Targeting the Four Pillars of Enterohepatic Bile Salt Cycling; Lessons From Genetics and Pharmacology
Abstract
Bile salts play a pivotal role in lipid homeostasis, are sensed by specialized receptors, and have been implicated in various disorders affecting the gut or liver. They may play a role either as culprit or as potential panacea. Four very efficient transporters mediate most of the hepatic and intestinal bile salt uptake and efflux, and are each essential for the efficient enterohepatic circulation of bile salts. Starting from the intestinal lumen, conjugated bile salts cross the otherwise impermeable lipid bilayer of (primarily terminal ileal) enterocytes through the apical sodium–dependent bile acid transporter (gene SLC10A2) and leave the enterocyte through the basolateral heteromeric organic solute transporter, which consists of an alpha and beta subunit (encoded by SLC51A and SLC51B). The Na+-taurocholate cotransporting polypeptide (gene SLC10A1) efficiently clears the portal circulation of bile salts, and the apical bile salt export pump (gene ABCB11) pumps the bile salts out of the hepatocyte into primary bile, against a very steep concentration gradient. Recently, individuals lacking either functional Na+-taurocholate cotransporting polypeptide or organic solute transporter have been described, completing the quartet of bile acid transport deficiencies, as apical sodium–dependent bile acid transporter and bile salt export pump deficiencies were already known for years. Novel pathophysiological insights have been obtained from knockout mice lacking functional expression of these genes and from pharmacological transporter inhibition in mice or humans. Conclusion: We provide a concise overview of the four main bile salt transport pathways and of their status as possible targets of interventions in cholestatic or metabolic disorders.
Abbreviations
-
- ASBT
-
- apical sodium–dependent bile acid transporter
-
- BSEP
-
- bile salt export pump
-
- FXR
-
- farnesoid X receptor
-
- GLP-1
-
- glucagon-like peptide 1
-
- KO
-
- knockout
-
- Mdr
-
- multidrug resistance protein
-
- NTCP
-
- Na+ taurocholate co-transporting polypeptide
-
- OATP
-
- organic anion transporting polypeptide
-
- OST
-
- organic solute transporter
-
- PBC
-
- primary biliary cholangitis
-
- TGR5
-
- G protein-coupled bile acid receptor
The enterohepatic circulation is a very efficient recycling system for bile salts containing most of the bile salt present in the body.(1) The entire bile salt pool circulates multiple times with each contraction of the gallbladder to the duodenum, through the ileum back to the liver and the biliary tract, to eventually be stored again for release during the next contraction (mostly after a meal) (Fig. 1). This implies that at least 20 g of bile salt passes the small intestinal every day, of which only less than 1 g/day is lost through fecal excretion (2%-5% per cycle). Some of the bile salts (mostly unconjugated) passively pass the epithelial lining of the colon, thereby escaping fecal excretion. Most of the bile salt, however, is actively taken up already in the ileum through the apical sodium–dependent bile acid transporter (ASBT, also referred to as the ileal bile acid transporter [IBAT]). We label this as the first of four pillars of the enterohepatic circulation, complemented by the second, the organic solute transporter (OST), facilitating efflux from enterocytes, the third, the Na+-taurocholate cotransporting polypeptide (NTCP), mediating hepatic uptake from the portal circulation, and finally the fourth, the bile salt export pump (BSEP), extruding bile salts from the hepatocyte into the canaliculi.
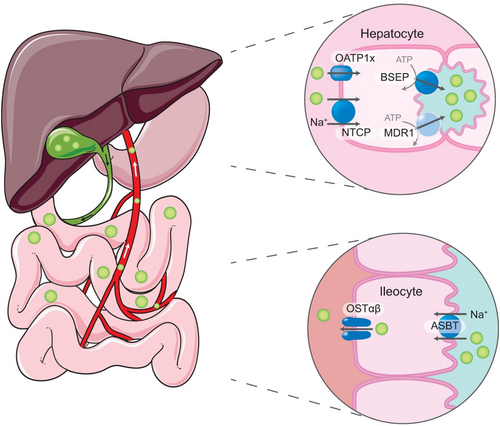
ASBT and BSEP deficiencies were identified in patients in 1997(2) and 1998,(3) respectively, but only recently individuals lacking functional NTCP(4) or OST(5, 6) were described. Genetic deficiencies of these transporters, either in humans or mice, have provided pathophysiological insights into the enterohepatic circulation of bile salts, and in their roles in health and disease. Apart from lipid solubilization in bile and intestine, bile salts also play a pivotal role as signaling molecules, both within and outside the enterohepatic circulation.(7, 8) This has led to the idea that pharmacological inhibition of some of these transporters could be used to ameliorate metabolic or cholestatic disorders. Here, we discuss the potential risks/limitations and advantages of these strategies.
ASBT
Oelkers et al. were the first to describe the consequences of genetic deficiency of ASBT, by identification of inactivating mutations in SLC10A2, the gene encoding ASBT, in patients with primary bile acid malabsorption.(2) These patients show defective conjugated bile salt absorption from the small intestine, leading to an increased spillover into the colon, causing diarrhea.(9) This disease can reliably be diagnosed by determining retention of 75Seleno-homotaurocholic acid in the body, but this procedure is not universally available. The pivotal role of ASBT in the enterohepatic cycling of bile salts is confirmed in ASBT knockout (KO) mice,(10) enabling the use of this model in studies on possible value of pharmacological ASBT inhibition as remedy. The first and most obvious disorder to mention is constipation, in which ASBT inhibition provided benefit.(11, 12) Second, ASBT inhibition was also successfully tested as therapy for hypercholesterolemia, as it results in stimulation of bile salt synthesis and therefore cholesterol catabolism,(13) and in strongly reduced transintestinal cholesterol excretion.(14) Note that similar mechanisms were evoked by surgically bypassing the ileum, a procedure designed in the 1960s to treat familial hypercholesterolemia.(15) In addition, increased levels of bile salts in the (large) intestine stimulates secretion of the incretin hormone glucagon-like peptide 1 (GLP-1), suggesting that ASBT inhibition could also have value in type 2 diabetes.(16) Furthermore, the increase in bile salts in the colon following ASBT inhibition stimulates the modification of bile salts by bacteria, increasing the (relative) amount of secondary bile salt species. The altered bile salt composition affects farnesoid X receptor (FXR) signaling. The combined effects of ASBT inhibition on lipid and glucose metabolism and signaling triggered the concept of using this approach to treat NAFLD. Studies in mice have suggested that an intestine-restricted ASBT inhibitor improves multiple aspects of NAFLD with restored glucose tolerance, reduced hepatic triglyceride, and total cholesterol concentrations.(17) Unfortunately, in a recent phase 2 trial in adults with NASH, this strategy failed to lower the amount of fat in the liver, or to have a beneficial effect on liver injury, while the main side effect of ASBT inhibition, diarrhea, was present in most tested individuals.(18)
Cholestatic disorders form a final set of conditions in which pharmacological ASBT inhibition could be beneficial, as treatment will reduce the hepatic bile salt load, leading to reduced liver injury. In multidrug resistance protein (Mdr) 2 KO mice, an animal model for bile salt–induced cholangiopathy, intestine-restricted pharmacological ASBT inhibition reduced cholestatic liver and bile duct damage.(19, 20) In humans, a trial was performed using similar inhibitors in patients with primary biliary cholangitis (PBC). Like in other cholestatic disorders, patients with PBC frequently suffer from chronic pruritus. Patients with PBC are commonly treated with ursodeoxycholate, but this is unfortunately largely ineffective against itch. The results of a phase 2 trial, with a reduction in itch as primary endpoint, were promising, as ASBT inhibition using GSK2330672 resulted in an approximate 35% reduction in pruritus, as assessed using the 5-D itch score, which was significantly different from the approximate 15% reduction in the placebo group.(21) ASBT inhibition is now also proposed as a nonsurgical intervention for other cholestatic disorders and has already been shown to reduce itch intensity in patients with Alagille syndrome.(22, 23) Unfortunately, diarrhea is reported as an adverse effect of ASBT inhibition in some, but not all, studies with patients with Alagille syndrome (ClinicalTrials.gov: NCT01903460).(22, 23) We recently proposed combination therapy of ASBT inhibitors with FXR agonism to reduce bile acid synthesis and potentially lower the risk of diarrhea.(24) Another strategy to reduce intestinal bile salt uptake with lowered incidence of diarrhea is the use of bile acid binding resins such as colesevelam. This increased fecal bile salt excretion and lowered cholestatic liver damage in Abcb4 KO mice.(25)
BSEP
The work of Bull and Thompson(26) and many others has been pivotal in the elucidation of genetic defects resulting in genetic forms of cholestasis, including progressive familial intrahepatic cholestasis (PFIC) type 2, where BSEP is mutated. BSEP deficiency has a wide spectrum of disease presentations varying from a mild or late-onset phenotype to early and severe liver damage. The incidence of HCC is increased and life expectation decreased when BSEP is nonfunctional or absent, compared with patients with residual BSEP function.(27) BSEP activity in patients with PFIC type 2 with specific mutations in the ABCB11 gene can partially be corrected using 4-phenylbutyrate(28) or ivacaftor (for specific missense mutations)(29) or agents inducing readthrough (for nonsense mutations).(30) Not only genetic defects, but also pharmacological (off-target) inhibition of BSEP transport function can cause cholestasis, and this leads to drug-induced liver injury.(31) Interestingly, the phenotype of the BSEP KO mouse model is rather mild, with impaired mitochondrial fatty acid β-oxidation,(32) but no signs of cholestasis, probably due to compensatory transport of (hydrophilic) bile salts through MDR1a/1b and to the more hydrophilic bile acid composition of murine bile compared with human bile.(33, 34) The canalicular targeting of MDR1 is impaired in BSEP deficiency, as was shown in Abcb11b mutant zebrafish and in a patient lacking BSEP protein due to nonsense mutations in ABCB11,(35) suggesting that strategies aimed at up-regulation or restoration of alternative transport routes could be beneficial in BSEP deficiency. A challenge with a bile duct ligation revealed that BSEP KO mice are largely protected against the liver injury.(36, 37) This may be explained by the enhanced synthesis of tetrahydroxy and pentahydroxy bile acids in these mice. This dramatically lowers the hydrophobicity of the bile salt pool, which decreases its detergent activity and mitigates damage to the hepatocytes and the biliary epithelium.
NTCP
The first individuals carrying mutations in SLC10A1 leading to NTCP deficiency were described by Vaz et al.(4) The most prominent biochemical feature was the extremely elevated total bile salt level in plasma (up to 1,500 μM; hypercholanemia), whereas clinical signs of jaundice, pruritus, or liver dysfunction were absent. Subsequently, multiple other NTCP-deficient individuals have been reported with isolated hypercholanemia as the common denominator, showing mild and transient neonatal hyperbilirubinemia and gallbladder anomalities but with no apparent long-term clinical consequences.(38-40) Pruritus was notably absent in these individuals, suggesting that isolated hypercholanemia may not be so detrimental as once thought. Interestingly, the p.Ser267Phe mutation in SLC10A1 (rs2296651), leading to transporter inactivation, is highly prevalent in East Asia, with an allele frequency of 8%-12% in individuals in Southern China.(41) The authors of the latter study suggest that NTCP deficiency leading to “hidden” hypercholanemia affects 0.64% of the Southern Han, 1.44% of the Dai Chinese population, and 1.21% of the Vietnamese population, indicating that a clinical presentation has a low penetrance, and NTCP deficiency is mostly asymptomatic. In contrast, SLC10A1 inactivity is likely even protective in certain conditions, and this variant may have exerted selection pressure in Southeast Asia. NTCP is the cell surface receptor for both HBV as well as HDV,(42, 43) and the p.Ser267Phe variant may provide protection against both HBV/HDV hepatocytic uptake and infection and HCC, which has been associated with these infections.(44-46) Myrcludex B (also called bulevirtide), a therapeutic peptide similar to the pre-S1 domain of the HBV/HDV envelope, competes with viral binding to NTCP and blocks both viral entry and hepatic bile salt uptake.(47-49) Myrcludex B has been tested in clinical trials in patients infected with HDV: It induces isolated hypercholanemia lasting for >12 hours per day without signs of pruritus.(47) Myrcludex B treatment also decreased HDV-RNA serum levels and induced alanine aminotransferase normalization under monotherapy in a phase 1b/2 trial.(49) In a phase 2 trial, 10 mg daily Myrcludex B combined with tenofovir resulted in a HDV-RNA negativation or decrease by ≥2 log10 from baseline in 77% of participants versus 4% in the tenofovir-only arm of the study (trial NCT03546621). The European Medicines Agency recently provided conditional marketing authorization for bulevirtide intended for the treatment of chronic HDV infection in adult patients with compensated liver disease, and indicated that no concerns exist on potential long-term safety/efficacy issues in relation to pediatric use in children infected with HDV (https://www.ema.europa.eu/en). Preclinical studies in mice offer promise for additional applications of NTCP inhibitors in conditions of hypercholesterolemia, obesity and cholestasis.(50-52)
NTCP-Deficient Mice
NTCP-deficient mice, first described by Slijepcevic et al.,(53) showed that some redundancy exists in hepatocytic uptake systems for bile salts in mice, due to the expression of other transporters of the organic anion transporting polypeptide (OATP) 1a/1b family. Most mice displayed no elevated bile salt levels at all, whereas a subset of these KO mice exhibited strong hypercholanemia.(54) This was independently confirmed by Mao et al.(55) This remarkable interindividual difference may be explained by a feed-forward mechanism in which elevated bile salt levels in plasma induce intestinal expression of FGF15.(53) This represses gene expression of Oatp1a/1b members in hepatocytes, leading to an almost complete block of hepatic bile salt uptake and therefore hypercholanemia, despite FGF15-induced dampening of bile acid synthesis.(53) Elevated levels of FGF15 may also explain the gallbladder abnormalities found in hyperchloremic 4-week-old NTCP KO mice.(39) In addition, NTCP deficiency induces bile salt sulfation, leading to increased elimination through urine, thus dampening the hypercholanemia.(55)
Protective Role of NTCP Deficiency in Metabolic Disease
NTCP deficiency may lead to partial protection against the deleterious effects of a high-calorie diet, as NTCP KO mice have a lower body weight gain and reduced hepatosteatosis on a high-fat diet (HFD).(51) This phenotype was explained by a reduced intestinal fat absorption and increased thermogenesis due to activation of brown adipose tissue.(51) Interestingly, NTCP/TGR5 (or GPBAR1, G protein-coupled bile acid receptor) double-KO mice revealed that the bile salt receptor TGR5 is completely dispensable for this dampened body weight gain, suggesting the presence of distinct bile salt sensory mechanisms.(51) Pharmacological inhibition of NTCP using Myrcludex B lowers body weight, dampens liver fat content, and induces GLP-1 in a Oat1p1a/1b-deficient mouse HFD model for obesity.(52) Interestingly, NTCP expression and hepatic bile salt uptake are down-regulated following Roux-en-Y gastric bypass, suggesting that this could contribute to some of the consequences of bariatric surgery on body weight and glucose handling.(56) Additionally, both NTCP deficiency and pharmacological NTCP inhibition reduce cholesterol levels in plasma in mice and humans.(44, 51, 52) In mice, NTCP inhibition leads to an enhanced biliary lipid/bile salt ratio.(57) Whether enhanced biliary cholesterol excretion contributes to the cholesterol lowering in plasma following NTCP deficiency awaits further exploration.
NTCP Inhibition Is Hepatoprotective in Cholestatic Conditions
Pharmacological NTCP inhibition using daily injections with Myrcludex B is hepatoprotective in various animal models for cholestasis.(50, 58) NTCP inhibition induced hypercholanemia, indicating effective reduction in hepatic bile salt uptake. This was paralleled by a relative increase in biliary phospholipid excretion (increased phospholipid/bile salt ratio), reduced liver enzymes, and lower expression of genes involved in inflammation and fibrosis in various cholestasis models. Mdr2 KO mice treated with Myrcludex B did not show any hypercholanemia and no hepatoprotective effect, likely due to relatively less repression of Oatp1a/1b-mediated bile salt uptake in this cholestasis model.(50)
Together, these studies suggest that pharmacological targeting of NTCP could have multiple applications in viral hepatitis and metabolic and cholestatic conditions. Furthermore, they suggest that the beneficial effects of NTCP inhibition are not only due to keeping bile salts out of the hepatocytes, but also attributable to the prolonged elevated bile salt levels in plasma.
OSTα-OSTβ
The heteromeric OSTαβ was identified in 2001 by Ballatori et al.(59) In a remarkable expression-cloning approach, two distinct nonhomologous subunits were identified that need to be co-expressed to form a functional bile salt transporter.(59) OSTα and OSTβ are encoded by two genes on separate chromosomes (SLC51A and SLC51B on Chr3 and Chr15, respectively). SLC51B deficiency was identified in 2018 in 2 brothers, clinically characterized by recessive inheritance, chronic diarrhea, severe fat-soluble vitamin deficiency, and features of cholestatic liver disease including elevated liver enzymes, particularly serum gamma-glutamyltransferase activity in serum.(5) In 1 of the siblings, a liver biopsy was taken that revealed mild portal fibrosis without steatosis or inflammation. Stool frequency declined with age. No additional patients have subsequently been identified yet. A KO animal model for SLC51B-deficiency is not yet published, so little is known about this novel genetic bile salt transport disorder. Only recently was a single patient with SLC51A deficiency identified.(6) A liver biopsy from this boy revealed a lobular architecture with periportal fibrosis, suggestive of early cirrhosis and minimal inflammation. His clinical presentation was more severe compared with the two cases of SLC51B deficiency, with easy bruising, two episodes of prolonged bleeding that required blood transfusions (each likely attributable to malabsorption of fat-soluble vitamin K), and failure to thrive. OSTα and OSTβ deficiency appears to have in common that patients display chronic malabsorptive diarrhea, but the number of cases are still too low to provide comprehensive disease insight.(6) OSTαβ does not exclusively transport bile salts, and its deficiency may affect transport of multiple compounds and drugs.(60) An animal model for OSTα deficiency was generated by two groups.(61, 62) Intestinal bile salt reabsorption is greatly reduced in these mice. OSTα KO mice have a much smaller bile salt pool size,(61, 62) related to strongly increased intestinal FXR activation.(63) They display a clear intestinal phenotype, with decreased villus length and a longer and thicker small intestine. The morphological phenotype with enterocyte damage is not due to FXR activation,(63) but restored following ASBT deletion, indicating that OSTαβ is pivotal to protect enterocytes from intracellular bile salt accumulation.(64) Whether hepatic OSTαβ is similarly critical to protect hepatocytes is less obvious. Both genes are expressed at a low level in normal conditions but up-regulated in cholestasis, in an FXR-dependent manner, suggesting a protective role.(60) However, challenging OSTα KO mice with a bile duct ligation(65) or cholate feeding(66) revealed that these mice are largely protected against cholestatic liver damage. The authors suggest that pharmacological OSTαβ inhibition may be beneficial under cholestatic conditions, as it leads to increased urinary bile salt excretion(65) and reduced intestinal uptake, inducing fecal bile salts excretion in early cholestasis. Furthermore, pharmacological OSTαβ inhibition induces intestine-specific FXR activation,(67) potentially leading to a hepatoprotective reduction of the bile salt pool and to target metabolic-syndrome associated diseases. Nevertheless, OSTαβ up-regulation following FXR activation will counteract some of its inhibition,(67) and the clinical phenotype of SLC51A/SLC51B deficiency with combined malabsorptive and cholestatic characteristics further argues against this approach.
Conclusions
The quartet of human bile acid transport deficiencies, the various animal models for these deficiencies, and the studies with pharmacological inhibition have yielded considerable novel pathophysiological insights into the enterohepatic circulation and (adaptive) consequences of disruptions at the four positions (Table 1). Nevertheless, multiple scientific questions remain. For example, can the benefits of NTCP inhibition seen in mice be transferred to the human situation? Why does OST deficiency appear to lead to cholestasis in humans, while both OSTα and OSTβ are normally only expressed at low levels in liver? Also illustrated by the multiple novel drugs targeting ASBT or NTCP in clinical trials, the field clearly remains highly dynamic.
| Transporter | Species | Deficiency or Inhibition Results in | Refs. |
|---|---|---|---|
| ASBT | Human | Defective intestinal bile salt absorption, causing diarrhea, decreased serum cholesterol levels; no improvement in NASH score after 48 weeks of treatment | 2, 18 |
| Rabbit, mouse | Increased bile salt synthesis and cholesterol catabolism; reduced transintestinal cholesterol excretion | 13, 14 | |
| Rat | Increased GLP-1 secretion due to increased bile salt load in the intestine | 16 | |
| Mouse | Restored glucose tolerance; reduced hepatic triglyceride and total cholesterol concentrations | 17 | |
| Mouse | Reduced cholestatic liver and bile duct damage | 19, 20 | |
| Human | Reduced pruritus in PBC and Alagille syndrome | 21, 22 | |
| BSEP | Human | Progressive familial intrahepatic cholestasis type 2 (BSEP deficiency) | 26 |
| Mouse | Impaired mitochondrial fatty acid β-oxidation; lower hydrophobicity of the bile salt pool, protecting the mice against liver damage during cholestasis | 32, 34, 36 | |
| NTCP | Human | Hypercholanemia without pruritus or liver dysfunction | 4 |
| Human | Protection against HDV/HBV infection and HCC | 44-46 | |
| Mouse | Normocholanemia; subset has hypercholanemia, elevated FGF15, and gallbladder thickening | 39, 53-55 | |
| Mouse | Hepatoprotective in cholestatic conditions | 50, 58 | |
| Mouse | Increased biliary phospholipid secretion | 39, 57 | |
| Mouse | Reduced obesity; reduced steatosis; reduced plasma cholesterol; increased GLP-1 | 51, 52 | |
| Human | Reduced plasma LDL-cholesterol | 44 | |
| Ostβ | Human | Diarrhea and fat-soluble vitamin deficiency, features of cholestatic liver disease | 5 |
| Ostα | Human | Diarrhea; periportal fibrosis suggestive of early cirrhosis; easy bruising/bleeding (each likely attributable to malabsorption of fat-soluble vitamin K) | 6 |
| Ostα | Mouse | Reduced intestinal bile salt reabsorption; reduced bile salt pool size; decreased villus length and a longer and thicker small intestine; protection against cholestatic liver injury | 61-63, 65, 66 |
| Ostαβ | Mouse | Intestinal FXR activation | 67 |
Author Contributions
All authors have contributed to the manuscript and agree with its content.




