Hepatocyte Nuclear Factor 4α Prevents the Steatosis-to-NASH Progression by Regulating p53 and Bile Acid Signaling (in mice)
Abstract
Background and Aims
Hepatocyte nuclear factor 4α (HNF4α) is highly enriched in the liver, but its role in the progression of nonalcoholic liver steatosis (NAFL) to NASH has not been elucidated. In this study, we investigated the effect of gain or loss of HNF4α function on the development and progression of NAFLD in mice.
Approach and Results
Overexpression of human HNF4α protected against high-fat/cholesterol/fructose (HFCF) diet–induced steatohepatitis, whereas loss of Hnf4α had opposite effects. HNF4α prevented hepatic triglyceride accumulation by promoting hepatic triglyceride lipolysis, fatty acid oxidation, and VLDL secretion. Furthermore, HNF4α suppressed the progression of NAFL to NASH. Overexpression of human HNF4α inhibited HFCF diet–induced steatohepatitis in control mice but not in hepatocyte-specific p53−/− mice. In HFCF diet–fed mice lacking hepatic Hnf4α, recapitulation of hepatic expression of HNF4α targets cholesterol 7α-hydroxylase and sterol 12α-hydroxylase and normalized hepatic triglyceride levels and attenuated steatohepatitis.
Conclusions
The current study indicates that HNF4α protects against diet-induced development and progression of NAFLD by coordinating the regulation of lipolytic, p53, and bile acid signaling pathways. Targeting hepatic HNF4α may be useful for treatment of NASH.
Abbreviations
-
- AAV
-
- adeno-associated virus
-
- ABCG
-
- ATP-binding cassette subfamily G
-
- Acat
-
- acyl-coenzyme a:cholesterol acyltransferase
-
- ALB
-
- albumin
-
- ALT
-
- alanine aminotransferase
-
- ApoB
-
- apolipoprotein B
-
- AST
-
- aspartate aminotransferase
-
- BA
-
- bile acid
-
- CES
-
- carboxylesterase
-
- Col1α1
-
- collagen type 1, alpha 1
-
- Col1α2
-
- collagen type 1, alpha 2
-
- CPT
-
- carnitine palmitoyltransferase
-
- CYP7A1
-
- cholesterol 7α-hydroxylase
-
- CYP8B1
-
- sterol 12α-hydroxylase
-
- FAO
-
- fatty acid oxidation
-
- FC
-
- free cholesterol
-
- FFA
-
- free fatty acid
-
- FXR
-
- farnesoid X receptor
-
- GC-MS
-
- gas chromatography–mass spectrometry
-
- h
-
- human
-
- H&E
-
- hematoxylin and eosin
-
- HFCF
-
- high-fat/cholesterol/fructose
-
- HNF4α
-
- hepatocyte nuclear factor 4α
-
- Mcp1
-
- monocyte chemoattractant protein 1
-
- mRNA
-
- messenger RNA
-
- MTTP
-
- microsomal triglyceride transfer protein
-
- NAFL
-
- nonalcoholic liver steatosis
-
- OCA
-
- obeticholic acid
-
- PDK4
-
- Pyruvate dehydrogenase lipoamide kinase isozyme 4
-
- PPARα
-
- peroxisome proliferator-activated receptor α
-
- ROS
-
- reactive oxygen species
-
- SHP
-
- small heterodimer partner
-
- Sma
-
- smooth muscle actin
-
- Smad
-
- mothers against decapentaplegic homolog
-
- SR-BI
-
- scavenger receptor class B type I
-
- TC
-
- total cholesterol
-
- TG
-
- triglyceride
-
- Tgfβ
-
- transforming growth factor β
-
- TGH
-
- triglyceride hydrolase/hydrolysis
-
- Timp1
-
- tissue inhibitor of metalloproteinase 1
NAFLD is a spectrum of liver diseases ranging from nonalcoholic liver steatosis (NAFL) to NASH, which may further progress to liver cirrhosis and hepatocarcinoma. Multiple mechanisms are involved in the development and progression of NAFLD. Although NAFL may be a protective mechanism to avoid lipotoxicity, NASH is the advanced form of NAFLD that needs medical attention. Several mechanisms, such as insulin resistance, abnormal apoptosis, reactive oxygen species (ROS), lipotoxicity, and inflammation, are believed to contribute to the progression of NAFL to NASH.(1-7) In addition, defective lipolysis also plays a role in the pathogenesis of NAFLD.(8-10)
Hepatocyte nuclear factor 4α (HNF4α) is a nuclear hormone receptor that is highly conserved between humans and mice. HNF4α is highly expressed in the liver but also found in the intestine, pancreas, and kidney.(11) HNF4α is constitutively active and usually binds to the promoters of genes to regulate their expression. Gene knockout studies demonstrate that HNF4α is required for the expression of a number of hepatic genes that regulate hepatocyte differentiation, energy metabolism, xenobiotic detoxification, bile acid (BA) synthesis, and plasma protein production.(12, 13) On a chow diet, liver-specific Hnf4α −/− mice display reduced plasma triglyceride (TG) and cholesterol levels, which are accompanied by reduced microsomal TG transfer protein (MTTP) and apolipoprotein B (ApoB) expression.(14) Acute loss of hepatic Hnf4α by short hairpin RNA also reduces plasma TG and cholesterol levels but increases hepatic TG accumulation.(15) In addition, loss of HNF4α results in reduced expression of genes involved in BA synthesis, such as cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CYP8B1),(16) and BA conjugation.(17) In patients with NASH or in obese mice, hepatic HNF4α expression is markedly reduced.(18) So far, the role of hepatic HNF4α in the progression of NAFL to NASH has not been investigated. Moreover, the mechanism underlying the regulation of hepatic TG levels by HNF4α has not been fully understood.
The tumor suppressor p53 is a primary stress sensor that controls a variety of biological processes. p53 expression or signaling is increased in the livers of patients with NAFLD or experimental NASH.(19-21) p53-deficent mice are resistant to methionine and choline-deficient diet–induced apoptosis and steatohepatitis.(19) Likewise, inhibition of p53 attenuates high-fat diet–induced liver steatosis and injury.(22) Consistent with these observations, activation of farnesoid X receptor (FXR) by obeticholic acid (OCA) inhibits diet-induced p53 activation and cell death and protects against the development of NASH.(23) BAs are endogenous ligands for FXR. Interestingly, p53 is shown to inhibit BA synthesis through inducing small heterodimer partner (SHP),(24) a nuclear hormone receptor that is also a target of FXR.(25) Nonetheless, it is unclear whether HNF4α regulation of the pathogenesis of NAFLD involves p53 and/or BAs.
In this report, we show that adeno-associated virus (AAV) 8–mediated overexpression of human (h) HNF4α (AAV8-albumin [ALB]-hHNF4α) in hepatocytes protected against high-fat/cholesterol/fructose (HFCF) diet–induced hepatosteatosis, inflammation, and fibrosis. In contrast, hepatocyte-specific Hnf4α−/− (Hnf4α Hep−/−) mice had aggravated steatohepatitis on an HFCF diet. Mechanistically, we show that HNF4α regulated hepatic TG accumulation by promoting TG hydrolase/hydrolysis (TGH), fatty acid oxidation (FAO), and VLDL secretion. We also show that overexpression of human HNF4α in hepatocytes protected against the development of steatohepatitis in p53fl/fl mice but not in hepatocyte-specific p53−/− (p53Hep−/−) mice. Lastly, recapitulation of hepatic CYP7A1 and CYP8B1 expression in Hnf4αHep−/− mice normalized hepatic TG levels and alleviated steatohepatitis. Our data suggest that HNF4α regulates the development and progression of NAFLD through coordinating modulation of lipolytic, p53, and BA signaling.
Materials and Methods
Mice and Diets
C57BL/6J mice (stock # 000664), Fxr−/− mice (stock # 004144), Hnf4α fl/fl mice (stock # 004665) and p53fl/fl mice (stock # 008462) were purchased from Jackson Laboratories (Bar Harbor, ME). Hnf4αfl/fl mice and Fxr−/− mice were cross-bred with C57BL/6J mice for at least 10 generations. AAV8-TBG-Null or AAV8-TBG-Cre (produced by Vector Biolabs) was injected to Hnf4α fl/fl mice or p53fl/fl mice to generate control mice (Hnf4αfl/fl mice or p53fl/fl mice), hepatocyte-specific Hnf4α−/− (Hnf4α Hep−/−) mice, or hepatocyte-specific p53−/− (p53Hep−/−) mice, respectively. All mice were kept in a temperature and humidity-controlled room with a 12-hour light/12-hour dark cycle and free access to water and food. The HFCF diet contained 40% fat/0.2% cholesterol (from TestDiet, AIN-76A), and 4.2% fructose (in drinking water). Mice were fed an HFCF diet for 20 weeks. Unless otherwise stated, male mice were used and were fasted for 5-6 hours before euthanasia. All the animal experiments were approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University.
AAVs
Human HNF4α, CYP7A1, CYP8B1, or p53 coding sequences were cloned into an AAV vector under the control of a mouse ALB promoter to generate AAV-ALB-hHNF4α, AAV-ALB-hCYP7A1, AAV-ALB-hCYP8B1, or AAV-ALB-hP53, respectively. AAV8-TBG-Null (control), AAV8-TBG-Cre, AAV8-ALB-Null (control), AAV8-ALB-hHNF4α, AAV8-ALB-hCYP7A1, AAV8-ALB-hCYP8B1, or AAV8-ALB-hP53 was produced using AAV serotype 8 and titrated by Vector Biolabs. Each mouse was IV injected with 3 × 1011 genome copies AAV and then fed a chow diet or an HFCF diet for up to 20 weeks.
OCA Treatment
OCA, a specific FXR agonist, was provided by Intercept Pharmaceuticals, Inc (San Diego, CA). Fxr+/+ or Fxr−/− mice were gavaged with either vehicle (0.5% carboxymethycellulose, Sigma) or OCA (30 mg/kg/day) for 7 days. In a different study, Hnf4αfl/fl mice or Hnf4αHep−/− mice were fed an HFCF diet for 16 weeks. In the last 30 days of the latter study, mice were also gavaged with either vehicle or OCA (20 mg/kg/day).
Messenger RNA and Quantitative Real-Time PCR
RNA was extracted using Trizol Reagent (Thermo Fisher), and messenger RNA (mRNA) levels were quantified by quantitative real-time PCR (qRT-PCR) using PowerUP SYBR Green Master Mix (Thermo Fisher) on a 7500 Real-Time PCR machine (Applied Biosystems). mRNA levels were normalized to 36B4.
Western Blot Assays
Western blot assays were performed using whole liver lysates or membrane extracts of the liver samples, as described.(26, 27) Antibodies against HNF4α (catalog # sc-374229) or p53 (catalog # sc-6243) were purchased from Santa Cruz Biotechnology. Antibodies against CYP7A1 (catalog # TA351400) or CYP8B1 (catalog # TA313734) were purchased from Origene. Antibodies against scavenger receptor class B type I (SR-BI) (catalog # NB400-101) or calnexin (catalog # NB100-1965) were purchased from Novus. Antibodies against phospho–mothers against decapentaplegic homolog (Smad) 2/3 (catalog # 8828), total Smad2/3 (catalog # 5678), cleaved caspase 3 (catalog # 9661) or total caspase 3 (catalog # 9662) were purchased from Cell Signaling Technology. Antibodies against carboxylesterase (CES) 1 (catalog # ab45957), CES2 (catalog # ab56528), acyl-coenzyme A:cholesterol acyltransferase (ACAT) 2 (catalog # ab23669), or tubulin (catalog # ab4074) were purchased from Abcam.
Analysis of Plasma or Hepatic Levels of Lipids, Hydroxyproline, ROS, Aspartate Aminotransferase, Alanine Aminotransferase, β-Hydroxybutyrate, and Apoptosis
Approximately 100 mg liver tissue was homogenized in methanol, and lipids were extracted in chloroform/methanol (2:1 vol/vol) as described.(28) TGs and cholesterol in the liver and plasma alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were measured using Infinity reagents from Thermo Scientific (Waltham, MA). Hepatic hydroxyproline level was quantified using a kit from Cell Biolabs (catalog # STA675). Hepatic ROS levels were measured using an OxiSelect In Vitro ROS/RNS Assay Kit (catalog # STA-347) from Cell Biolabs (San Diego, CA) as described.(29) Plasma β-hydroxybutyrate level was determined using a kit from Pointe Scientific (Canton, MI). Apoptosis was determined using a kit from Abcam (catalog # ab206386).
TGH Activity Assays
Hepatic microsome proteins were isolated and TGH activity was measured using 3H-triolein as substrate as described.(30)
FAO
Primary hepatocytes were isolated and cultured in DMEM in 12-well dishes. FAO was performed using [3H]palmitate as substrate as described.(31, 32)
Oil Red O, Hematoxylin and Eosin, or Sirius Red Staining
Liver was fixed in 4% formalin and then embedded in optimal cutting temperature compound or paraffin. Oil Red O, hematoxylin and eosin (H&E), or Sirius Red staining was performed as described.(32)
Analysis of Fatty Acid Levels and Composition
Hepatic total free fatty acids (FFAs) were measured using a kit from Wako Chemicals USA (Richmond, VA). Hepatic fatty acid composition was analyzed by gas chromatography–mass spectrometry (GC-MS) as described.(30)
Statistical Analysis
Statistical significance was analyzed using unpaired Student t test or ANOVA (GraphPad Prism, CA). All values are expressed as mean ± SEM. Differences were considered statistically significant at P < 0.05.
Results
HNF4α is an essential regulator of hepatic TGH activity and FAO. Hepatic TG level is controlled by TG synthesis, lipolysis, and VLDL secretion. Studies have shown that HNF4α regulates hepatic expression of MTTP and apoB as well as VLDL secretion.(14, 15) So far, the role of hepatic HNF4α in lipolysis or FAO has not been examined. Because adenoviruses are known to cause significant liver inflammation, we generated AAV8-ALB-hHNF4α, which expressed human HNF4α specifically in hepatocytes. On a chow diet, AAV8-mediated overexpression of human HNF4α reduced hepatic TG and FFA levels by ~33% (Fig. 1A, left and middle panels). Analysis of hepatic fatty acid composition by GC-MS showed that human HNF4α overexpression reduced the levels of C16:0, C18:1, C18:2, and C20:4 fatty acyl-CoA by 50%-90% (Fig. 1A, right panel). Analysis of hepatic mRNA levels by qRT-PCR showed that human HNF4α induced the expression of genes involved in BA biosynthesis (Cyp7a1, Cyp8b1), lipolysis (Ces1, Ces2), FAO (peroxisome proliferator-activated receptor α [Pparα], carnitine palmitoyltransferase 1 [Cpt1], Cpt2, Pyruvate dehydrogenase lipoamide kinase isozyme 4 [Pdk4]), and VLDL secretion (Mttp, Apob) (Fig. 1B). There was no change in hepatic mRNA levels of genes involved in de novo lipogenesis (DNL) (sterol regulatory element binding protein 1c, fatty acid synthase [Fasn], acetyl-CoA carboxylase 1 [Acc1]) (Fig. 1B) or hepatic levels of total cholesterol (TC), free cholesterol (FC), or cholesteryl esters (CE) (Supporting Fig. S1A). We have shown that CES1 and CES2 have TGH activity, whose overexpression lowers hepatic TG levels and stimulates FAO.(30, 32) In line with these findings, overexpression of human HNF4α increased hepatic TGH activity by 76% (Fig. 1C, left panel) and FAO by 28% (Fig. 1C, right panel).
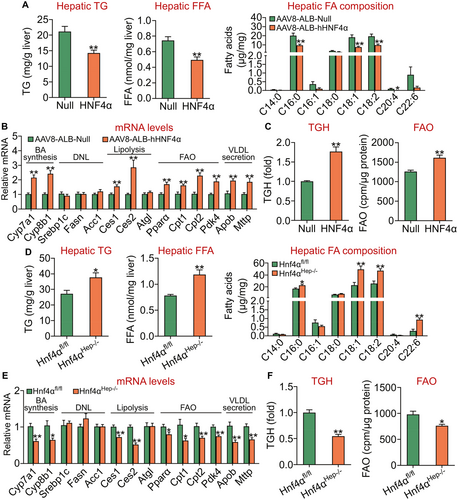
In contrast to what was observed in mice overexpressing human HNF4α in the liver, hepatocyte-specific loss of Hnf4α increased hepatic levels of TG by 39% and FFA by 52% (Fig. 1D, left and middle panels). Hepatic C16:0, C18:1, C18:2, and C22:6 fatty acyl-CoA levels were increased by 135% to 320% (Fig. 1D, right panel). Hnf4αHep−/− mice also had reduced expression of Cyp7a1, Cyp8b1, Ces1, Ces2, Pparα, Cpt1, Cpt2, Pdk4, Mttp, or Apob (Fig. 1E). There was no change in hepatic expression of genes involved in DNL (Fig. 1E) or hepatic levels of TC, FC, or CE (Supporting Fig. S1B). In addition, hepatocyte-specific loss of Hnf4α reduced hepatic TGH activity by 45% (Fig. 1F, left panel) and FAO by 22% (Fig. 1F, right panel).
Taken together, our gain and loss-of-function data demonstrate that HNF4α is an essential regulator of hepatic TGH activity, which may account, at least in part, for the regulation of hepatic TG levels by HNF4α.
Hepatocyte-specific overexpression of human HNF4α is sufficient to protect against HFCF diet–induced NASH. We have shown that hepatic HNF4α expression is markedly reduced in patients with NAFLD or high-fat diet–fed mice.(18) So far, it remains to be determined whether gain of HNF4α function is sufficient to protect from diet-induced steatosis or steatohepatitis. To address this question, we injected C57BL/6J mice IV with AAV8-ALB-Null or AAV8-ALB-hHNF4α, which were then fed an HFCF diet for 20 weeks. Injection of AAV8-ALB-hHNF4α led to a 5.9-7.5-fold increase in hepatic HNF4α protein level at 8, 16, or 20 weeks (Supporting Fig. S2A,B). Overexpression of human HNF4α prevented HFCF diet–induced increases in hepatic TG or FFA levels (Fig. 2A), and hepatic C16:0, C16:1, or C18:1 fatty acyl-CoA levels (Fig. 2B). Oil Red O and H&E staining also confirmed a reduction in hepatic neutral lipid accumulation in mice overexpressing HNF4α (Fig. 2C). In addition, human HNF4α induced Ces1 and Ces2 mRNA and protein levels and expression of genes involved in FAO (Pparα, Cpt1, Cpt2, Pdk4) or VLDL secretion (Mttp, Apob) (Fig. 2D). Consistent with these data, human HNF4α overexpression increased hepatic TGH activity by 60% (Fig. 2E) and FAO by 45% (Fig. 2F) in mice fed an HFCF diet.
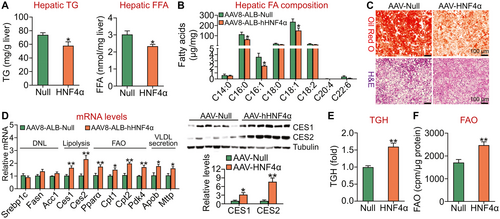
We also investigated the effect of gain of hepatic HNF4α function on the development of NASH. Overexpression of human HNF4α reduced plasma ALT levels by 35% but had no effect on plasma AST levels (Fig. 3A), suggesting that gain of hepatic HNF4α function protects against diet-induced liver injury. Overexpression of human HNF4α significantly inhibited the expression of genes involved in inflammation or fibrogenesis (Tnfα, Il-6, Il-1β, monocyte chemoattractant protein 1 [Mcp1], F4/80, p53, α-smooth muscle actin (Sma), tissue inhibitor of metalloproteinase 1 (Timp1), collagen type 1, alpha 1 [Col1α1], collagen type 1, alpha 2 [Col1α2]) (Fig. 3B). HNF4α also induced the expression of genes involved in cholesterol catabolism (Cyp7a1, Cyp8b1), HDL uptake (scavenger receptor group B type 1 [Scarb1]), cholesterol efflux (ATP-binding cassette subfamily G [Abcg]8), or cholesterol esterification (Acta1, Acat2) with genes involved in cholesterol synthesis or LDL uptake (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, 3-hydroxy-3-methyl-glutaryl-coenzyme A synthase [Hmgcs], LDL receptor [Ldlr]) being unchanged (Fig. 3B). Consistent with the changes in mRNA levels, hepatic protein levels of HNF4α, CYP7A1, CYP8B1, SR-BI, and ACAT2 were increased by ≥2-fold (Fig. 3C, left panels). In line with the changes in gene expression, hepatic TC and FC levels were decreased by >38%, whereas hepatic BA levels were increased by 142% (Fig. 3C, right panels).
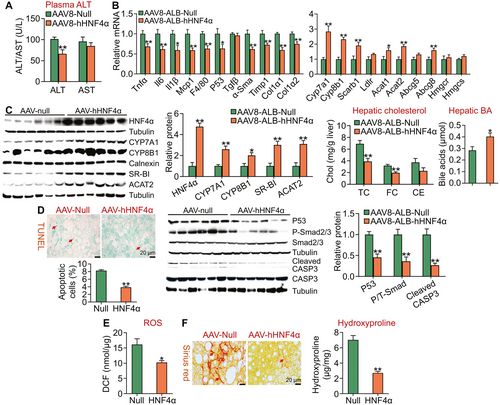
Apoptosis and ROS play an important role in promoting the progression of NAFL to NASH. Hepatocyte-specific expression of human HNF4α in an HFCF diet–induced NASH model reduced hepatic apoptosis by >50% (Fig. 3D, left panel) and hepatic protein levels of p53, phospho-Smad2/3, and cleaved caspase 3 by >50% (Fig. 3D, middle and right panels). In addition, human HNF4α also reduced hepatic ROS levels (Fig. 3E). Consistent with the reduced apoptosis and ROS levels, human HNF4α overexpression inhibited hepatic fibrogenesis, with hepatic hydroxyproline levels being reduced by >60% (Fig. 3F, left and right panels).
Collectively, the data of Figs. 2 and 3 demonstrate that hepatocyte-specific overexpression of human HNF4α protects against diet-induced NASH by a mechanism likely involving induction of lipolysis and inhibition of inflammation, apoptosis, and ROS production.
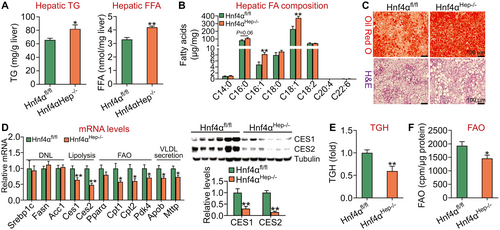
HNF4α is essential for protection against HFCF diet–induced NASH. To investigate the effect of loss of HNF4α on diet-induced NASH, we fed Hnf4αfl/fl mice and Hnf4αHep−/− mice an HFCF diet for 20 weeks. Loss of hepatic Hnf4α raised hepatic levels of TG and FFA (Fig. 4A) as well as C16:0, C16:1, and C18:1 fatty acyl-CoA levels by 32% (P = 0.06), 73%, and 63%, respectively (Fig. 4B). Oil Red O or H&E staining confirmed more neutral lipid accumulation in Hnf4αHep−/− mice (Fig. 4C). Analysis of hepatic gene expression showed that hepatic mRNA levels of Ces1, Ces2, Cpt1, Cpt2, Pdk4, Mttp, or Apob were significantly reduced (Fig. 4D, left panel) and that hepatic CES1 or CES2 protein levels were reduced by ≥70% (Fig. 4D, right panel). In addition, loss of hepatic Hnf4α reduced hepatic TGH activity by 40% (Fig. 4E) and FAO by 24% (Fig. 4F).
Loss of HNF4α also raised plasma ALT levels by 29% (Fig. 5A) and induced a number of genes involved in inflammation or fibrogenesis (Tnfα, Il-6, Il-1β, Mcp1, F4/80, p53, transforming growth factor β (Tgfβ), α-Sma, Timp1, Col1α1, Col1α2) (Fig. 5B, left panel) but reduced hepatic expression of genes involved in BA/cholesterol metabolism (Cyp7a1, Cyp8b1, Scarb1, Ldlr, Acat2, Abcg5, Abcg8, Hmgcs) (Fig. 5B, right panel). In line with the changes in mRNA levels, Hnf4αHep−/− mice had a 45%-82% reduction in protein levels of HNF4α, CYP7A1, CYP8B1, SR-BI, or ACAT2 (Fig. 5C, left panels) and an increase in hepatic TC and FC levels but a decrease in hepatic BA levels (Fig. 5C, right panels).
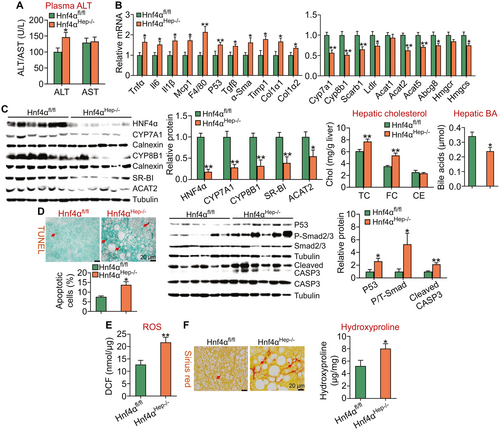
In addition, Hnf4αHep−/− mice had an 82% increase in hepatic apoptosis (Fig. 5D, left panel) and a >2-fold increase in hepatic protein levels of p53, phospho-Smad2/3, and cleaved caspase 3 (Fig. 5D, middle and right panels). Finally, loss of HNF4α also increased hepatic ROS levels by 170% (Fig. 5E) and raised hepatic fibrogenesis and hydroxyproline levels by 154% (Fig. 5F, left and right panels). Thus, loss of HNF4α aggravates the development of diet-induced NASH.
Human HNF4α protects against diet-induced NASH through inhibition of p53. p53 is a key regulator of apoptosis and the progression of NAFLD.(19) Our data have shown that HNF4α represses hepatic p53 expression by >50% (Fig. 3D), suggesting that p53 may play an important role in HNF4α-mediated protection against NASH. To test this hypothesis, we injected p53fl/fl mice and p53Hep−/− mice IV with AAV8-ALB-Null or AAV8-ALB-hHNF4α, which were then fed an HFCF diet for 20 weeks. Overexpression of human HNF4α reduced plasma ALT levels in p53fl/fl mice but not in p53Hep−/− mice (Fig. 6A). In addition, hepatic mRNA levels of inflammatory genes (Tnfα, Il-6, Il-1β, Mcp1) (Fig. 6B) or fibrogenic genes (Tgfβ, Timp1, α-Sma, Col1α1, Colα2) (Fig. 6C) were reduced in p53fl/fl mice but not in p53Hep−/− mice. Consistent with the changes in gene expression, hepatic hydroxyproline levels were reduced in p53fl/fl mice but not in p53Hep−/− mice (Fig. 6D, left panel). Interestingly, HNF4α overexpression reduced hepatic TG, FFA, and cholesterol levels in both p53fl/fl mice and p53Hep−/− mice (Fig. 6D middle and right panels). Overexpression of human HNF4α also inhibited apoptosis (Fig. 6E) and reduced the protein levels of phospho-Smad2/3 and cleaved caspase 3 (Fig. 6F) in p53fl/fl mice but not p53Hep−/− mice.
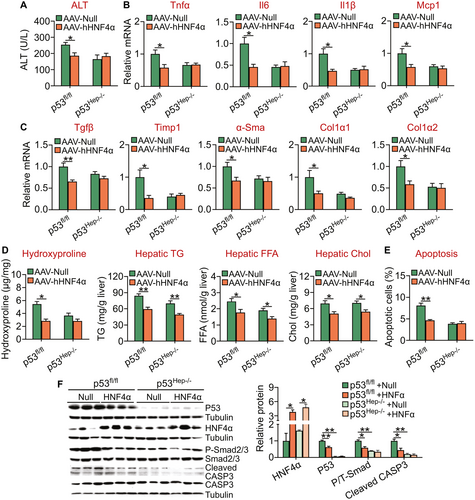
To further investigate whether HNF4α -mediated inhibition of p53 played a role in the development of NAFLD, we generated AAV8-ALB-p53 that overexpressed human p53, which was IV injected into C57BL/6J mice together with AAV8-ALB-Null or AAV8-ALB-hHNF4α. The mice were then fed an HFCF diet for 20 weeks. Our data showed that recapitulation of hepatic p53 expression level by 76% of the control mice (Supporting Fig. S3A) partially restored plasma ALT levels (Supporting Fig. S3B), hepatic TG, cholesterol, and hydroxyproline levels (Supporting Fig. S3C-E), and hepatic fibrogenesis in mice overexpressing HNF4α (Supporting Fig. S3F). In addition, partial recapitulation of hepatic p53 expression level also partially restored hepatic expression of proinflammatory and fibrogenic genes (Tnfα, Il-6, Il-1β, Mcp1, Timp1, α-Sma, Col1α1, Col1α2) (Supporting Fig. S4A and S4B) and fully restored apoptosis (Supporting Fig. S4C and S4D) and phospho-Smad2/3 and cleaved caspase 3 levels in the liver (Supporting Fig. S4E,F).
Taken together, these data demonstrate that gain of hepatic HNF4α function protects against steatohepatitis through, at least in part, inhibition of p53.
Loss of HNF4α aggravates the development of NASH by inhibiting BA signaling. BAs are endogenous ligands for FXR whose pharmacological activation inhibits p53 activity and protects against cell death and the development of NASH.(23) Because CYP7A1 and CYP8B1 are key enzymes in the BA biosynthetic pathway and are reduced in Hnf4αHep−/− mice, we hypothesized that the reduced BA synthesis/signaling might be responsible, at least in part, for the aggravated steatohepatitis in these mice. To test this hypothesis, we IV injected AAV8-ALB-Null or AAV8-ALB-hCYP7A1 plus AAV8-ALB-hCYP8B1 into Hnf4α fl/fl mice or Hnf4αHep−/− mice, which were then fed an HFCF diet for 20 weeks. In Hnf4αHep−/− mice, exogenous expression of human CYP7A1 and CYP8B1 fully normalized hepatic CYP7A1 and CYP8B1 protein expression and hepatic BA levels (Fig. 7A) and also partially normalized hepatic p53 protein expression (Fig. 7A, left panel; Supporting Fig. S5). In addition, exogenous expression of human CYP7A1 and CYP8B1 in Hnf4αHep−/− mice also fully normalized hepatic levels of TC, FC (Fig. 7B), TG, FFA (Fig. 7C), and FAO (Fig. 7D) but failed to normalize hepatic TGH activity (Fig. 7E). Consistent with these data, hepatic expression of Pparα, Cpt1, Cpt2, or Pdk4, but not Ces1 or Ces2, was normalized in Hnf4αHep−/− mice that expressed human CYP7A1 and CYP8B1 (Fig. 7F).
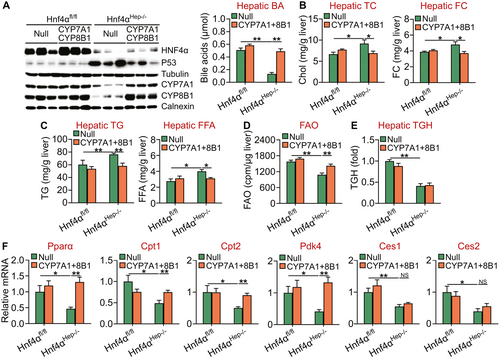
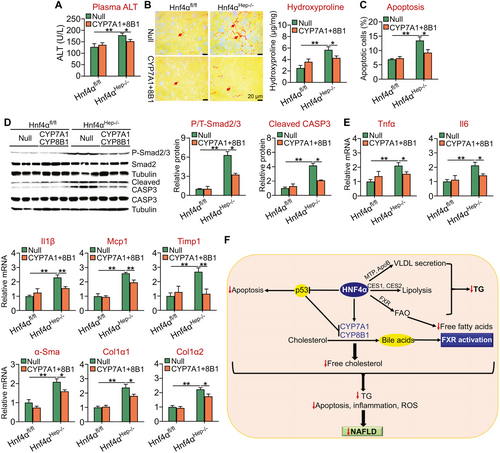
Furthermore, recapitulation of hepatic CYP7A1 and CYP8B1 expression in Hnf4αHep−/− mice also partially or fully normalized plasma ALT levels (Fig. 8A) as well as hepatic levels of hydroxyproline or fibrosis (Fig. 8B), apoptosis (Fig. 8C), phospho-Smad2/3, cleaved caspase 3 (Fig. 8D), and proinflammatory or fibrogenic genes (Tnfα, Il-6, Il-1β, Mcp1, Timp1, α-Sma, Col1a1, Col1a2) (Fig. 8E).
Expression of CYP7A1 and CYP8B1 produces BAs, which are endogenous ligands for FXR. Indeed, recapitulation of hepatic CYP7A1 and CYP8B1 expression in mice lacking HNF4α induced the expression of FXR target genes, including Shp, BA export protein, fibroblast growth factor 21 (Fgf21), and organic solute transporter α(25, 33) (Supporting Fig. S6). Treatment of C57BL/6 mice with either vehicle or the FXR agonist OCA induced hepatic genes involved in FAO, including Pparα, Cpt1, Cpt2, and Pdk4 (Supporting Fig. S7A). Consistent with these later data, OCA treatment induced FAO in Fxr+/+ hepatocytes but not in Fxr−/− hepatocytes (Supporting Fig. S7B). These data may explain why expression of CYP7A1 and CYP8B1 induces FAO.
To investigate whether activation of FXR by a synthetic FXR agonist can mimic the effects of CYP7A1 and CYP8B1 overexpression, we fed Hnf4αfl/fl mice and Hnf4αHep−/− mice an HFCF diet for 20 weeks. In the last 30 days, mice were also gavaged with either vehicle or OCA. Activation of FXR in Hnf4αHep−/− mice reduced plasma ALT level as well as hepatic TG and FFA levels (Supporting Fig. S8A-C) but induced hepatic mRNA levels of Shp (a known FXR target gene), Pparα, Cpt1, Cpt2, and Pdk4 (Supporting Fig. S8D). In addition, activation of FXR in Hnf4αHep−/− mice also inhibited liver fibrosis (Supporting Fig. S9A,B) and reduced hepatic mRNA levels of proinflammatory and fibrogenic genes (Tnfα, Il-6, Il-1β, Mcp1, Timp1, Col1a1, Col1a2) (Supporting Fig. S9C,D). Thus, the data of Supporting Figs. S6-S9 suggest that recapitulation of hepatic CYP7A1 and CYP8B1 expression exerts their beneficial effects in mice lacking HNF4α likely by increasing BAs and subsequent activation of FXR.
Taken together, the data of Figs. 7 and 8 and Supporting Figs. S6-S9 show that recapitulation of hepatic CYP7A1 and CYP8B1 expression in Hnf4αHep−/− mice partly or fully prevents diet-induced NASH, which can be mimicked by activation of FXR through a synthetic agonist. Thus, disruption of hepatic BA signaling may account, at least in part, for the aggravated NASH in mice lacking Hnf4α.
Discussion
Studies have shown that acute or chronic loss of hepatic HNF4α increases hepatic TG levels in chow-fed mice(14, 15) and that hepatic HNF4α expression is markedly reduced in patients with NASH and in NASH mouse models.(18) However, it remains to be determined whether gain or loss of hepatic HNF4α expression plays a role in Western diet–induced development or progression of NAFLD. In the current study, we provide compelling evidence demonstrating that HNF4α is required and also sufficient to protect against diet-induced development and progression of NASH by integrating lipolytic, p53, and BA signaling (Fig. 8F).
The development of NAFLD often initiates from TG accumulation in the liver. The lipolytic pathway is an important mechanism of the development and progression of NAFLD.(8-10) Our gain and loss-of-function studies demonstrate that HNF4α is a critical regulator of hepatic TGH activity. Importantly, HNF4α also increases FAO, allowing released FFAs to be oxidized so that no toxic FFAs are accumulated in the liver. Our data suggest that the induction of FAO by HNF4α is likely through activation of FXR, as HNF4α promotes activation of FXR and FXR activation induces FAO. We and others have shown that HNF4α regulates VLDL secretion.(14, 15) Thus, HNF4α regulates hepatic TG levels, likely by modulating both TGH and VLDL secretion (Fig. 8F).
The mechanism underlying the progression of NAFLD from NAFL to NASH has not been well understood. It is believed that abnormal mitochondrial activity, apoptosis, ROS production, lipotoxicity, and inflammation are involved in the progression of NAFLD. p53 is well known for its role in inducing apoptosis and inhibiting tumorigenesis. Mice deficient in p53 are resistant to diet-induced steatohepatitis,(19) highlighting a critical role of apoptosis in the progression of NAFLD. Our data show that HNF4α inhibits hepatic p53 expression and that overexpression of human HNF4α attenuates diet-induced NASH in the control mice but not in mice lacking hepatocyte p53. Importantly, partial recapitulation of hepatic p53 protein expression is sufficient to restore hepatic apoptosis level and prevent HNF4α-mediated amelioration of steatohepatitis. Interestingly, p53 also plays a role in HNF4α-mediated inhibition of proinflammatory and fibrogenic gene expression, the TGFβ/Smad pathway, and caspase 3 cleavage. The TGFβ/Smad pathway is known to promote liver fibrosis.(34, 35) These data highlight an important role of p53 in the progression of NAFL to NASH. In addition to inhibition of p53 expression, HNF4α also reduces hepatic levels of FC and palmitate, which have been shown to cause lipotoxicity and apoptosis.(7, 36-39) The reduction in hepatic FC and palmitate levels may result from increased conversion of FC to BAs and increased FAO, respectively. Thus, a reduction in hepatic FC and palmitate levels may also play a role in HNF4α-mediated protection against the development of steatohepatitis (Fig. 8F).
Studies have shown that activation of FXR inhibits diet-induced p53 signaling and NASH.(23) Loss of hepatic HNF4α inhibits both CYP7A1 and CYP8B1 expression, therefore suppressing BA synthesis and FXR signaling. Interestingly, normalization of hepatic CYP7A1 and CYP8B1 expression in mice lacking Hnf4α completely normalizes hepatic TG levels and also partially attenuates diet-induced p53 expression and steatohepatitis. The reduced hepatic TG levels may partly result from normalized FXR signaling, as activation of FXR is known to lower hepatic TG levels.(40) Activation of FXR is also shown to inhibit inflammation.(40) Importantly, treatment of mice lacking Hnf4α with a synthetic FXR agonist mimics the effects of CYP7A1 and CYP8B1 normalization. Thus, the impaired BA/FXR signaling plays a role in the pathogenesis of NASH in Hnf4αHep−/− mice (Fig. 8F). Although both HNF4α and FXR inhibit p53, they also inhibit inflammation and regulate many other pathways. As a result, HNF4α overexpression or FXR activation is not expected to cause tumorigenesis.
In summary, our studies have demonstrated that HNF4α is a key regulator of the development and progression of NASH. HNF4α regulates hepatic TG accumulation by regulating VLDL secretion and lipolysis (Fig. 8F). On the other hand, HNF4α also regulates apoptosis, BA synthesis, and FAO. HNF4α inhibits apoptosis by suppressing p53 expression. HNF4α promotes BA production by inducing CYP7A1 and CYP8B1 expression, leading to reduced hepatic FC levels and activation of FXR. Activation of FXR stimulates FAO and reduces hepatic palmitate levels. The reduced FC and palmitate levels can reduce lipotoxicity. Thus, HNF4α inhibits the progression of NAFL to NASH likely by suppressing apoptosis, inflammation, and ROS production (Fig. 8F). Because hepatic HNF4α expression is markedly reduced in patients with NASH, targeting hepatic HNF4α may represent a promising approach for treatment of NASH.
Author Contributions
Y.Y.X. and Y.Z. designed the studies. Y.Y.X., Y.D.Z., S.H., Y.X., D.S., X.P., F.C.B., S.C., R.G., and L.Y. performed the studies. Y.Y.X. and Y.Z. analyzed the data and wrote the manuscript. All the authors approved the final version of manuscript.




