A microRNA-7/growth arrest specific 6/TYRO3 axis regulates the growth and invasiveness of sorafenib-resistant cells in human hepatocellular carcinoma
Potential conflict of interest: Nothing to report.
Supported by grants from the Medical Research Commercialisation Fund, the National Health and Medical Research Council, and the Cancer Council of Western Australia. Grant No. 1084964 to PJL, 2015-2017.
Abstract
Sorafenib remains the only approved drug for treating patients with advanced hepatocellular carcinoma (HCC). However, the therapeutic effect of sorafenib is transient, and patients invariably develop sorafenib resistance (SR). Recently, TYRO3, a member of the TYRO3-AXL-MER family of receptor tyrosine kinases, was identified as being aberrantly expressed in a significant proportion of HCC; however, its role in SR is unknown. In this study, we generated two functionally distinct sorafenib-resistant human Huh-7 HCC cell lines in order to identify new mechanisms to abrogate acquired SR as well as new potential therapeutic targets in HCC. Initially, we investigated the effects of a microRNA (miR), miR-7-5p (miR-7), in both in vitro and in vivo preclinical models of human HCC and identified miR-7 as a potent tumor suppressor of human HCC. We identified TYRO3 as a new functional target of miR-7, which regulates proliferation, migration, and invasion of Huh-7 cells through the phosphoinositide 3-kinase/protein kinase B pathway and is markedly elevated with acquisition of SR. Furthermore, miR-7 effectively silenced TYRO3 expression in both sorafenib-sensitive and sorafenib-resistant Huh-7 cells, inhibiting TYRO3/growth arrest specific 6-mediated cancer cell migration and invasion. Conclusion: We identified a mechanism for acquiring SR in HCC that is through the aberrant expression of the TYRO3/phosphoinositide 3-kinase/protein kinase B signal transduction pathway, and that can be overcome by miR-7 overexpression. Taken together, these data suggest a potential role for miR-7 as an RNA-based therapeutic to treat refractory and drug-resistant HCC. (Hepatology 2018;67:216-231)
-
- Abbreviations: AFP
-
- alpha-fetoprotein
-
- AKT
-
- protein kinase B
-
- ANOVA
-
- analysis of variance
-
- CXCL
-
- chemokine (C-X-C motif) ligand
-
- EGF
-
- epidermal growth factor
-
- EGFR
-
- epidermal growth factor receptor
-
- ERK
-
- extracellular signal regulated kinases
-
- FGFR4
-
- fibroblast growth factor receptor 4
-
- GAS6
-
- growth arrest specific 6
-
- HCC
-
- hepatocellular carcinoma
-
- IL
-
- interleukin
-
- miR-7
-
- miR-7-5p
-
- miRNA
-
- microRNA
-
- mRNA
-
- messenger RNA
-
- NC
-
- negative control
-
- NFκB
-
- nuclear factor kappa B
-
- PI3-Kinase
-
- phosphoinositide 3-kinase
-
- qRT-PCR
-
- quantitative reverse-transcription polymerase chain reaction
-
- RelA
-
- transcription factor p65
-
- RTK
-
- receptor tyrosine kinase
-
- siRNA
-
- short interfering RNA
-
- SR
-
- sorafenib resistance
-
- TAM
-
- TYRO3/AXL/MER tyrosine kinase receptor
-
- TCGA
-
- The Cancer Genome Atlas
-
- UTR
-
- untranslated region
Liver cancer ranks sixth among all malignancies in the world and is the second leading cause of cancer-related mortality.1 The rising incidence of hepatocellular carcinoma (HCC), the most common primary malignant neoplasm of the liver, has been linked to hepatitis B or C infection and increasing worldwide obesity associated with fatty liver disease.2 In 2017, it is estimated that 40,710 patients will be diagnosed with HCC in the United States, and more than 70% of them will eventually die from this disease.2 Less than 30% of patients with HCC can be managed by curative therapy, and sorafenib, a multikinase inhibitor, is the only available drug for managing patients with advanced tumors.3 The clinical benefit of sorafenib is modest, adverse effects are common, and almost all patients become refractory within a few months, which is associated with a very poor prognosis.4 Recently, regorafinib, another multikinase inhibitor, was approved as a second-line therapy by the U.S. Food and Drug Administration for patients with HCC resistant to sorafenib.5 Although regorafinib can improve overall survival modestly (an increase in median overall survival from 7.8 months to 10.6 months), it is associated with a range of side effects, and some severe drug-associated toxicity has been reported.4 Based on this evidence, there is an urgent need to better understand the molecular mechanisms underlying the development of sorafenib resistance (SR) and to identify novel therapeutic targets to improve clinical outcome.
Recently, TYRO3, a member of the TYRO3-AXL-MER (TAM) family of transmembrane tyrosine kinase receptors was found to be aberrantly expressed in HCC.6 Of 55 HCCs, 42% had elevated expression of TYRO3 (>2-fold) compared to adjacent normal tissue, and higher levels of TYRO3 were associated with higher levels of serum alpha-fetoprotein (AFP) and an increased tumor diameter. These data suggested that inhibition of TYRO3 and its signaling pathways in tumors with high expression could be of therapeutic benefit in HCC. The role of TYRO3 in SR is unknown.
Oligonucleotide therapeutics is an emerging field being driven by advances in technology resulting in new RNA-based drug therapies as an alternative to conventional chemical inhibitors or antibodies (e.g., RNA interference to proprotein convertase subtilisin/kexin type 9 to treat hypercholesterolemia).7 This technology includes the short interfering RNAs (siRNAs), anti-sense oligonucleotides, and microRNAs (miRNAs).8 Our interest in the RNA-based drug development field has been with miRNAs, which are a family of endogenously transcribed ∼22 nucleotide noncoding RNAs that posttranscriptionally regulate gene expression by inducing either translational repression or message decay.9 Abnormal expression of several miRNAs has been observed in HCC.10-13 Here, we focused on a tumor suppressor miRNA, miR-7-5p (miR-7), which is down-regulated in several human cancers, including HCC, and has been shown to regulate a number of oncogenic signal transduction pathways, such as the epidermal growth factor receptor (EGFR) signaling pathway, as well as the phosphoinositide 3-kinase/protein kinase B (PI3-Kinase/AKT) and RAF-MEK- extracellular signal-regulated kinase (ERK) pathways.13-17 We aimed to explore the role of miR-7 in HCC and in sorafenib-resistant HCC and to identify new targets that could be useful clinically. Although the EGFR is frequently overexpressed in HCC and therefore an excellent target, to date anti-EGFR treatments have not been found to benefit patients. Given that miRNAs target multiple messenger RNAs (mRNAs) in multiple pathways, we hypothesized that miR-7 would simultaneously target the EGFR and several other pathways in HCC, resulting in enhanced inhibition of tumor growth.
We demonstrate that miR-7 is a potent inhibitor of HCC cell growth in vitro and in vivo and that TYRO3 is a direct miR-7 gene target. Furthermore, TYRO3 was found to be an important mediator of SR in HCC cells. miR-7 significantly reduced TYRO3 expression in parental and sorafenib-resistant HCC cells. Thus, miR-7 could potentially be exploited as a novel RNA-based therapeutic for HCC, with specific emphasis on patients with sorafenib-resistant tumors.
Materials and Methods
CELL CULTURE
The Huh-7 and Hep3B cell lines used in this study were kindly provided by Associate Professor Nicholas Shackel, Centenary Institute, Sydney, Australia. Two sorafenib-resistant sublines were established, namely Huh-7/SR1 and Huh-7/SR2, by culturing the cells in increasing doses of sorafenib. The origin and culture conditions of these cells are detailed in the Supporting Materials and Methods.
REAGENTS
A full list of reagents is included in the Supporting Materials and Methods.
CELL VIABILITY, PROLIFERATION, AND COLONY FORMATION ASSAY
To determine the sensitivity of HCC cell lines to sorafenib, miR-7, and TYRO3, cells were treated with increasing concentrations of drug, miRNA, and siRNA, or after transfection with a single concentration of miRNA/siRNA, and the effect on cell viability was measured over time, as described.18 Two-dimensional and three-dimensional colony formation assays were performed, as described.15 Further details are outlined in the Supporting Materials and Methods.
MIGRATION AND INVASION ASSAYS
Migration and invasion were assessed using transwell assays with polycarbonate membrane inserts with 8.0 μm pore size (Costar) coated with or without Matrigel (Corning), as described in Supporting Materials and Methods.
RNA EXTRACTION, QUANTITATIVE REVERSE-TRANSCRIPTION POLYMERASE CHAIN REACTION, AND miRNA TARGET VALIDATION (LUCIFERASE REPORTER ASSAYS)
Full methods are described in Supporting Materials and Methods.
WESTERN BLOT ANALYSIS
Immunoblotting was performed using antibodies detailed in the Supporting Materials and Methods. Densitometry was performed using ImageJ version 1.51h software (http://rsb.info.nih.gov/ij/).
PHOSPHO-RECEPTOR TYROSINE KINASE ARRAY
Phospho-receptor tyrosine kinase (Phospho-RTK) array (R&D Systems) was performed according to the manufacturer's instructions. Lysates were prepared from Huh-7 cells transfected with 30 nM miRNA precursor molecules for 48 hours, and 500 μg protein was used for each array. Densitometry was performed using ImageJ version 1.48 software (http://rsb.info.nih.gov/ij/).
ORTHOTOPIC LIVER CANCER MODEL
For details on methodology, please see Supporting Materials and Methods.
PUBLIC ARRAY DATA
To investigate the clinical significance of TYRO3 expression in human HCC, a Kaplan-Meier survival curve was plotted using data sets from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/, TCGA-LIHC, RNASeqV2 (data type), Illumina HiSeq 2000 (platform), downloaded and accessed October 2015) in the R survival package. For information about patient samples and expression analysis, refer to Supporting Materials and Methods.
STATISTICAL ANALYSIS
All results are represented as mean ± SEM except for quantitative reverse-transcription polymerase chain reaction (qRT-PCR), which is presented as mean ± SEM of ΔΔCt. Graph Pad Prism (version 7.0, GraphPad Software) was used to calculate statistical significance between test (e.g., miR-7-5p/siTYRO3) and control (e.g., miR-negative control [NC]/NC siRNA) treated groups using the Student t test (one-tailed, unpaired), one-way analysis of variance (ANOVA), two-way ANOVA, ANOVA with repeated measures, and Mann-Whitney U test as appropriate. P < 0.05 was considered statistically significant wherein *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Results
miR-7 IS A POTENT SUPPRESSOR OF HCC GROWTH IN VITRO AND IN VIVO
The constitutive activation of EGFR in HCC leads to persistent downstream signaling through the PI3-Kinase and AKT, p21-activated kinases, and Raf/MEK/ERK pathways, transducing growth signals to tumor cells and promoting tumor progression and dissemination.19 As miR-7 is down-regulated in HCC,13, 20 its replacement should reduce EGFR signaling together with other pathways containing miR-7 targets. We initially evaluated the effects of miR-7 overexpression in vitro using a human HCC-derived cell line Huh-7 and found that miR-7 produced a significant antiproliferative (P = 0.0435), antimigratory (P < 0.0001), and anti-invasive (P = 0.0024) phenotype in Huh-7 cells and reduced their clonogenic capacity (P < 0.01) (Fig. 1A, panel i-iv; Supporting Fig. S1A). miR-7 was cytotoxic at very low concentrations to Huh-7 (50% effective concentration [EC50] = 1.514 nM), in a well-differentiated human hepatocellular carcinoma derived cell line; HEPG2 (EC50 = 2.115 nM) and human hepatoma derived cell line; and PLC/PRF/5 (Alexander cell) (EC50 = 0.4812 nM) cells (Fig. 1B; Supporting Fig. S1B, panel i and ii). The growth inhibitory effect of miR-7 on HCC cell viability was unrelated to the level of endogenous miR-7, which varies across the three cell lines (Supporting Fig. S1B, panel iii). miR-7 induced a G0/G1 cell-cycle arrest associated with apoptosis, as evidenced by an increase in caspase3/7 activity and cleaved poly(adenosine diphosphate ribose) polymerase protein in a western blot (Supporting Fig. S1C).

miR-7 is a potent tumor suppressor of HCC viability in vitro and in vivo. (A) The antitumorigenic effect of miR-7 on human HCC-derived cell line Huh-7 was initially investigated in an in vitro system using (i) an MTS cell titer assay for proliferation, (ii) transwell assays without/with matrigel coating to assess cell migration and (iii) invasion, and (iv) clonogenic assay to assess their tumorigenic potentials. (B) Evaluation of the EC50 of miR-7 by MTS assay. (C) The study design demonstrating the time frame at which either miR-7 or the negative control was administered intravenously to an orthotopic model of HCC (n = 8/ group). (D) Measurement of serum AFP at day 4, 7, 10, and 14 posttreatment. (E) Representative livers of treated mice showing tumor burden and (F) weights of the liver tumors. All in vitro assays were carried out on 3 independent days with technical repeats. *P < 0.05, **P < 0.01, and ****<0.0001, by one-way repeated measure ANOVA (A) and Mann-Whitney U test (B,C). ****P < 0.0001 for all in vivo experiments by one-way repeated measure ANOVA. Abbreviations: i.v., intravenous; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
We next explored the effects of miR-7 in an orthotopic model of human HCC, using a NOV340-miR-7 mimic, as described in the Materials and Methods. The NOV340-miR-7 mimic had a similar effect on Huh-7 cell viability as the Ambion miR-7 mimic used for the in vitro work (Supporting Fig. S1D). The initial in vivo investigation found that miR-7 expression was enriched in both murine liver and human HCC cell line-derived tumor tissues following tail vein injection of the NOV340-miR-7 formulation (Supporting Fig. S1E). We then conducted a short miR-7 replacement therapy trial in which nonobese diabetic SCID IL2rγ null [NOD.Cg-PrKdcSCIDil2rgtm1wjl/SzJ] or NSG immunodeficient mice received Huh-7 cells orthotopically; once the cells reached a critical mass (equivalent to AFP secretion of 1,000 ng/mL), they commenced a 2-week alternate day intravenous treatment regimen of miR-7 or control (Fig. 1C). Analysis of the mice at 14 days showed that the tumor burden in the mice receiving miR-7 was dramatically reduced, accompanied by markedly lower AFP levels (almost 100-fold lower) (Fig. 1D). This was associated with a major reduction in tumor size and weight (Fig. 1F,G; Supporting Fig. S1F, panel i). Remarkably, in the miR-7 recipient group (n = 8), no visible tumors were observed macroscopically in six of the mice, explaining why there are only two tumor weights plotted for miR-7 in Fig. 1F (Supporting Fig. S1E,F panel ii). Taken together, these data indicate that miR-7 is a potent inhibitor of human HCC cell proliferation, migration, and invasion in vitro and tumor growth in vivo.
THE TAM FAMILY MEMBER TYRO3 IS ELEVATED IN HCC AND IS A NOVEL miR-7 TARGET GENE
Given the magnitude of effect induced by miR-7 in HCC, we set out to identify new targets of the miRNA within the RTK family, using an unbiased phospho-RTK antibody array. Densitometry analysis of the 42-member RTK array showed that miR-7 reduced the amount of phosphorylated EGFR and insulin-like growth factor 1 receptor, consistent with each being a validated target of miR-7 (Fig. 2A, #2 and #5, respectively). In addition, miR-7 reduced the levels of phosphorylated fibroblast growth factor receptor 4 (FGFR4) and TYRO3 (Fig. 2A, #3 and #6, respectively), and Target Scan analysis revealed FGFR4 and TYRO3 contain putative miR-7 seed sequences in their 3' untranslated region (UTR) (Supporting Fig. S2A) that are well conserved across primates. FGFR4 has been implicated in HCC and is a potential therapeutic target.21 Small molecule inhibitors have been developed against FGFR4 (e.g., BLU9931), and much is known about its expression in HCC.22 In contrast, interest in TYRO3 in HCC is just emerging, and given its association with resistance to both chemotherapy and targeted therapies in chronic myeloid leukemia and ovarian cancer,23, 24 we speculated that aberrant TYRO3 expression might be an important determinant of SR in HCC. To date, there are no specific small molecule TYRO3 inhibitors that have been developed as they either target all of the TAM family members or Axl alone. Thus, miR-7, by directly targeting TYRO3, could represent an alternative approach to specifically inhibit TYRO3 expression in HCC.

TYRO3 is elevated in HCC and is a novel miR-7 target gene. (A) Phospho-RTK array of miR-7 versus miR-NC-treated Huh-7 cells. Top panel shows the differential phosphorylation of a number of receptor tyrosine kinases, and the bottom panel corresponds to the densitometry calculations: 1, Control; 2, P-EGFR; 3, P-FGFR4; 4, P-Insulin R; 5, P-IGF1R; 6, P-TYRO3 (p-Dtk). (B) Effect of miR-7 overexpression on (i) TYRO3 mRNA and (ii) protein, respectively, in Huh7 cells. (C) Luciferase reporter assay using the 3‘-UTR of TYRO3 as a target of miR-7. Upper panel (i) shows the sequence of the seed site mutated within the TYRO3 3’-UTR, bottom panel (ii) shows luciferase assay. (D,E) TYRO3 expression in HCC versus normal surrounding tissue and associated Kaplan-Meier survival curves in patients with HCC from the TCGA cohort (n = 368). ***P < 0.001 by paired Student t test, from three independent experiments with technical repeats. Abbreviation: IGF1R, insulin-like growth factor 1 receptor.
When miR-7 was overexpressed in Huh-7 or HEP3B cells, we observed TYRO3 mRNA and protein expression were significantly reduced (Fig. 2B; Supporting Fig. S2B). Furthermore, we identified TYRO3 as a direct functional gene target of miR-7 by using a luciferase reporter assay with constructs containing either the wild-type or mutant TYRO3 3'-UTR (Fig. 2C). Transient overexpression of miR-7 significantly reduced luciferase activity of the wild-type TYRO3 but not the mutant compared to the miR-NC (Fig. 2C, panel ii). Additional clinical validation of the importance of TYRO3 expression in HCC was achieved within a cohort of 368 patients from the TCGA; this showed that TYRO3 expression is significantly increased in tumor compared to surrounding normal tissue (Fig. 2D). Further, patients with HCC with higher expression of TYRO3 had a significantly worse prognosis and reduced survival (Fig. 2E).
TYRO3 REGULATES PROLIFERATION, MIGRATION, AND INVASION OF HCC CELLS THROUGH THE PI3-KINASE/AKT PATHWAY
To evaluate the effects of regulating TYRO3 expression in HCC (Huh-7 and HEP3B cells), we used two commercially available TYRO3 siRNAs (named #5 and #6), each targeting different regions at the 3′-end of TYRO3 mRNA. This region is distinctive among all three transcript variants ensuing from alternative 5′-end splicing, which can regulate subsequent biological function.25 We found a functional difference between the two siRNAs in migration assays whereby TYRO3 #6 siRNA did not alter migration in HEP3B cells (Supporting Fig. S3). However, using both the xCELLigence and transwell migration assay, TYRO3 siRNA #6 was found to be more potent than TYRO3 siRNA #5 in Huh-7 cells as it decreased both migration (P < 0.0001) and invasion (P = 0.0043) (Fig. 3B,C; Supporting Fig. S3). TYRO3 function has been previously examined in HEP3B but not Huh-7 cells.6 Thus, we elected to investigate TYRO3 function in Huh-7 cells for all the functional assays in this study. Transient knockdown of TYRO3 in Huh-7 cells significantly reduced their rate of proliferation (P < 0.05) (Fig. 3A). To identify TYRO3-induced signaling pathways involved in oncogenic processes in HCC, the siRNA-mediated TYRO3-depleted Huh-7 cells were analyzed for various downstream signaling molecules. For the PI3-Kinase/AKT pathway, the amount of phosphorylated AKT (serine 473) is considerably reduced in TYRO3-deficient Huh-7 cells, whereas phosphatase and tensin homolog (a negative regulator of the pathway19) remained intact (Fig. 3D). In contrast, for the nuclear factor kappa B and RAF/MEK/ERK pathways, phosphorylated transcription factor p65 (RelA; serine 536) and phosphorylated ERK1/2 (threonine 202/204) are increased on TYRO3 depletion (Fig. 3D, ERK1/2 increased with siTYRO3 #6 only). These results suggest that the canonical PI3-Kinase/AKT pathway transduces the protumorigenic signaling of TYRO3 in HCC. Furthermore, in cells in which TYRO3 expression is reduced, alternative pathways may become active, such as RelA/nuclear factor kappa B signaling. Of note, we have previously reported that RelA is a direct target of miR-7.15
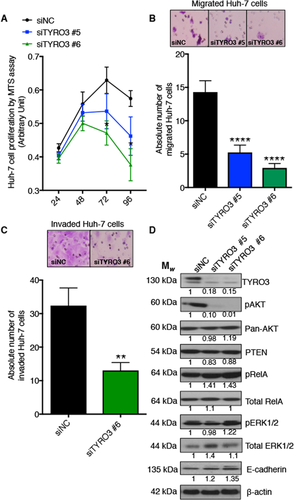
TYRO3 regulates proliferation, migration, and invasion of HCC cells through the PI3-Kinase/AKT pathway. Huh-7 cells were treated with siRNA to TYRO3, and the effects of subsequent TYRO3 depletion were evaluated. (A) Cell proliferation assay; (B) migration assay in matrigel; (C) two-dimensional invasion assay. (D) Immunoblot analysis of PI3-Kinase/AKT, NFκB, and MAPK/ERK signaling pathways. Western blots quantified by relative band densitometry using Image J (version 1.51h) by normalizing the bands to β-actin and then to the negative control. All experiments were performed on 3 independent days with technical repeats. Error bars represent mean ± SEM and *P < 0.05, **P < 0.01, ****P < 0.001 by one-way repeated measure ANOVA (A) and Mann-Whitney U test (B,C). Abbreviations: MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NFκB, nuclear factor kappa B; PTEN, phosphatase and tensin homolog.
ENDOGENOUS miR-7 REGULATES THE TYRO3-MEDIATED METASTATIC PHENOTYPE IN HCC
To comprehend the biological significance underlying the miR-7/TYRO3 axis in Huh-7 cells, “rescue of function” experiments were conducted whereby the endogenous miR-7 levels were depleted using anti-miR-7 for 48 hours followed by sequential depletion of TYRO3 for a further 24 hours (Supporting Fig. S4A). We hypothesized that if TYRO3 is a physiological direct target of miR-7, elimination of endogenous miR-7 would induce a phenotype characteristic of TYRO3 overexpression. Subsequent introduction of TYRO3 siRNAs should antagonize this gain of function. We found by using anti-miR-7, depletion of endogenous miR-7 did not affect Huh-7 cell proliferation (Fig. 4A; Supporting Fig. S4B) but significantly stimulated their migration and invasion (Fig. 4C,D). When the cells lacking endogenous miR-7 were exposed to TYRO3 siRNA, we observed anti-miR-7 rescued the level of TYRO3 protein, consistent with anti-miR-7 nullifying the effect of any endogenous miR-7 (Supporting Fig. S4C). Subsequent depletion of TYRO3 in these cells did not alter Huh-7 cell viability (Fig. 4B) but significantly antagonized the extent of migration and invasion provoked by anti-miR-7 (Fig. 4C,D). We further observed that the TYRO3-dependent prometastatic effect in HCC is partly mediated by altered expression of E-cadherin (Supporting Fig. S5; Fig. 3D). Taken together, these data provide additional evidence for an important inverse regulation relationship between miR-7 and TYRO3 in HCC.

Knockdown of endogenous miR-7 in Huh-7 cells induced a promigratory and pro-invasive phenotype that was rescued by sequential depletion of TYRO3. Huh-7 cells were treated with anti-miR-7 or anti-miR controls for 48 hours and then with TYRO3 siRNA for 24 hours, and effects of these treatments assessed in vitro. (A,B) Cell proliferation assay, (C) migration assay, and (D) invasion assay. (E,F) qRT-PCR for chemokines: (i) CXCL10 and (ii) IL-8 expression. All experiments were performed on 3 independent days with technical repeats. The error bars represent mean ± SEM of ΔCT. **P < 0.01, and ****P < 0.0001 by paired Student t test. (A-D)All experiments were performed on 3 independent days with technical repeats. Error bars represent mean ± SEM, and *P < 0.05 and ****P < 0.0001 by two-way ANOVA with post-hoc correction by the Tukey method. Abbreviation: MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
miR-7 REVERSES THE REBOUND SURGE OF PROINFLAMMATORY CHEMOKINES UPON TYRO3 DEPLETION IN Huh-7 CELLS
Loss of TAM receptors in HCC has been associated with altered expression of some key proinflammatory cytokines and chemokines,26 which could contribute to increased growth. Thus, we investigated the regulation of expression of chemokine (C-X-C motif) ligand (CXCL)10 and interleukin (IL)-8/CXCL8, two well-characterized HCC-associated chemokines with putative miR-7 binding sites. We found with qRT-PCR that TYRO3 depletion significantly increased expression of CXCL10 (rho = 0.943; P = 0.017) and IL-8 (rho = 0.886; P = 0.033), suggestive of a rebound surge of proinflammatory chemokines (Fig. 4E). Furthermore, overexpression of miR-7 significantly decreased the expression of IL-8 (P = 0.025) and CXCL10 (P = 0.0034) in Huh-7 cells (Fig. 4F). Based on these findings, we concluded that miR-7 not only fine tunes TYRO3 expression in Huh-7 cells but also down-regulates key proinflammatory chemokines. This effect of miR-7, combined with the other data above, serves to illustrate how broad its effects are and also exemplifies its potency as an inhibitor of HCC cell viability.
SORAFENIB RESISTANCE IN HCC IS OVERCOME BY miR-7 SUPPRESSION OF TYRO3 FUNCTION
To understand the mechanisms driving acquired resistance to sorafenib in HCC, two sorafenib-resistant cell lines Huh-7/SR1 (EC50 = 42.92 μM) and Huh-7/SR2 (EC50 = 75.94 μM) were established (Fig. 5A). miR-7 expression was significantly reduced in both sorafenib-resistant cell lines (Fig. 5B, panel i), whereas TYRO3 expression was markedly increased in the more resistant Huh-7/SR2 cell line (Fig. 5B, panel ii). We found that the EGFR pathway becomes less active during acquiring SR (Fig. 5C). The expression of TYRO3 protein is elevated in the Huh-7/SR2 cell line consistent with its mRNA profile and is accompanied by higher levels of phospho-RelA(Fig. 5C). Phospho-AKT and phospho-ERK1/2 were differentially expressed between the two sorafenib-resistant lines (Fig. 5C). In addition, we found increased TYRO3 expression in HEP3B cells, and these cells were less sensitive to sorafenib than the parental Huh-7 cells, with an EC50 of ∼14 μM (Supporting Fig. S6). Further characterization showed the two resistant cell lines were functionally very distinct from their precursors. Compared to the parental cells, the Huh-7/SR1 cells were more proliferative whereas the Huh-7/SR2 cell lines were less proliferative in nature (Fig. 5D, panel i). Both sorafenib-resistant lines were more migratory and invasive than the parental cell line (Fig. 5D, panel ii and iii). Aberrant activation of TYRO3 is reported to stimulate epithelial–mesenchymal transition in colorectal cancer and regulate tumor dissemination.27 We observed that Huh-7/SR2 cells lost their epithelial polarity by expressing more mesenchymal cell markers (α-smooth muscle actin and vimentin) and less epithelial E-cadherin compared to their parental counterparts (Fig. 5E, panel i and ii). The sorafenib-resistant cells also transcribed more TWIST1 (Fig. 5E, panel iii). Furthermore, depleting TYRO3 levels in both the parental and Huh-7/SR2 cells reduced their viability in a dose-dependent manner, and the EC50 of siTYRO3 in the Huh-7/SR2 cells was much less than that of the parental cells (0.009 nM versus 2.516 nM) (Fig. 5F, panel i and ii). Finally, by using concentrations near the EC50 of siTYRO3, we found that combination therapy of siTYRO3 with sorafenib (5.5 μM, ∼highest achievable dose in plasma) significantly increased the cytotoxic effect of sorafenib in the parental Huh-7 cell line (Fig. 5F, panel iii), although this effect was not synergistic (using the Bliss Additivity model28).
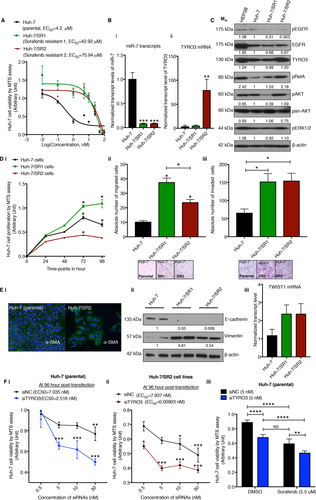
Sorafenib resistance in HCC is overcome through suppression of TYRO3 function. (A) EC50 calculations of sorafenib treatment assessed by cell titer assay in Huh-7 parental and sorafenib-resistant cell lines. (B) qRT-PCR for (i) miR-7 and (ii) TYRO3 mRNA in parental and sorafenib-resistant cell lines. (C) Immunoblot analysis of phospho-EGFR, EGFR, TYRO3, and their downstream signaling pathways pAKT, pERK, and pRelA in Huh-7 parental and sorafenib-resistant cells. Western blots quantified by relative band densitometry by normalizing the bands to β-actin and then to the parental control (using Image J version 1.51h). (D) Phenotypic evaluation of two sorafenib-resistant cell lines by (i) cell proliferation, (ii) transwell migration, and (iii) invasion assays. (E) (i) Evaluation of the EMT marker α-smooth muscle actin by immunofluorescence immunocytochemistry. (ii) Immunoblot for markers of EMT (E-cadherin and vimentin) in the parental and sorafenib-resistant cell lines. (iii) qRT-PCR for Twist mRNA from each of the same lines. (F) Cell proliferation assay following titration of TYRO3 siRNA in (i) parental cells and (ii) Huh-7/SR2 cells. (iii) Cell proliferation assay of combining 5 nM siTYRO3 and 5.5 µM sorafenib in Huh-7 parental cells. All experiments were performed on 3 independent days with technical repeats. Error bars represent mean ± SEM. *P < 0.05, ***P < 0.001, and ****P < 0.0001 by one-way repeated measure ANOVA (D), one-way ANOVA with post-hoc corrections by Dunnett's method (B, Dii and iii), and two-way ANOVA with post-hoc correction by Tukey method (E,F). Abbreviations: α-SMA, α-smooth muscle actin; DMSO, dimethyl sulfoxide; EMT, epithelial–mesenchymal transition; MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; pEGFR, phospho-EGFR.
We next investigated the effects of miR-7 on the sorafenib-resistant cells. We observed that similar to siTYRO3, miR-7 significantly reduced viability of both Huh-7/SR1 and Huh-7/SR2 cells in a dose-dependent fashion and markedly attenuated their migration and invasion (Fig. 6A,B). miR-7 reinforced E-cadherin expression in the Huh-7/SR2 cell line and induced a global reduction of key survival molecules (TYRO3, phospho-AKT, panAKT, phospho-ERK, and total RelA), previously shown to be up-regulated in these cells (Fig. 6C, and compare to Fig. 5C). We next stimulated the Huh-7/SR2 cells with the ligand to TYRO3, namely growth arrest specific 6 (GAS6), in the presence and absence of miR-7. We observed that in the absence of miR-7, GAS6 significantly stimulated the migration and invasion of the Huh-7/SR2 cell lines, and this effect was dramatically reduced in the presence of miR-7 (Fig. 6D). Further, in the presence of miR-7, the GAS6-induced increase in migration and invasion was abolished, consistent with miR-7 effectively inhibiting the GAS6-TYRO3 pathway.29 Finally, when we treated sorafenib-resistant Huh-7 cells with a constant dose of miR-7 and increasing doses of sorafenib, the EC50 curve shifted significantly downwards (Fig. 6E,F). The Bliss Additivity model28 demonstrated synergy between miR-7 (at 5 nM) and sorafenib in both sorafenib-resistant cell lines (Supporting Table S1 and S2; 0.1-75 μM and 25 μM sorafenib in SR2 and SR1, respectively).
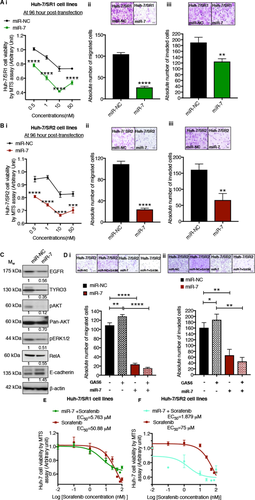
miR-7 abolished the TYRO3-dependent increase in invasiveness in sorafenib-resistant Huh-7 cells. The tumor-suppressive function of miR-7 on the two sorafenib-resistant Huh-7 cell lines (A) SR1 and (B) SR2 was investigated by (i) MTS cell titer assay for cytotoxicity and (ii) by transwell assays to determine migration and (iii) invasive capacity. (C) Western blot analysis of the effect of miR-7 transfected into Huh-7/SR2 cells. (D) (i) Migration and (ii) invasion assay of Huh-7/SR2 cells following stimulation of TYRO3 by its ligand GAS6. (E,F) Cell viability assays of the combination of miR-7 with sorafenib in Huh-7/SR1 and Huh-7/SR2 cells. After transfecting miR-7 into the cells (5 nM), they were treated with increasing doses of sorafenib (0.01 to 100 μM). EC50 counts were determined by MTS cell viability assay after 48 hours of treatment with sorafenib. All experiments were performed on 3 independent days with technical repeats. Error bars represent ± SD, and *P < 0.05, **P < 0.01, and ****P < 0.0001 by Mann-Whitney U test (Aii and iii; Bii and iii) and two-way ANOVA (Ai,Bi,D) with post-hoc correction by the Tukey test. Abbreviation: MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
Discussion
HCC-targeted therapies, such as sorafenib, which are directed against multiple growth factor receptors or downstream signaling kinases, do offer some therapeutic benefit, although the acquisition of therapeutic resistance is a major clinical problem. Here, we show that miR-7 is a potent inhibitor of HCC growth in vitro and in an orthotopic liver model of HCC in vivo. A significant component of that action is by direct inhibition of TYRO3 expression and its downstream signaling network, driven predominantly by the canonical PI3-Kinase/AKT pathway. Unexpectedly, TYRO3 expression is also amplified in sorafenib-resistant HCC cells and was associated with their mesenchymal phenotype. We therefore propose that combining miR-7 with TYRO3 inhibition will afford greater inhibition of HCC cell growth and represent a strategy to overcome SR (see Fig. 7).
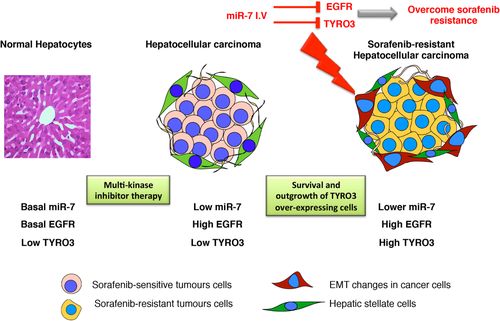
A proposed model for how miR-7 rescues sorafenib resistance in HCC. Neoplastic transformation reduces endogenous levels of miR-7 in hepatocytes with subsequent elevated levels of its target genes. EGFR is the most well-characterized gene target of miR-7 that is frequently elevated in HCC and with acquisition of SR. Aberrant activation of TYRO3 signaling is a novel mechanism of acquiring SR in Huh-7 cells and is a new target of miR-7. Administration of miR-7 inhibits cancer cell proliferation in an orthotopic model of human HCC and overcomes SR in vitro by targeting both the EGFR and TYRO3 signaling pathways.
The interaction of miR-7 with members of the EGF/EGFR pathway in a number of solid tumors, such as glioblastoma,16 melanoma,15 and HCC,13 and its ability to sensitize cancer cells to radiotherapy30 and targeted therapies (erlotinib)18 demonstrate its potential as a therapeutic strategy. In this study, we report another novel target of miR-7 belonging to the TAM family of RTKs, TYRO3, and identified it as a key driver of the oncogenic PI3-Kinase/AKT pathway in HCC. There is increasing emphasis on developing TAM receptor inhibitors for the treatment of cancer, and Axl inhibitors have recently entered phase I clinical trial for treatment of several different solid tumors.31 The early data suggest that these small molecule Axl inhibitors show some therapeutic efficacy toward HCC, especially as Axl is reported elevated in preclinical models of human HCC and in patient samples.32, 33
Our TCGA HCC data demonstrated that TYRO3 is aberrantly overexpressed in HCC and associated with a poor prognosis. Our results also suggest that TYRO3 may play an important pro-proliferative and pro-invasive role in parental as well as sorafenib-resistant cells. Significantly, inhibition of TYRO3 not only reduced proliferation, migration, and invasion of sorafenib-sensitive HCC cell lines through massive inhibition of the phospho-AKT-pathway but also significantly inhibited growth of sorafenib-resistant cells in a dose-dependent fashion. Hitherto, we did not observe any synergism between simultaneous inhibition of TYRO3 and sorafenib therapy in the resistant cell lines, potentially because of a subtle paradoxical increase in phospho-ERK levels following loss of TYRO3, which has been implicated in conferring SR.34
TYRO3 is reported to modulate several oncogenic pathways, including survival, altered cellular morphology, cell-cycle transition, proliferation, adhesion, motility, and release of a number of inflammatory cytokines and is observed elevated in 42% of HCC cases.6, 35, 36 It is also known to induce neoplastic transformation in nonmalignant cells and becomes activated in dormant tumors about to proliferate.37, 38 In the presence of limited therapeutic options for patients with HCC, TYRO3 represents a promising option for developing new HCC-targeted drug therapies. In that context, TYRO3-specific inhibitors are being identified and tested as new-targeted therapies for HCC.39 In the absence of any small molecule inhibitors of TYRO3, therapeutic targeting of TYRO3 and its downstream signaling pathways by miR-7 is an exciting prospect for treating sorafenib-sensitive and sorafenib-resistant HCC.
In HCC, SR may be inherent wherein the cancer cells typically express less phospho-ERK and phosphatase and tensin homolog but more phospho-AKT.40, 41 More commonly, however, resistance is acquired where the cells augment critical cell-autonomous survival mechanisms by, for example, increasing expression of EGFR42 or exploiting noncancerous cells of the tumor microenvironment,43 to sustain a drug-resistant tumor-permissive niche. Surprisingly, in the resistant cell lines the EGF/EGFR pathway was minimally active compared to GAS6/TYRO3-driven PI3-Kinase/AKT signaling, the latter accompanied by elevated phospho-RelA, indicative of additional TYRO3-independent complementary pathways activated in these cells. miR-7 retained its cytotoxicity in the sorafenib-resistant cells at a low concentration, significantly reduced their migratory and invasiveness in vitro, and was synergistic with sorafenib. Furthermore, a reciprocal association exists between expression of miR-7 and its target genes in the sorafenib-resistant cells, underpinning the potential clinical importance of miR-7 in refractory tumors.
The negative association between TYRO3 and CXCL10, and TYRO3 and IL-8 is concerning because CXCL10 is a homing factor of immune cells to the tumor microenvironment and IL-8 is a well-characterized pro-angiogenic factor in HCC.44, 45 A higher level of CXCL10 is reported in recurrent HCC, while elevated IL-8 is observed in patients having poor overall survival.44, 46 Thus, although TYRO3 siRNA inhibited HCC cell growth and phospho-Akt, there was an accompanying increase in each of CXCL10 and IL-8, in part negating the effects of siRNA to TYRO3. In contrast, miR-7 significantly inhibited phospho-Akt and expression of both CXCL10 and IL-8 in HCC, the latter each containing a miR-7 target site in their 3'-UTR. These data emphasize a major advantage of using an miRNA as therapeutic in HCC because it simultaneously targets multiple members of multiple signaling pathways.
With the availability of very few therapeutic interventions for patients with HCC and an inescapable poor patient outcome, there is an urgent need to develop newer treatment options. RNA-based therapeutics is a rapidly emerging field, with particular reference to hepatic disorders because of the recent phase 2 clinical trial success of specifically delivering siRNAs to hepatocytes.7, 47 These exciting developments in RNA-based therapeutics result from synthesizing oligonucleotides with second-generation chemistry, which are more stable than previously, and using conjugates that target the hepatic asialoglycoprotein receptors, which retain their expression even during development of HCC.7, 47 Thus, generation of second-generation stable miR-7 mimics, which would be delivered efficiently to human hepatocytes, could become an option as an miRNA replacement therapy for HCC.
In conclusion, we identified an miR-7/GAS6/TYRO3 axis that regulates the growth, migratory, and invasive phenotype of parental and sorafenib-resistant HCC cells, predominantly through inhibition of the PI3-Kinase/AKT pathway. Given the recent advances in RNA-based therapies and improved delivery methods, these data lay a foundation for using miR-7 in the treatment of advanced and sorafenib-resistant HCC, potentially in combination with a small molecule inhibitor of TYRO3.
Acknowledgment
We thank Dr. Shane Colley and Dr. Andrew Woo for proof reading this manuscript. We also thank Associate Professor Nicholas Shackel of Centenary Institute, Sydney, for gifting the Huh-7 and HEP3B cell lines to do our research. We thank David Brown, ex-Mirna Therapeutics, for advice and technical help for some of the studies.
REFERENCES
Author names in bold designate shared co-first authorship.




