and found that iNOS protein expression was elevated in hepatocytes of male alcohol-fed knockout mice compared to wild-type controls. iNOS expression was equally high in tumor cells of both wild-type and knockout mice (Fig. 6B). iNOS expression in the liver correlated with tumor number in contrast to Sod1, which did not show significant correlation with tumor number in any of the groups (Fig. 6C). These data suggest that iNOS expression and activity increase in alcohol-fed knockout mice and may contribute to elevated cell death, inflammation, and tumor growth in these mice. This hypothesis is in accordance with previous findings on the role of iNOS in tumor growth.(34)
We next assessed the downstream effects of iNOS activation, NO levels, and protein nitrosylation. We found that PRMT1-knockout livers had elevated NO levels in the presence or absence of alcohol (Fig. 6D). In the absence of alcohol, knockout mice have no significant elevation of protein nitrosylation, possibly due to compensatory mechanisms. However, in the presence of alcohol, an increase in ROS production (Fig. 5D) results in a dramatic increase in protein nitrosylation, possibly due to more peroxynitrite intermediate. We found that alcohol-fed knockout mice had an increase in protein S-nitrosylation (attachment of a NO group to cysteine to form an S-nitroso-cysteine [SNO]) measured either by SNO-specific antibody or using a biotin switch assay method (Fig. 6E). Similarly, we found an increase in 3-nitrotyrosine-positive staining in the livers of knockout mice fed alcohol (Supporting Fig. S5).
We found that the PRMT1-ADMA-NO-SNO pathway is relevant in human liver as well. We analyzed PRMT1 expression and protein SNO in explant livers of patients with cirrhosis and found a strong negative correlation between PRMT1 protein levels and SNO levels in these samples (Fig. 6F), suggesting that hepatic PRMT1 regulates protein nitrosylation in livers of patients with cirrhosis.
Hepatic iNOS Activity Contributes to the Sensitivity of PRMT1-Knockout Hepatocytes to Oxidative Stress
Next, we examined the relative contribution of the PRMT1-ADMA-NO pathway to oxidative stress-induced hepatocyte death, inflammation, and tumor development. We used a specific iNOS inhibitor, 1400 W, to reduce NO levels in PRMT1-knockout hepatocytes and found that iNOS inhibition did not alter the survival of wild-type hepatocytes treated with various concentrations of hydrogen peroxide. In contrast, PRMT1-knockout hepatocyte survival was significantly elevated in the presence of iNOS inhibitor (Fig. 7A), suggesting that iNOS activation contributes to oxidative stress sensitivity of PRMT1 knockout hepatocytes. To confirm this finding, we tested whether reducing ADMA levels and thus elevating NO in wild-type hepatocytes can increase their sensitivity to oxidative stress. To do so, we overexpressed dimethylarginine dimethylaminohydrolase 1 (Ddah1) and Ddah2 in wild-type hepatocytes. These enzymes promote ADMA degradation.(32) We found that Ddah1 and to a lesser extent Ddah2 overexpression resulted in reduced survival of wild-type hepatocytes in the presence of hydrogen peroxide (Fig. 7B).
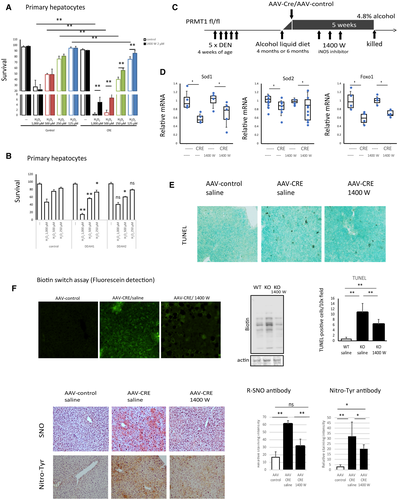
To test the role of iNOS activation in vivo, we used a model of alcohol-associated HCC development described by Ambade et al.(8) Briefly, mice received five doses of DEN and were fed an alcohol liquid diet for 5 weeks. This model recapitulates several features of alcohol-associated HCC, including elevated inflammation, proliferation, and fibrosis as early as 4 months of age.(8) Additionally, we gave mice AAV-control or AAV-CRE on the first day of the alcohol diet to induce PRMT1 knockout in hepatocytes. These mice also received three doses of iNOS inhibitor or saline control. We used two time points to assess the contribution of the PRMT1-iNOS pathway in early and later stages of tumor development (Fig. 7C). The iNOS inhibitor did not affect mRNA levels of the oxidative stress response genes Sod1, Sod2, and Foxo1 (Fig. 7D) but reduced the number of apoptotic hepatocytes in knockout-mice livers (Supporting Fig. S6). We assessed cell death in the livers of these mice using TUNEL staining. Wild-type mice showed very little cell death in the livers after 5 weeks of alcohol feeding. In contrast, knockout mice had an elevated number of TUNEL-positive cells. Knockout mice that received the iNOS inhibitor showed a 2-fold reduction in the number of TUNEL-positive cells (Fig. 7E). In agreement with these data, treated mice had lower levels of serum ALT, but the difference did not reach statistical significance (Supporting Fig. S6).
Taken together, these data demonstrate that iNOS activation in PRMT1-knockout mice results in greater sensitivity to oxidative stress-induced cell death in vitro and in vivo.
Inhibition of iNOS Protects Alcohol-Fed PRMT1-Knockout Mice From Liver Inflammation and Tumor Development in the Presence of Alcohol
Finally, we assessed the levels of protein SNO, 3-nitrotyrosine, and liver inflammation in these mice. We found that similar to the situation in 8-month-old mice (Fig. 6E), the precancer model PRMT1-knockout mice demonstrated a dramatic elevation of SNO levels measured by specific antibody or using the biotin switch assay and nitrotyrosine levels (Fig. 7F). This increase was dependent on iNOS activation because iNOS inhibitor treatment abrogated this effect (Fig. 7F).
To assess liver inflammation, we measured liver cytokine gene expression. We observed that in this model there was elevated cytokine mRNA expression in the knockout mice, similar to that seen in the previous model (Fig. 8A). iNOS inhibition decreased this inflammation both in female and male mice.
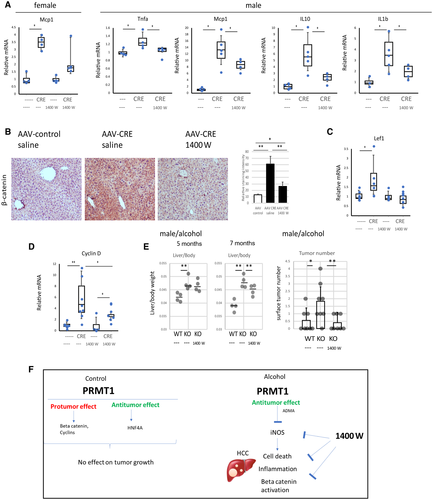
Finally, we examined the effect of iNOS inhibition on proliferation. We found that treatment reduced accumulation of β-catenin (Fig. 8B) and prevented elevation of its downstream target gene Lef1 (Fig. 8C) in the livers of PRMT1-knockout mice. Similarly, we found reduced cyclin D1 expression in the animals that received the iNOS inhibitor compared to saline alone (Fig. 8D). Increased proliferation in the knockout mice correlated with an increase in liver to body weight ratios and the tumor numbers in male mice. Inhibitor treatment reduced the increase in liver weight and prevented the increase in tumor numbers (Fig. 8E).
Taken together, the iNOS inhibitor treatment in this model of HCC can in part rescue the phenotype of knockout mice fed alcohol. Specifically, it can reduce cell death, inflammation, proliferation, and tumor development.
Discussion
PRMT1 regulates multiple aspects of liver biology, including cell proliferation,(19) fatty acid metabolism,(35) glucose metabolism,(36) innate immune response,(37) oxidative stress response, and apoptosis.(38) However, it role in liver cancer development is controversial.
HCC is the third most common cause of cancer-related death.(1, 2) Unlike other cancers with known risk factors, causes of HCC are not completely understood. Most patients with HCC have a history of chronic liver disease and cirrhosis.(3) Commonly, the HCC is associated with hepatitis B and C and chronic alcohol drinking.(4) HCC development is a multistep process that involves genetic and epigenetic modifications in the liver that lead to malignant transformation of hepatocytes.(6)
Alcohol promotes liver tumors by multiple mechanisms.(7-10) Recently, several mouse models were developed to study the mechanism of alcohol effects on cancer development in the liver. All models involved a combination of one or multiple injections of DEN with Lieber-DeCarli alcohol feeding of mice for 5-16 weeks.(7, 8, 28) The Lieber-DeCarli feeding model is an accepted model of ALD that results in features of human ALD, including steatosis, inflammation, and liver fibrosis.(39) Results of these studies suggested that alcohol promotes HCC progression in mice through increasing hepatocyte proliferation,(7, 8) increasing inflammation and tumor-associated macrophage infiltration,(8, 28) and promoting epithelial-mesenchymal transition.(8, 28) However, relative contributions of these pathways are unknown.
In our study, we employed a single injection of DEN at 2 weeks of age followed by Lieber-DeCarli alcohol feeding beginning at 6 months of age. This model was described by Yan et al.(28) We continued alcohol feeding for a shorter period of time (7 weeks instead of 2.5 months) due to the high sensitivity of PRMT1-knockout mice to alcohol.(18) This duration of alcohol feeding was not sufficient to promote tumors in wild-type mice. In contrast, in PRMT1-knockout mice, alcohol feeding resulted in a significant increase in tumor number.
Analysis of PRMT1-dependent changes in alcohol-fed mice suggested that increased tumorigenesis in PRMT1-knockout mice was due to enhanced oxidative and nitrosative stress in livers of knockout mice fed alcohol, which resulted in hepatocyte death and inflammation. Increased hepatocyte death promoted immune cell infiltration in the liver and in tumors (TAMs), which enhanced tumor growth through an increase in WNT ligand production and β-catenin activation. In this work, we did not examine other TAM-mediated functions that regulate tumor growth(40) and focused on β-catenin because it had been described as a mechanism of alcohol-induced HCC development.(7)
PRMT1 is known to negatively regulate NOS activity. Additionally, in alcohol-fed male mice, PRMT1 knockout also resulted in up-regulated Nos2 mRNA and protein level in contrast to chow-fed or pair-fed mice. Specifically, iNOS expression was increased in hepatocytes. Possibly this is due to inflammation in the liver and activation of nuclear factor kappa B (NF-κB) in hepatocytes. iNOS up-regulation can contribute to additional nitrosative stress in alcohol-fed mice.
By blocking nitrosative stress in the liver by iNOS inhibitor administration to PRMT1-knockout mice, we could reduce cell death and inflammation in the liver as well as WNT/β-catenin activation and tumor growth, suggesting that WNT pathway activation and proliferation were secondary to inflammation (Fig. 8F). These data are in accordance to reports that low-grade cell death in the liver promotes HCC development.(41)
Taken together, we found that PRMT1 protects liver from alcohol-associated HCC development. Interestingly, we did not find a significant difference between wild-type and PRMT1-knockout mice fed a chow diet or control liquid diet despite the changes in gene expression of several relevant genes, such as cyclins, β-catenin (protumor role of PRMT1), and Hnf4a (tumor suppressor role of PRMT1).
In addition to targets analyzed in this study, PRMT1 has multiple protein targets, including transcription factors (FOXOs, c-Myc, nuclear erythroid 2 p45-related factor 2 [NRF2], p53, NF-κB, zinc finger protein GLI1 [Gli]) and signaling molecules (amplitude-shift keying [ASK], tumor necrosis factor receptor–associated factor 6 [TRAF6], EGFR).(12) These targets often have opposing roles in the pathways relevant to cancer development. Thus, we hypothesized that the role of PRMT1 depends on other factors, such as environmental conditions, for example, the presence of alcohol. Our data suggest that in chow-fed mice, protumor and antitumor functions of PRMT1 nullify each other. In contrast, in the presence of alcohol, PRMT1-dependent oxidative and nitrosative stress shifts the equilibrium toward PRMT1 being a tumor suppressor. Additionally, we explored tumor development in chow-fed mice at a single time point. We could find a different effect of PRMT1 knockout on tumor growth at different time points.
We found several sex differences in our HCC model. As expected, female mice had fewer tumors. PRMT1 knockout resulted in a similar 4-fold to 5-fold increase in the liver injury marker (ALT). However, an increase in tumor number and inflammation-related gene expression changes induced by PRMT1 knockout in female mice had a lower magnitude, and in some cases, for example, Nos2, Axin2, and Lef1 expression, female mice did not show a significant change. These data suggest that sex differences in inflammation and β-catenin signaling might contribute to the difference in tumor development between male and female mice. Previous results similarly indicated that male-specific HCC development is linked to increased inflammation.(42)
The PRMT1-ADMA-NO pathway is well characterized in the cardiovascular disease field.(12) In contrast, the role of PRMT1 in NO production in the liver has not been previously studied. We found that PRMT1 regulated NO levels in the liver in the presence and absence of alcohol. However, only in the presence of alcohol did PRMT1 knockout result in the accumulation of S-nytrosylated and 3-nitrotyrosine-modified proteins in hepatocytes. iNOS inhibition using a specific inhibitor abolished the increase in SNO and 3-nitrotyrosine accumulation and prevented downstream liver inflammation.
Elevated SNO can directly contribute to tumor growth by regulating the function of several cancer-related proteins, such as β-catenin, signal transducer and activator of transcription 3 (STAT3), EGFR, and others.(43-45) A large number of studies suggest the role of protein nitrosylation in HCC development.(34, 46) S-Nitrosoglutathione (GSNO) reductase (GSNOR; also known as alcohol dehydrogenase class III) is an enzyme that degrades the nonprotein S-nitrosothiol GSNO, thus regulating protein SNO.(34) Mice deficient in GSNOR show increased protein SNO and tissue injury and develop spontaneous liver tumors. An increase in HCC in GSNOR−/− mice is abolished by genetic deletion of iNOS.(34)
iNOS is often increased (both mRNA and protein) in the hepatocytes of patients with chronic hepatitis B or C virus infection, hemochromatosis, and alcoholic cirrhosis. Furthermore, iNOS is elevated in the tumor cells of patients with HCC. iNOS inhibition significantly reduced tumor growth, metastases, tumor initiation, and self-renewal in several tumor types,(47) suggesting that there are multiple mechanisms of iNOS in promoting tumor development.
GW274150 is a selective and orally bioavailable inhibitor of human iNOS and is currently used in clinical trials for migraine and arthritis. The general NOS inhibitor NG-monomethyl-L-arginine (L-NMMA) is in phase I and II clinical trials for patients with melanoma, nonsmall-cell lung cancer, head and neck squamous cell carcinoma, and others (clinicaltrials.gov).
Taken together, previous results and data presented in this study suggest that selective iNOS inhibition is a promising strategy for treatment of ALD and alcohol-associated HCC, specifically in a subset of patients with reduced liver PRMT1 and high iNOS activity levels (Fig. 6F).