Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2−/−) mice
Potential conflict of interest: Y.Z., T.T., J.L.K., and Mayo Clinic have a financial interest related to this research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic conflict of interest policies.
Supported by grants of the Swiss National Science Foundation (Schweizerischer Nationalfond SNF; PZ00P3_154691 to J.C.M.), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK057993 to N.F.L.), the National Institute on Aging (NIA; AG013925 to J.L.K.), the Connor Group, the Noaber and Glenn Foundations, the National Institutes of Health (NIH; DK63947 to G.J.G.), the University of Zürich (Forschungskredit), the Max and Martha Dangel Foundation, and the Stiftung zur Krebsbekämpfung.
Abstract
Cholangiocyte senescence has been linked to primary sclerosing cholangitis (PSC). Persistent secretion of growth factors by senescent cholangiocytes leads to the activation of stromal fibroblasts (ASFs), which are drivers of fibrosis. The activated phenotype of ASFs is characterized by an increased sensitivity to apoptotic stimuli. Here, we examined the mechanisms of apoptotic priming in ASFs and explored a combined targeting strategy to deplete senescent cholangiocytes and ASFs from fibrotic tissue to ameliorate liver fibrosis. Using a coculture system, we determined that senescent cholangiocytes promoted quiescent mesenchymal cell activation in a platelet-derived growth factor (PDGF)-dependent manner. We also identified B-cell lymphoma-extra large (Bcl-xL) as a key survival factor in PDGF-activated human and mouse fibroblasts. Bcl-xL was also up-regulated in senescent cholangiocytes. In vitro, inhibition of Bcl-xL by the small molecule Bcl-2 homology domain 3 mimetic, A-1331852, or Bcl-xL-specific small interfering RNA induced apoptosis in PDGF-activated fibroblasts, but not in quiescent fibroblasts. Likewise, inhibition of Bcl-xL reduced the survival and increased apoptosis of senescent cholangiocytes, compared to nonsenescent cells. Treatment of multidrug resistance 2 gene knockout (Mdr2−/−) mice with A-1331852 resulted in an 80% decrease in senescent cholangiocytes, a reduction of fibrosis-inducing growth factors and cytokines, decrease of α-smooth muscle actin–positive ASFs, and finally in a significant reduction of liver fibrosis. Conclusion: Bcl-xL is a key survival factor in ASFs as well as in senescent cholangiocytes. Treatment with the Bcl-xL-specific inhibitor, A-1331852, reduces liver fibrosis, possibly by a dual effect on activated fibroblasts and senescent cholangiocytes. This mechanism represents an attractive therapeutic strategy in biliary fibrosis. (Hepatology 2018;67:247-259).
Abbreviations
-
- ASF
-
- activated stromal fibroblast
-
- Bak
-
- Bcl-2-antagonist/killer 1
-
- Bax
-
- Bcl-2-associated X protein
-
- Bcl-2
-
- B-cell lymphoma 2
-
- Bcl-xL
-
- B-cell lymphoma-extra large
-
- BH3
-
- Bcl-2 homology domain 3
-
- BSA
-
- bovine serum albumin
-
- CCA
-
- cholangiocarcinoma
-
- CK19
-
- cytokeratin 19
-
- CXCL1
-
- chemokine (C-X-C motif) ligand 1
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- ECM
-
- extracellular matrix
-
- hFBs
-
- human fibroblasts
-
- HFF-1
-
- human foreskin fibroblasts
-
- HSCs
-
- hepatic stellate cells
-
- IHC
-
- immunohistochemistry
-
- IL
-
- interleukin
-
- IR
-
- ionizing radiation
-
- Mcl-1
-
- myeloid cell leukemia sequence 1
-
- NHCs
-
- normal human cholangiocytes
-
- PARP
-
- poly (ADP-ribose) polymerase
-
- PDGF
-
- platelet-derived growth factor
-
- PSC
-
- primary sclerosing cholangitis
-
- SASP
-
- senescence-associated secretory phenotype
-
- siRNA
-
- small interfering RNA
-
- αSMA
-
- α-smooth muscle actin
-
- TGFβ
-
- transforming growth factor beta
-
- TNFα
-
- tumor necrosis factor alpha
-
- TUNEL
-
- terminal deoxynucleotidyl transferase dUTP nick end labeling
-
- WT
-
- wild type
Primary sclerosing cholangitis (PSC) is a chronic idiopathic cholangiopathy. Although the pathogenesis is still poorly understood, several steps in the disease progression have been identified. One model of pathogenesis suggests that cholangiocyte injury initiates an inflammatory cascade that involves other cell types, such as fibroblasts, in the periductal stroma. This interplay of injured epithelial cells and subsequently activated stromal fibroblasts leads to the characteristic fibrotic reaction that further promotes injury by causing biliary strictures and cholestasis. Recent studies have demonstrated that cholangiocyte senescence is a prominent feature in PSC. These senescent cholangiocytes are characterized by growth arrest, but also exhibit features of the senescence-associated secretory phenotype (SASP), including increased secretion of signaling molecules and growth factors such as platelet-derived growth factor (PDGF).1, 2
Periductal stromal fibroblasts in the liver are responsible for the synthesis, deposition, and remodeling of the extracellular matrix (ECM) and play a pivotal role in tissue repair. Upon tissue injury and through their interplay with epithelial cells, fibroblasts become activated by growth factors such as PDGF.3-5 Persistent activation of stromal fibroblasts can lead to fibrosis.6 Activated stromal fibroblasts (ASFs) exhibit increased sensitivity to apoptotic stimuli.7 Indeed, states of cell activation or injury can result in increased apoptotic sensitivity, also termed “apoptotic priming.”8 Cellular activation leads to an imbalance of proapoptotic and antiapoptotic B-cell lymphoma 2 (Bcl-2) proteins, often with an overweight of proapoptotic family members. In this state, minimal further proapoptotic stimuli are sufficient to drive activated cells into cell death. However, the molecular mechanisms of fibroblast activation and apoptotic priming in the liver are not well understood.
Bcl-2 proteins are key regulators of cellular apoptosis. The Bcl-2 protein family comprises proapoptotic and antiapoptotic members. Among the most prominent antiapoptotic proteins are Bcl-2, B-cell lymphoma-extra large (Bcl-xL), and myeloid cell leukemia sequence 1 (Mcl-1). Proapoptotic family members include the effector proteins Bcl-2-associated X protein (Bax) and Bcl-2-antagonist/killer 1 (Bak), which upon activation form pores in the outer mitochondrial membrane. Additionally, the Bcl-2 homology domain 3 (BH3)-only proteins inhibit antiapoptotic family members and “fine tune” the apoptotic machinery. Antiapoptotic proteins of the Bcl-2 family keep Bax and Bak in check, whereas BH3-only proteins in turn regulate the activity of antiapoptotic Bcl-2 proteins.9 In a healthy cell, these proapoptotic and antiapoptotic factors are in a finely regulated balance to ensure cell survival.
We have previously shown, in a murine model of cholangiocarcinoma (CCA), that ASFs in the tumor microenvironment can be selectively targeted with BH3 mimetics,7 small molecules that mimic BH3 protein function and have proapoptotic effects. This results in reduced tumor volume and prolonged survival of animals. The senescent cholangiocyte phenotype characteristic of PSC1 may maintain cellular activation in the microenvironment by secretion of activating mediators, thereby fueling the development of biliary fibrosis. Interestingly, several senescent cell types seem to depend on Bcl-xL for survival.10, 11 Very recently, small molecules that selectively induce apoptosis in senescent cells have gained interest. The elimination of senescent cells by so-called “senolytics” may reduce detrimental aging processes.12 Thus, ASFs as well as senescent human cholangiocytes might be sensitive to proapoptotic or senolytic effects of BH3 mimetics, respectively.
In this study, we examined the mechanism of PDGF-mediated apoptotic priming in ASFs. Furthermore, we explored whether BH3 mimetics might have a dual therapeutic effect on ASFs as well as on senescent cholangiocytes in the multidrug resistance 2 gene knockout (Mdr2−/−) mouse model of biliary liver fibrosis, thereby reducing liver injury and fibrosis.
Materials and Methods
IN VITRO EXPERIMENTS
Fibroblasts were activated with 50 ng/mL of recombinant human PDGF-BB (eBioscience, San Diego, CA) for 24 hours. Nontransformed, low-passage (less than passage 15) normal human cholangiocytes (NHCs) were induced to senesce with ionizing radiation (IR; 10 Gy), a well-documented approach to induce cellular senescence in vitro.13 For apoptosis induction, fibroblasts were treated for 24 hours with the following proapoptotic BH3 mimetics: 1 μM of navitoclax (ABT-263, specific for Bcl-2, Bcl-xL, and Bcl-ω), 1 μM of A-1195425 (ABT-199, specific for Bcl-2), 1 μM of A-1331852 (specific for Bcl-xL), and 10 μM of A-1210477 (specific for Mcl-1). NHCs were treated with 1 μM of A-1331852 for 1 hour, 48 hours post-IR treatment. The set of BH3 mimetics was kindly provided by AbbVie International (Chicago, IL).
COCULTURE EXPERIMENTS
NHCs were seeded on the inserts of transwell plates (six- or 24-wells plate), and were induced to senescence by IR. NHCs remained in culture for 4 days to achieve maximum percentage of senescence (60%) as measured by beta-galactosidase staining. Human hepatic stellate cells (HSCs; LX-2) were seeded on the bottom of separate plates (six- or 24-wells plate). LX-2 cells were incubated with a PDGF neutralizing antibody (R&D Systems, Minneapolis, MN) at the recommended concentration from 1 hour before the start of the coculture. NHCs and senescent cholangiocytes were incubated with LX-2 in the presence or absence of PDGF antibody for 24 hours before harvesting proteins.
For a complete list of utilized cell lines see the Supporting Information.
SMALL INTERFERING RNA
Cells were transfected with 10 nM of Silencer Select Pre-Designed small interfering RNA (siRNA) targeting Bcl-xL or Silencer Select Negative Control No. 2 (both from Invitrogen, Carlsbad, CA) for 24-48 hours. Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA) was used according to the manufacturer's protocol.
IN SITU COSTAINING OF mRNA AND PROTEIN
Detection of p16Ink4a mRNA and cytokeratin 19 (CK19) protein in formalin-fixed, paraffin-embedded mouse liver tissue was performed as described.14 Briefly, tissues were deparaffinized, rehydrated, and subjected to heat-mediated antigen retrieval in 0.01 M of sodium citrate buffer (pH 6.0). Slides were allowed to cool, washed, and incubated in prehybridization solution (3% bovine serum albumin [BSA] in 4× SSX) for 20 minutes at 55°C. Tissues were then incubated for 1 hour at 55°C with a fluorescein-labeled p16Ink4a LNA probe (Exiqon, Vedbaek, Copenhagen) diluted to 25 nM in hybridization buffer (10% dextran sulfate in 4× SSC). Slides were washed and subjected to a tyramide signal amplification step (PerkinElmer, Waltham, MA). Slides were washed again, blocked in 4% BSA, and incubated overnight at 4°C with a primary antibody to CK19 (Abcam, Cambridge, UK). After overnight incubation, slides were washed and incubated with a secondary antibody (Alexa Fluor 568; Thermo Fisher Scientific, Waltham, MA), washed again, and mounted in Prolong Gold with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Waltham, MA). Slides were analyzed on a Zeiss 710 Confocal Microscope.
Mdr2−/− RODENT MODEL OF PSC
Mdr2−/− mice were kindly provided by Frank Lammert (Saarland University Medical Center, Department of Medicine II, Homburg, Germany). Balb/cJ wild-type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All protocols were approved by the local animal care and use committees (264/2013). Twelve-week-old female Balb/cJ WT mice and Mdr2−/− mice were treated during the light cycle with 50 mg/kg/d of navitoclax (ABT-263), 25 mg/kg/d of A-1331852 (Bcl-xL inhibitor), or vehicle for 14 days by daily oral gavage. Finally, blood was taken for liver enzyme quantification. For fibrosis quantification, animals were sacrificed and livers were excised and snap-frozen in liquid nitrogen. For immunohistochemistry (IHC), liver sections were also paraffin-embedded and frozen in Tissue-Tek O.C.T. compound (Sakura, Alphen aan den Rijn, The Netherlands).
SIRIUS RED STAINING
Paraformaldehyde-fixed, paraffin-embedded liver tissue sections (3 μm) were deparaffinized, hydrated, and incubated in picrosirius red solution (0.5% picrosirius red in picric acid; both from Sigma-Aldrich, St. Louis, MO). Following a washing step, sections were incubated in Weigert's hematoxylin (Sigma-Aldrich, St. Louis, MO). Sections were rinsed and differentiated with hydrochloric acid. Sections were then dehydrated and mounted. The percentage of Sirius Red–positive area was quantified using ImageJ (NIH, Bethesda, MD) and calculated from 10 portal fields (magnification, ×20) in each animal.
HYDROXYPROLINE QUANTIFICATION
Hydroxyproline content was quantified colorimetrically using the Hydroxyproline Assay Kit (Sigma-Aldrich, St. Louis, MO). Briefly, 100 mg of liver tissue from the left liver lobe was homogenized in 1 mL of H2O bidest. using GentleMacs Tissue Homogenizer (Miltenyi Biotec, Bergisch Gladbach, Germany) and subsequently hydrolyzed in 1 mL of 12 M of hydrochloric acid at 120°C for 3 hours. The hydrolysate was centrifuged at 13,000g for 10 minutes and 20 μL of the supernatant were transferred to a microtiter plate and evaporated to dryness at 60°C. The lyophilisate was rehydrated in 100 μL of Chloramine T/Oxidation Buffer and incubated at room temperature for 5 minutes. Following addition of 100 μL of dimethylaminobenzaldehyde/perchloric acid/isopropanol solution, samples were incubated at 60°C for 90 minutes. Absorbance was read at 561 nm (Synergy 2; BioTek, Winooski, VT). Hydroxyproline concentration was calculated from a standard curve prepared with high-purity hydroxyproline.
STATISTICAL ANALYSIS
Data represent at least three independent experiments and are expressed as mean ± SD. Statistical significance was determined using two-tailed Student t tests or analysis of variance. Graphs were generated using GraphPad Prism software (version 6; GraphPad Software Inc., La Jolla, CA).
Further materials and methods are described in the Supporting Information.
Results
SENESCENT CHOLANGIOCYTES EXHIBIT AN ALTERED Bcl-2 PROTEIN PROFILE AND ARE SENSITIVE TO Bcl-xL INHIBITION
In general, senescent cells up-regulate the antiapoptotic Bcl-2 proteins, Bcl-ω and Bcl-xL, to maintain their viability.10 In order to study the expression of Bcl-2 family members in senescent cholangiocytes, NHCs were experimentally induced to senescence. Western blotting analysis confirmed that antiapoptotic Bcl-xL is up-regulated in senescent cholangiocytes as compared to nonsenescent cells whereas antiapoptotic Bcl-2 and proapoptotic Bak are unchanged (Fig. 1A).
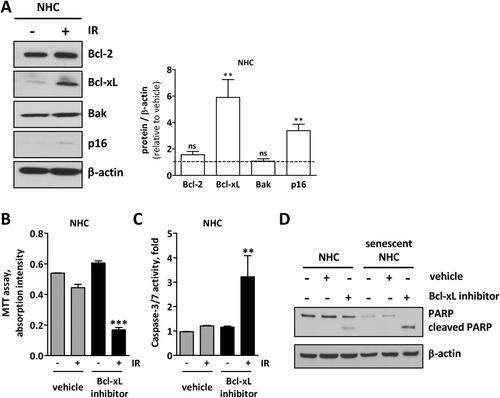
We then asked whether senescent cholangiocytes need Bcl-xL for their survival. Therefore, we exposed IR-treated senescent cholangiocytes to the selective Bcl-xL inhibitor, A-1331852, and monitored both apoptosis and cell viability. Whereas nonsenescent NHCs showed no alterations, senescent cholangiocytes had a reduced level of viable cells (Fig. 1B) and a high level of caspase-3/7 activity (Fig. 1C) after only 1 hour of A-1331852 treatment. Enhanced apoptosis sensitivity in senescent cholangiocytes was confirmed by both cleaved poly (ADP-ribose) polymerase (PARP; Fig. 1D) and apoptotic nuclei (Supporting Fig. S1), suggesting that only senescent cholangiocytes are sensitive to A-1331852-induced apoptosis.
IN VITRO ACTIVATED FIBROBLASTS ARE PRIMED FOR APOPTOSIS AND SENSITIVE TO BH3 MIMETICS
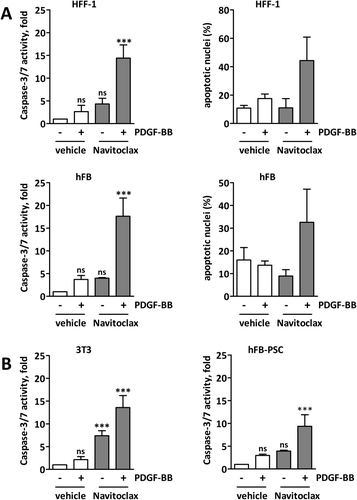
In order to investigate apoptotic priming in activated fibroblasts, we stimulated the human (human foreskin fibroblast [HFF]-1) fibroblasts with PDGF-BB to achieve an activated phenotype (Supporting Fig. S2A,B) and subsequently exposed them to the proapoptotic BH3 mimetic navitoclax in vitro. Pretreatment with PDGF-BB significantly increased the sensitivity of fibroblasts to navitoclax-mediated apoptosis as assessed biochemically by caspase-3/7 activity and morphologically using DAPI staining for apoptotic nuclei, whereas nonactivated fibroblasts did not respond to navitoclax (Fig. 2A and Supporting Fig. S2C). Treatment of PDGF-BB–pretreated human primary fibroblasts (hFBs) revealed the same results (Fig. 2A and Supporting Fig. S2C). Furthermore, treatment of 3T3 mouse fibroblasts and human liver fibroblasts obtained from a PSC patient (hFB-PSC) with navitoclax significantly increased caspase-3/7 activity in PDGF-BB-pretreated fibroblasts as compared to quiescent fibroblasts (Fig. 2B). These data show that quiescent fibroblasts can be activated in vitro, and that activated fibroblasts can be targeted with the BH3 mimetic navitoclax.
ACTIVATION OF STROMAL FIBROBLASTS DECREASES Bcl-2 PROTEIN
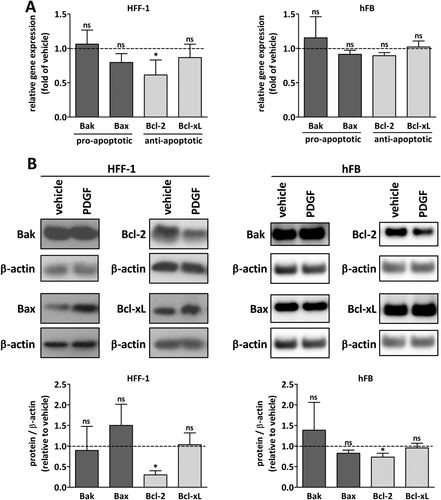
We have previously shown that alteration of the Bcl-2 family is responsible for apoptotic priming in cancer-associated fibroblasts.15 Bcl-2 profiling of PDGF-activated fibroblasts revealed a down-regulation of Bcl-2 whereas Bcl-xL protein was unchanged (Fig. 3 and Supporting Fig. S3). Proapoptotic Bax was increased in HFF-1 cells, but not changed in activated primary fibroblasts (Fig. 3B).
SURVIVAL OF ACTIVATED FIBROBLASTS IS Bcl-xL DEPENDENT
To explore the hypothesis that loss of Bcl-2 protein renders activated fibroblasts dependent on other antiapoptotic Bcl-2 proteins for survival, we exposed PDGF-activated human and mouse fibroblasts to specific Bcl-2 protein inhibitors. The specific inhibition of Bcl-xL solely induced apoptosis in PDGF-activated fibroblasts, whereas specific inhibition of Bcl-2 itself and Mcl-1 did not result in cell death (Fig. 4A). Furthermore, treatment of different human and mouse cell lines and primary human fibroblasts with the Bcl-xL inhibitor, A-1331852, did result in significant apoptosis induction in PDGF-activated cells as assessed biochemically by caspase-3/7 activity and morphologically using DAPI staining, whereas nonactivated fibroblasts did not respond to Bcl-xL inhibition (Fig. 4B,C and Supporting Fig. S4).
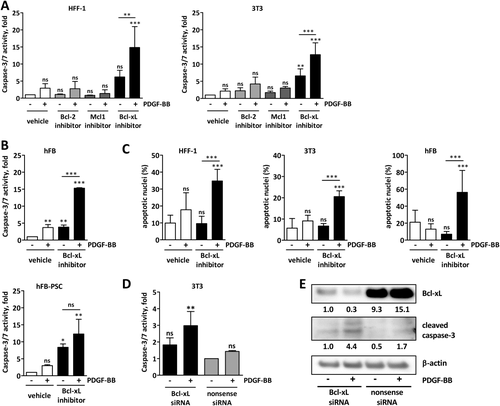
To confirm the Bcl-xL dependency of ASFs, we performed a siRNA knockdown of Bcl-xL in human and mouse fibroblasts. Consistent with our previous observations, treatment of these cells with PDGF induced apoptosis in fibroblasts with Bcl-xL knockdown as shown by increased caspase-3/7 activity and cleaved caspase-3 by western blotting, whereas nonsense siRNA-treated cells did not respond to PDGF treatment (Fig. 4D,E). This confirms that the survival of activated fibroblasts is exquisitely dependent on Bcl-xL.
SENESCENT CHOLANGIOCYTES EXHIBIT A SECRETORY PHENOTYPE AND INDUCE FIBROBLAST ACTIVATION IN VITRO
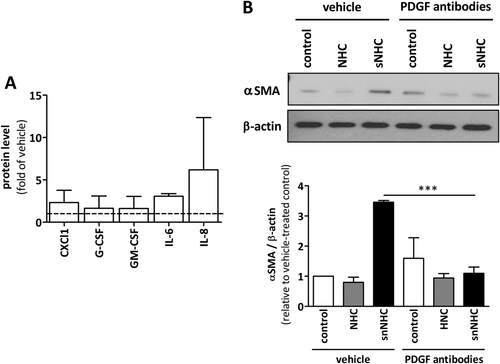
Senescent cholangiocytes are characteristically found in PSC. Analysis of cell culture media from normal and IR-induced senescent NHCs revealed an increased secretion of chemokine (C-X-C motif) ligand 1 (CXCL1), interleukin (IL)-6, and IL-8 by senescent NHCs (Fig. 5A). Cocultivation of the HSC line, LX-2, with senescent NHCs induced activation of these cells as observed by increased α-smooth muscle actin (αSMA), compared to cocultivation with normal NHCs (Fig. 5B). Addition of an anti-PDGF antibody almost completely abrogated this activation. These data confirm that senescent cholangiocytes induce fibroblast activation that is blocked by anti-PDGF antibodies.
Bcl-xL INHIBITION IN THE Mdr2−/− PSC MOUSE MODEL DEPLETES SENESCENT CHOLANGIOCYTES
Based on our in vitro data, we treated Mdr2−/− mice with the selective Bcl-xL inhibitor, A-1331852, to deplete senescent cholangiocytes as well as ASFs and ideally to reduce biliary liver fibrosis. In the case of cholangiocytes, the number of p16Ink4a-positive senescent cholangiocytes was significantly reduced in animals treated with A-1331852 (Fig. 6A). In addition, a reduction of SASP proteins in the sera of treated animals was observed (Supporting Fig. S5B).
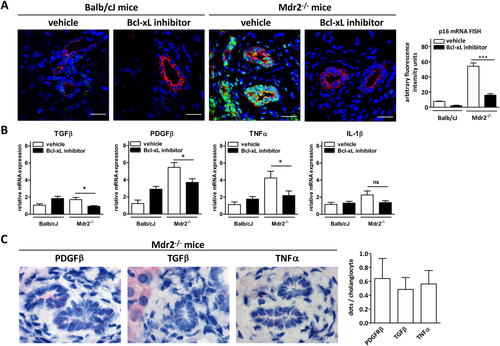
RNAscope in situ hybridization of liver tissue sections confirmed the expression of profibrotic transforming growth factor beta (TGFβ), PDGFβ, and tumor necrosis factor alpha (TNFα) by cholangiocytes (Fig. 6C). Treatment of Mdr2−/− mice with Bcl-xL inhibitor A-1331852 led to a significant reduction of expression of profibrotic factors, including TGFβ, PDGF, and TNFα, in the liver as compared to vehicle-treated mice (Fig. 6B).
SELECTIVE INHIBITION OF Bcl-xL REDUCES LIVER FIBROSIS IN Mdr2−/− MICE
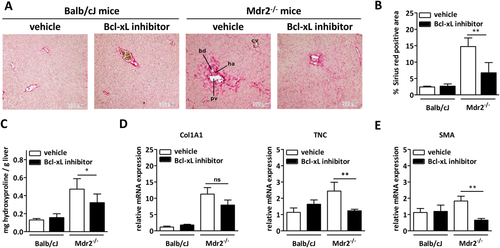
In line with the reduction of senescent cholangiocytes, we found significantly reduced expression of the fibroblast activation marker, αSMA (Fig. 7E), and a decrease in overall fibrotic tissue in A-1331852-treated animals as compared to vehicle-treated controls (Fig. 7A). Morphometric analysis of Sirius Red–stained liver sections revealed a significant reduction of periductal fibrosis (Fig. 7B). Quantification of collagen by measuring hydroxyproline confirmed reduction of fibrosis in treated animals (Fig. 7C). Furthermore, Collagen 1A1 and Tenascin C mRNA expression in liver was reduced in Mdr2−/− mice treated with Bcl-xL inhibitor, as compared to vehicle-treated animals (Fig. 7D).
To better characterize the Mdr2−/− mouse model of PSC, hematoxylin-eosin stained tissue sections were examined for inflammation and signs of cholestasis. Mild portal inflammation, but no signs of cholestasis or bilirubinostasis, was observed in Mdr2−/− mice.
To assess for cholangiocyte proliferation and ductular reaction, IHC for Ki67 and sex-determining region Y box 9 (Supporting Fig. S7) was performed. Morphometry of these stainings showed a significantly reduced ductular reaction after treatment with A-1331852.
Complementing terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining did not show increased cell death of normal bile duct epithelia or hepatocytes following treatment with A-1331852 (Supporting Fig. S6).
Discussion
This study provides mechanistic insights into the interaction of senescent cholangiocytes and stromal fibroblasts. Our data indicate that: (1) senescent cholangiocytes and activated fibroblasts depend on Bcl-xL for survival; (2) activation of stromal fibroblasts by PDGF causes loss of prosurvival Bcl-2 with resulting Bcl-xL dependency and apoptotic priming; (3) senescent cholangiocytes activate fibroblasts in a PDGF-dependent manner; and (4) specific inhibition of Bcl-xL by the BH3 mimetic, A-1331852, reduces both senescent cholangiocytes and ASFs, thereby alleviating biliary fibrosis in Mdr2−/− mice.
A large percentage of cholangiocytes in livers of patients with PSC exhibit a senescent phenotype and SASP.1 Attributed to secreted signaling molecules, SASP-expressing cholangiocytes alter the periductal microenvironment and induce activation of stromal fibroblasts.1, 2 Recruitment and activation of stromal fibroblasts by PDGF has been shown in both cancer and benign, chronic fibrosing conditions.16-18 Besides other growth factors, PDGF is released into the microenvironment by senescent/SASP signals to stromal fibroblasts. As shown, PDGF activates stromal fibroblasts, induces proliferation and ECM deposition, but also primes these fibroblasts for apoptosis.15 We demonstrated earlier that ASFs become selectively sensitive to the proapoptotic BH3 mimetic navitoclax and undergo apoptosis upon treatment.7 As shown here and in previous studies,15 PDGF-mediated fibroblast activation results in down-regulation of the antiapoptotic regulator, Bcl-2. This primes ASFs for apoptosis and renders their survival dependent on the complementary prosurvival factor, Bcl-xL. Consequently, inhibition of Bcl-xL with specific BH3 mimetics effectively induces apoptosis in these cells. Interestingly, after siRNA knockdown of Bcl-xL, PDGF becomes an inducer of apoptosis in stromal fibroblasts and results in increased cell death.
Recent data show that, like ASFs, senescent cells depend on Bcl-xL as a pivotal survival factor10, 11 and can be efficiently eliminated by the poly-specific BH3 mimetic, navitoclax (ABT-263).19 This provides the rationale to target both cell types, Bcl-xL-dependent ASFs and Bcl-xL-dependent senescent cholangiocytes, with the specific BH3 mimetic, A-1331852, as a dual treatment strategy for biliary fibrosis in PSC. Indeed, in the Mdr2−/− mouse model of PSC, inhibition of Bcl-xL by A-1331852 significantly reduced both the number of p16Ink4a-positive senescent cholangiocytes and fibrosis. This reduction in senescent cholangiocytes was reflected by a reduction of SASP-specific signaling molecules in the sera of treated animals (Supporting Fig. S5B). At the same time, we observed a reduction in pathological ductular reaction following Bcl-xL inhibition, whereas liver parenchyma and normal bile ducts appeared unaffected (Supporting Fig. S7).
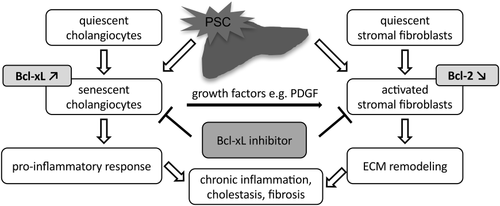
There was no significant improvement of lab values in animals (Supporting Fig. S5A). This was not unexpected, given that the approach to inhibit Bcl-xL does not prevent ongoing biliary tract injury, but eliminates cells that have already suffered damage or have already been activated. Moreover, the ultimate goal of PSC therapy is the reduction of hepatobiliary fibrosis. We propose a pathogenetic pathway of epithelial injury, ensuing epithelial senescence, development of an SASP, and subsequent persistent stromal cell activation that results in fibrosis, which further aggravates epithelial injury (Fig. 8). To interrupt this process appears to be a promising therapeutic approach.
Earlier unspecific Bcl-2 inhibitors like Navitoclax showed dose-limiting hematological toxicities. Neutropenia was the primary limiting side effect, particularly after combination with standard chemotherapeutics. Pronounced thrombocytopenia was also observed, but not dose limiting. Although still limited, current data indicate that BCL-xL–selective inhibitors such as A-1331852 may actually have an improved safety profile and increased efficacy.20 In addition, the desired therapeutic effects are attributed to the cytotoxic action of A-1331852 rather than a cytostatic one, therefore intermittent application of the drug for treatment of a human disease seems feasible to reduce potential hematological side effects.
Normal hepatocytes and normal biliary epithelia did not show increased cell death following A-1331852 treatment as assessed by TUNEL staining. This supports the idea that no relevant liver toxicity has to be expected. Moreover, there was a trend to improved liver values in animals treated with A-1331852 when compared to animals that received vehicle control (Supporting Fig. S5B).
Similar observations have been made previously in CCA with regard to depletion of ASFs. From our point of view, the current findings underline a link between biliary fibrosis and cancer development. The Bcl-xL inhibitor promotes a possible cancer preventive approach in PSC and as such has clinical relevance.
We therefore suggest combined targeting of both ASFs and senescent cholangiocytes with BH3 mimetics as a novel therapeutic strategy. Similar mechanisms of interaction between injured, senescent epithelia that activate stromal cells with resulting apoptotic priming may be a pathogenic mechanism in other forms of liver fibrosis and even in other organs. In this regard, Bcl-xL–specific inhibitors may have relevance in antifibrotic treatment strategies beyond the liver.
REFERENCES
Author names in bold designate shared co-first authorship.




