Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study
Potential conflict of interest: Harrys A. Torres consults and has received grants from Gilead, Merck, and Vertex. Anna S. Lok has received grants from Bristol-Myers Squibb and Gilead.
This study was presented in part at The Liver Meeting 2016, November 11-15, 2016, Boston, MA.
Abstract
Hepatitis C virus (HCV) reactivation in patients receiving cancer treatment has been reported in retrospective studies. We sought to determine prospectively the incidence, predictors, and clinical significance of HCV reactivation during cancer treatment. HCV-infected patients receiving cancer treatment at our institution between November 2012 and July 2016 were studied. Reactivation was defined as an increase in HCV-RNA ≥1 log10 IU/mL over baseline and hepatitis flare as an increase in alanine aminotransferase to ≥3 times the upper limit of normal. One hundred patients were studied, 50 with hematologic malignancies and 50 with solid tumors. Reactivation occurred in 23 (23%) patients, including 18 (36%) patients with hematologic malignancies and 5 (10%) patients with solid tumors. In univariate analysis, patients with reactivation were more likely than those without reactivation to have prolonged lymphopenia (median, 95 versus 22 days; P = 0.01) and to have received rituximab (44% versus 9%; P < 0.0001), bendamustine (22% versus 0%; P < 0.001), high-dose steroids (57% versus 21%; P = 0.001), or purine analogs (22% versus 5%; P = 0.02). Rituximab (odds ratio = 9.52; P = 0.001), and high-dose steroids (odds ratio = 5.05; P = 0.01) retained significance in multivariable analysis. Of the 23 patients with reactivation, 10 (43%) had hepatitis flare. No patient with reactivation experienced liver failure or liver-related death within 36 weeks after initiation of cancer treatment. Fourteen patients with hepatitis flare, six of whom had reactivation, required discontinuation or dose reduction of cancer treatment. Conclusion: HCV reactivation occurred in 23% of HCV-infected patients receiving cancer treatment, and most had an unremarkable clinical course. However, reactivation can affect the cancer treatment plan. Our findings suggest that HCV infection should not contraindicate cancer therapy and infected patients should have access to multiple cancer treatments with close monitoring while receiving regimens associated with HCV reactivation. (Hepatology 2018;67:36-47).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HCVr
-
- HCV reactivation
-
- IQR
-
- interquartile range
-
- JAK
-
- Janus kinase
Hepatitis C virus (HCV) infection is the most common blood-borne infection in the United States, where at least 3.5 million people are currently infected.1 HCV infection is observed in 1.5% to 32% of cancer patients around the world, depending on the geographic area and type of cancer studied.2-5
Among patients with cancer receiving chemotherapy, liver dysfunction caused by hepatitis B virus (HBV) reactivation is a significant problem,6-8 occurring in 14% to 72% of patients who did not receive prophylactic antiviral therapy and leading to liver failure in 13% of cases and death in 6% of cases.6, 8-10 In contrast, the incidence and consequences of HCV reactivation (HCVr) during cancer treatment remain poorly defined. HCVr appears to be less common and to have less severe consequences than HBV reactivation,3, 7, 11-13 with only a few fatal cases of fulminant hepatitis attributed to HCV having been reported.14, 15 In a retrospective study of 308 HCV-infected cancer patients, we observed that 11% experienced hepatitis flare during cancer treatment; however, the true incidence of HCVr could not be determined, because HCV-RNA monitoring was not performed in many patients.11
The use of chemotherapy in HCV-infected patients can be challenging.5, 16 Oncologists extrapolate what is known about HBV reactivation and often exclude HCV-infected cancer patients from receiving many kinds of chemotherapy. For instance, approximately 48% of early-phase cancer clinical trials at MD Anderson Cancer Center exclude HCV-infected patients. Thus, to fill the critical void in understanding of HCVr, we conducted this prospective observational study to determine the incidence, predictors, and clinical significance of HCVr in patients receiving cancer treatment.
Materials and Methods
PATIENTS
Cancer patients at least 18 years of age who were referred to the HCV clinic at The University of Texas MD Anderson Cancer Center from November 2012 through July 2016 and were scheduled to receive cancer treatment (chemotherapy [including targeted therapy] with or without radiotherapy) were enrolled in an observational study. Patients with hematologic malignancies or solid tumors were included.
To avoid the confounding effect on liver chemistry tests caused by tumors infiltrating the liver, we excluded from our analysis patients with hepatocellular carcinoma or metastases in the liver (Fig. 1). Patients with undetectable HCV-RNA were also excluded.
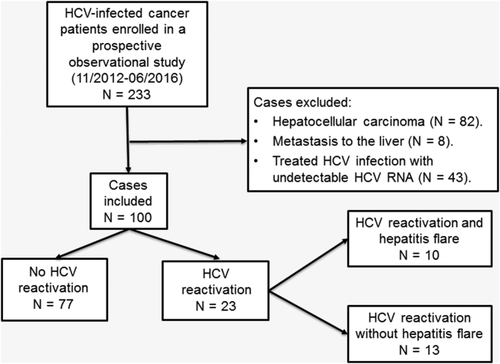
Serum alanine aminotransferase (ALT) levels were measured regularly during cancer treatment. The MD Anderson HCV clinic protocol recommends quantifying serum HCV-RNA level before the initiation of chemotherapy, at 12-week intervals after initiation of cancer treatment, and at the time of hepatitis flare. The protocol for this observational study was approved by the MD Anderson Institutional Review Board. Written informed consent was obtained from each patient.
HCV-RNA QUANTIFICATION
To measure HCV-RNA in serum, we used the COBAS AmpliPrep/COBAS TaqMan HCV test (version 2.0, Roche Molecular Systems, Branchburg, NJ) with a quantification range of 15 to 100,000,000 IU/mL (1.18 log10 to 8.00 log10 IU/mL). HCV-RNA was measured during the routine follow-up of the patients in the clinic, irrespective of the cancer management.
CHEMOTHERAPY REGIMENS STUDIED
Only the last chemotherapy regimen was studied for each patient. However, if different chemotherapy regimens were given less than 3 months apart, we analyzed the cumulative effect of these regimens on HCVr. Patients receiving any type of cancer treatment, including maintenance chemotherapy, were included.
DEFINITIONS
As described previously,11 we defined HCVr as an increase in HCV-RNA level of ≥1 log10 IU/mL from baseline HCV-RNA following institution of cancer treatment, because chronically infected patients generally have stable HCV-RNA levels that vary within 0.5 log10 IU/mL.17
Hepatitis flare was defined as an increase in ALT level to ≥3 times the upper limit of normal in the absence of liver infiltration by tumor, use of hepatotoxic drugs other than chemotherapeutics,18 or other active systemic infection (bacterial or fungal infection or infection with hepatitis A virus, HBV, hepatitis E virus, or human immunodeficiency virus).
Lymphopenia was defined as an absolute lymphocyte count of ≤1000 cells/μL or less. Duration of lymphopenia was measured from the start date of lymphopenia to the date of resolution of lymphopenia or 12 weeks after discontinuation of cancer treatment, whichever occurred first.
Liver fibrosis was staged by liver biopsy in patients who had adequate platelets and normal coagulation, and who consented to the procedure. Cirrhosis was diagnosed on the basis of liver biopsy or a combination of clinical findings (e.g., ascites, encephalopathy, or jaundice), serum biomarker (Prometheus Fibrospect II [Prometheus Laboratories, San Diego, CA]), and radiologic findings (e.g., hepatic nodularity). As reported previously, acute liver failure in patients with chronic liver disease was defined as the development of jaundice (serum bilirubin level >5 mg/dL) and coagulopathy (international normalized ratio ≥1.5) complicated within 4 weeks by clinical ascites and/or encephalopathy.19
High-dose steroids were defined as cumulative administration of the equivalent of >600 mg of prednisone within 6 months after the start of cancer treatment. We divided chemotherapy regimens into the following subcategories: alkylating agents (cyclophosphamide, ifosfamide, melphalan, bendamustine, busulfan), platinum-containing agents (cisplatin, carboplatin, oxaliplatin), pyrimidine compounds (fluorouracil, capecitabine, cytarabine, gemcitabine, decitabine), purine analogs (mercaptopurine, fludarabine, cladribine, clofarabine), folate antagonists (methotrexate, pemetrexed), anthracyclines (daunorubicin, doxorubicin, epirubicin, idarubicin, bleomycin), topoisomerases (topotecan, etoposide, irinotecan), mitotic inhibitors (paclitaxel, docetaxel, vinblastine, vincristine), cytidine analogues (azacitidine, decitabine), immunosuppressants (tacrolimus, cyclosporine), immunomodulatory drugs (lenalidomide, thalidomide), and tamoxifen. Targeted therapies other than rituximab (e.g., cetuximab, bortezomib, alemtuzumab) were grouped together.
OUTCOMES
Primary outcomes were the occurrence of HCVr and hepatitis flare from the start date of cancer treatment to 36 weeks (three clinic visits at 12-week follow-up interval) after the start date. Secondary outcomes were discontinuation or dose reduction of chemotherapy due to hepatitis flare, liver failure, and death within 36 weeks after the start of cancer treatment.
STATISTICAL ANALYSIS
Patient characteristics were analyzed using descriptive statistics. We used a chi-square or Fisher's exact test to compare categorical variables and a Wilcoxon Mann-Whitney test to compare continuous variables. We used exact logistic regression analysis to determine independent predictors of HCVr. A Spearman rank correlation test was used to assess the correlation between the changes (peak values minus baseline values) in HCV-RNA and ALT levels. All statistical tests were two-sided and conducted using SPSS version 23.0 (IBM Corp., Armonk, NY). P < 0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS
A total of 233 patients were enrolled, and 133 were excluded for various reasons (Fig. 1), leaving 100 patients in the analysis.
The patient characteristics are detailed in Table 1. Of the 100 patients, 73 were men and 27 were women. The median age was 59 years (interquartile range [IQR], 54-63). Sixty-one patients were white, 26 were black, nine were Hispanic, and four were classified as “Other.” Fifty patients had hematologic malignancies, and 50 had solid tumors. Seventy-eight patients were infected with HCV genotype 1; of 71 patients tested, 44 had rs12979860 (formerly known as interleukin 28 B) genotype CT. Among 38 patients with liver biopsy performed, 15 (39%) had advanced liver disease (Metavir score F3 or F4). At enrollment, 13 patients had cirrhosis (based on liver biopsy in five patients, and on a combination of clinical, laboratory, or radiologic findings in eight patients), and 70 patients were HCV treatment-naïve. Five patients received only maintenance chemotherapy, four received only targeted therapy, and two received only radiotherapy.
| Characteristic | Values |
|---|---|
| Age at cancer treatment initiation, median (IQR), years | 59 (54-63) |
| Sex | |
| Men | 73 (73) |
| Women | 27 (27) |
| Race/ethnicity | |
| Non-Hispanic | 91 (91) |
| White | 61 (67) |
| Black | 26 (29) |
| Othera | 4 (4) |
| Hispanic | 9 (9) |
| Cancer type | |
| Hematologic | 50 (50) |
| Solid | 50 (50) |
| Hematologic cancer type | |
| Multiple myeloma | 16/50 (32) |
| Leukemia | 17/50 (34) |
| Lymphoma | 16/50 (32) |
| Otherb | 1/50 (2) |
| Solid cancer type | |
| Gastrointestinalc | 15/50 (30) |
| Head and neck | 10/50 (20) |
| Lung | 10/50 (20) |
| Breast | 5/50 (10) |
| Melanoma | 3/50 (6) |
| Otherd | 7/50 (14) |
| HCV genotype | |
| 1 | 78/99 (78) |
| 2 | 15/99 (15) |
| 3 | 3/99 (3) |
| 4 | 2/99 (2) |
| 6 | 1/99 (1) |
| rs12979860 genotypee | |
| CC | 20/71 (28) |
| CT | 44/71 (62) |
| TT | 7/71 (10) |
| HCV treatment-naïve | 70 (70) |
| HBV coinfection | |
| Anti-HBc positive | 25/97 (26) |
| HBsAg-positive | 1/100 (1) |
| HIV infection | 0/97 (0) |
| Cirrhosis at enrollmentf | 13 (13) |
| Liver biopsy performed | 38 |
| Metavir score F1-F2 | 23 (61) |
| Metavir score F3-F4 | 15 (39) |
- Data are presented as n (%) unless noted otherwise.
- a Asian and Middle Eastern.
- b Diamond-Blackfan anemia with hematopoietic cell transplantation.
- c Colorectal cancer (n = 5), anal cancer (n = 3), esophageal cancer (n = 3), pancreatic cancer (n = 2), cholangiocarcinoma (n = 1), and gastrointestinal stromal tumor (n = 1).
- d Prostate (n = 2), gynecologic (n = 2), testicular (n = 1), urothelial (n = 1), and thyroid (n = 1).
- e Formerly known as interleukin-28B.
- f Diagnosed on the basis of liver biopsy (n = 5) or radiologic and clinical findings (n = 8).
- Abbreviations: Anti-HBc, hepatitis B core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range.
INCIDENCE OF HCVr
All 100 patients had HCV-RNA level measured at baseline, and 74 had HCV-RNA measured at least twice after cancer treatment initiation. In addition, 71 patients had HCV-RNA measured at least once during cancer treatment, and 87 patients had HCV-RNA measured at least once after cancer treatment. HCVr was detected in 23 patients (incidence [95% confidence interval] = 23% [15%-31%]) in the entire cohort, including 18 (36% [22%-50%]) with hematologic malignancies and 5 (10% [1%-19%]) with solid tumors (Fig. 2). The incidence of HCVr was highest among patients receiving regimens containing bendamustine (5/5 [100%]), rituximab (10/17 [59%]), purine analogs (5/9 [56%]), or high-dose steroids (13/29 [45%]) (Table 2). Details on the 23 patients with HCVr are shown in Table 3.
| Cancer Type or Drugs Received | HCVr Incidence Rate, n (%) | 95% CI for Estimated HCVr Incidence Rate | Median Change in HCV-RNA Level, log10 IU/mL (IQR) |
|---|---|---|---|
| All patients | 23/100 (23) | 15%-31% | 0.2 (−0.1-0.82) |
| Cancer type | |||
| Hematologic | 18/50 (36) | 22%-50% | 0.44 (−0.14-1.10) |
| Solid | 5/50 (10) | 1%-19 % | 0.04 (−0.32-0.39) |
| Drugs received | |||
| Rituximab | 10/17 (59) | 33%-85% | 1.05 (0.47-1.22) |
| High-dose steroid | 13/29 (45) | 26%-64% | 0.54 (−0.12-1.43) |
| Alkylating agenta | 9/29 (31) | 13%-48% | 0.35 (−0.07-1.06) |
| Cyclophosphamide or ifosfamide | 3/17 (18) | 0.35 (−0.16-0.75) | |
| Melphalan | 3/12 (25) | 0.1 (−0.24-0.25) | |
| Bendamustine | 5/5 (100) | 1.23 (1.13-2.0) | |
| Platinumb | 3/32 (9) | 0%-20% | −0.09 (−0.37-0.33) |
| Antimetabolite | 11/40 (28) | 13%-42% | 0.25 (−0.38-1.05) |
| Pyrimidine compoundc | 9/32 (28) | 0.33 (−0.35-1.05) | |
| Purine analogd | 5/9 (56) | 0.96 (0.11-1.36) | |
| Folate antagoniste | 2/7 (29) | 0.39 (−0.14-0.39) | |
| Anthracyclinf | 4/13 (31) | 2%-60% | 0.35 (−0.04-0.75) |
| Topoisomeraseg | 2/8 (25) | 0%-63% | −0.01 (−0.37-0.71) |
| Mitotic inhibitorh | 3/20 (15) | 0%-32% | 0.27 (−0.34-0.75) |
| Monoclonal antibodyi | 7/22 (32) | 11%-53% | 0.37 (−0.04-1.10) |
- a Cyclophosphamide, ifosfamide, melphalan, bendamustine, busulfan.
- b Cisplatin, carboplatin, or oxaliplatin.
- c Fluorouracil, capecitabine, cytarabine, gemcitabine, or decitabine.
- d Mercaptopurine, fludarabine, cladribine, or clofarabine.
- e Methotrexate or pemetrexed.
- f Daunorubicin, doxorubicin, epirubicin, idarubicin, or bleomycin.
- g Topotecan, etoposide, or irinotecan.
- h Paclitaxel, docetaxel, vinblastine, or vincristine.
- i Cetuximab, ruxolitinib, trastuzumab, bortezomib, carfilzomib, bevacizumab, dasatinib, ipilimumab, dabrafenib, trametinib, vismodegib, alemtuzumab, ofatumumab, pazopanib, or regorafenib.
- Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HCVr, HCV reactivation; IQR, interquartile range.
| Patient No. | Age, Years | Sex | Cirrhosis | Type of Cancer | Chemotherapy Regimen | HCV-RNA Change, log10 IU/mL | Peak of ALT, U/L | Peak of Bilirubin,mg/dL | Peak of INR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Female | No | MM | Bortezomib, cyclophosphamide, lenalidomide + HDS | 1.08 | 134 | 1.1 | 1.12 |
| 2 | 80 | Female | No | MM | Thalidomide | 2.86 | 241 | 1 | 1.03 |
| 3 | 63 | Male | No | MM | Gemcitabine, busulfan, melphalan + HDS | 1.58 | 124 | 8.8a | 2.53a |
| 4 | 69 | Male | No | WM | R- bortezomib | 1.12 | 69 | 0.7 | 1.09 |
| 5 | 57 | Male | No | DLBCL | R-gemcitabine, and ofatumumab | 1.1 | 395 | 1.1 | 1.05 |
| 6 | 53 | Male | No | DLBCL | R-lenalidomide followed by R-bendamustine, fludarabine, methotrexate | 1.21 | 111 | 1.2 | 1.08 |
| 7 | 59 | Male | No | DLBCL | R-CHOP followed by R-ICE | 1.0 | 252 | 1.4 | 1.1 |
| 8 | 25 | Male | No | DLBCL | R-ICE followed by R-vorinostat, gemcitabine, busulfan, melphalan | 1.99 | 539 | 8.3 | 1.25 |
| 9 | 70 | Male | No | FL | R-bendamustine | 2.39 | 1160 | 14 | 1.32 |
| 10 | 61 | Female | No | MZL | R-bendamustine | 1.05 | 66 | 0.8 | 1.03 |
| 11 | 65 | Male | No | MCL | R-ibrutinib | 1.1 | 137 | 0.7 | 1.12 |
| 12 | 52 | Male | No | MCL | R-bendamustine followed by R-fludarabine, melphalan, methotrexate | 1.62 | 163 | 2.7 | 1.27 |
| 13 | 62 | Male | Yes | AML | Cladribine, idarubicin, cytarabine + HDS | 1.52 | 138 | 1.6 | 1.22 |
| 14 | 57 | Female | No | AML | Cytarabine, idarubicin, clofarabine + HDS | 1.0 | 888 | 1.8 | 1.24 |
| 15 | 57 | Male | No | AML | Decitabine followed by cladribine, idarubicin, and cytarabine | 2.08 | 247 | 7.9a | 1.68a |
| 16 | 62 | Male | Yes | CLL | Alemtuzumab + HDS | 1.35 | 152 | 1.8 | 1.12 |
| 17 | 63 | Male | Yes | CLL | R-bendamustine | 1.23 | 104 | 1.5 | 1.23 |
| 18 | 69 | Male | No | MDS | HDS followed by cyclosporine | 1.68 | 349 | 6 | 1.07 |
| 19 | 41 | Female | No | Esophageal | Docetaxel, 5-FU, oxaliplatin, HDS | 2.96 | 46 | 0.5 | 1.32 |
| 20 | 54 | Male | No | Gastroesophageal | Docetaxel, 5-FU + HDS | 1.52 | 453 | 0.9 | 0.98 |
| 21 | 56 | Male | No | Anal | Mitomycin, 5-FU | 1.09 | 78 | 0.7 | 1.05 |
| 22 | 61 | Male | No | Germ cell | Ruxolitinib | 1.38 | 134 | 0.8 | 1.14 |
| 23 | 56 | Male | No | Melanoma | Ipilimumab followed by HDS | 1.43 | 493 | 0.9 | 1.13 |
- a Neither of these patients had clinical ascites and/or encephalopathy and thus did not fulfill the Asian Pacific Association for the Study of the Liver criteria for acute liver failure in patients with chronic liver disease.
- Abbreviations: 5-FU, fluorouracil; AML, acute myeloid leukemia; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CLL, chronic lymphocytic lymphoma; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HDS, high-dose steroids; ICE, ifosfamide, carboplatin, etoposide; INR, international normalized ratio; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MZL, marginal zone lymphoma; R, rituximab; WM, Waldenstrom's macroglobulinemia.
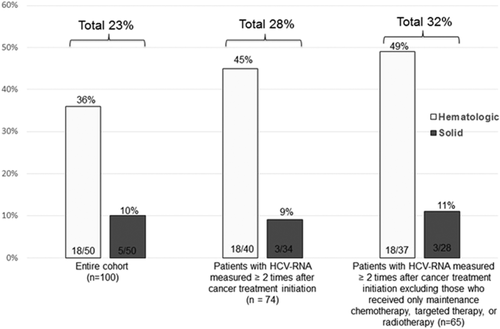
Among the 74 patients with HCV-RNA measured at least twice after cancer treatment initiation, 21 (28% [18%-39%]) had HCVr detected, including 18 of 40 (45% [29%-61%]) with hematologic malignancies and 3 of 34 (9% [0%-19%]) with solid tumors. When patients who received only maintenance chemotherapy, targeted therapy, or radiotherapy were excluded, HCVr was detected in 21 (32% [20%-44%]) of the remaining 65 patients, including 18 of 37 (49% [32%-65%]) with hematologic malignancies and 3 of 28 (11% [0%-22%]) with solid tumors (Fig. 2).
PREDICTORS OF HCVr
Compared with patients without HCVr, patients with HCVr were more likely to have hematologic malignancies (78% versus 42%; P = 0.002), particularly lymphoma (50% versus 22%; P = 0.05); have lower baseline HCV RNA (median [IQR] = 5.9 [5.3-6.2] versus 6.6 [6.1-6.9] log10 IU/mL; P < 0.0001); have less frequent advanced liver disease by liver biopsy (15% versus 48%; P = 0.02); and have received rituximab (44% versus 9%; P < 0.0001), high-dose steroids (57% versus 21%, P = 0.001), bendamustine (22% versus 0%; P < 0.001), or purine analogs (22% versus 5%; P = 0.02) (Table 4). Patients with HCVr also had a lower lymphocyte nadir (median [IQR], 260 [30-530] versus 530 [210-1110] cells/μL; P = 0.03) and more prolonged lymphopenia (95 [7-200] versus 22 [0-79] days; P = 0.01) after cancer treatment (Table 4). On multivariable analysis, only rituximab (odds ratio = 9.52; 95% confidence interval = 2.19-49.12; P = 0.001) and high-dose steroids (odds ratio = 5.05; 95% confidence interval = 1.40-20.23; P = 0.01) were significantly associated with HCVr (Table 5).
| Characteristic | No. (%) of Patients With HCVr (n = 23) | No. (%) of Patients Without HCVr (n = 77) | P |
|---|---|---|---|
| Age at initiation of cancer treatment in years, median (IQR), years | 58 (54-63) | 59 (54-63) | 0.88 |
| Male sex | 18 (78) | 55 (71) | 0.51 |
| White | 16 (70) | 45 (59) | 0.46 |
| Hematologic cancer | 18 (78) | 32 (42) | 0.002 |
| Lymphoma | 9/18 (50) | 7/32 (22) | 0.005 |
| HCV | 0.56 | ||
| Genotype 1 | 17 (74) | 61 (80) | |
| Non–genotype 1 | 6 (26) | 15 (20) | |
| rs12979860a | 0.21 | ||
| Genotype CC | 7/17 (41) | 13/54 (24) | |
| Non–genotype CC | 10/17 (59) | 41/54 (76) | |
| HBcAb-positive | 5/23 (22) | 20/74 (27) | 0.61 |
| HBV DNA detected | 0/4 | 0/18 | |
| Cirrhosis at enrollment | 3 (13) | 10 (13) | >0.99 |
| Liver biopsy, Metavir score F3-F4 | 2/13 (15%) | 13/25 (52%) | 0.02 |
| Chemotherapy | 23 (100) | 72 (94) | 0.58 |
| Radiotherapy | 2 (9) | 17 (22) | 0.22 |
| Hematopoietic cell transplantation | 3 (13) | 6 (8) | 0.42 |
| Drugs received | |||
| Rituximab | 10 (44) | 7 (9) | <0.0001 |
| Rituximab cumulative dose, mg, median (IQR) | 3894 (2987-5068) | 2950 (1850-4584) | 0.48 |
| High-dose steroid | 13 (57) | 16 (21) | 0.001 |
| Steroid cumulative dose, mg, median (IQR) | 850 (250-1425) | 488 (300-700) | 0.10 |
| Alkylating agentb | 9 (39) | 20 (26) | 0.22 |
| Cyclophosphamide or ifosfamide | 3 (13) | 14 (18) | 0.75 |
| Melphalan | 3 (13) | 9 (12) | >0.99 |
| Bendamustine | 5 (22) | 0 | <0.0001 |
| Busulfan | 2 (9) | 2 (3) | 0.23 |
| Platinumc | 3 (13) | 29 (38) | 0.02 |
| Antimetabolite | 11 (48) | 28 (36) | 0.38 |
| Pyrimidine compoundd | 9 (39) | 23 (30) | 0.40 |
| Purine analoge | 5 (22) | 4 (5) | 0.02 |
| Folate antagonistf | 2 (9) | 5 (7) | 0.65 |
| Anthracycling | 4 (17) | 9 (12) | 0.48 |
| Topoisomeraseh | 2 (9) | 6 (8) | >0.99 |
| Mitotic inhibitori | 3 (13) | 17 (22) | 0.55 |
| Monoclonal antibodyj | 7 (30) | 15 (20) | 0.26 |
| Immunosuppressant (tacrolimus or cyclosporine) | 1 (4) | 1 (1) | 0.40 |
| Cytidine analogue (azacitidine or decitabine) | 1 (4) | 4 (5) | >0.99 |
| Immunomodulatory drugk | 3 (13) | 4 (5) | 0.19 |
| Tamoxifen | 0 | 4 (5) | 0.57 |
| Baseline HCV-RNA, log10 IU/mL, median (IQR) | 5.9 (5.3-6.2) | 6.6 (6.1-6.9) | <0.0001 |
| ALT, IU/mL | |||
| Baseline, median (IQR) | 71 (53-88) | 52 (32-69) | 0.04 |
| Peak, median (IQR) | 152 (111-395) | 74 (52-126) | <0.0001 |
| Lymphopenia | 21 (91) | 49 (64) | 0.01 |
| Nadir lymphocyte count, cells/μL, median (IQR) | 260 (30-530) | 530 (210-1110) | 0.03 |
| Duration of lymphopenia, days, median (IQR) | 95 (7-200) | 22 (0-79) | 0.01 |
| Hepatitis flare | 10 (43) | 12 (16) | 0.006 |
| Cancer treatment discontinuation or dose reduction due to hepatitis flare | 6 (26) | 8 (10) | 0.08 |
| Liver failure | 0 | 2 (3) | >0.99 |
| Death within 36 weeks of peak HCV-RNAl | 3 (13) | 5 (6) | 0.38 |
- a Formerly known as interleukin-28B.
- b Cyclophosphamide, ifosfamide, melphalan, bendamustine, or busulfan.
- c Cisplatin, carboplatin, or oxaliplatin.
- d Fluorouracil, capecitabine, cytarabine, gemcitabine, or decitabine.
- e Mercaptopurine, fludarabine, cladribine, or clofarabine.
- f Methotrexate or pemetrexed.
- g Daunorubicin, doxorubicin, epirubicin, idarubicin, or bleomycin.
- h Topotecan, etoposide, or irinotecan.
- i Paclitaxel, docetaxel, vinblastine, or vincristine.
- j Cetuximab, ruxolitinib, trastuzumab, bortezomib, carfilzomib, bevacizumab, dasatinib, ipilimumab, dabrafenib, trametinib, vismodegib, alemtuzumab, ofatumumab, pazopanib, or regorafenib.
- k Thalidomide, lenalidomide, or pomalidomide.
- l Cause of death was progressive cancer (n = 4), bacterial/fungal pneumonia and progressive disease (n = 4), and unknown (n = 4).
- Abbreviations: HbcAb, hepatitis B core antibody; HbsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HCVr, HCV reactivation; IQR, interquartile range.
| Potential Predictor | OR (95% CI) | P |
|---|---|---|
| High-dose steroids (>600 mg equivalent prednisone versus <600 mg equivalent) | 5.05 (1.40-20.23) | 0.01 |
| Rituximab use (yes versus no) | 9.52 (2.19-49.12) | 0.001 |
| Baseline HCV-RNA (>6 log10 IU/mL versus <6 log10 IU/mL) | 0.12 (0.03-0.46) | <0.001 |
- All variables with P < 0.2 in univariate analysis were included in the model (steroid use, rituximab use, platinum use, purine compound use, bendamustine use, type of malignancy [solid versus hematologic], baseline HCV-RNA level, nadir of lymphopenia, and duration of lymphopenia). Liver fibrosis stage by liver biopsy was not included because only 38 patients underwent liver biopsies.
- Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio.
FACTORS ASSOCIATED WITH HEPATITIS FLARE IN PATIENTS WITH HCVr
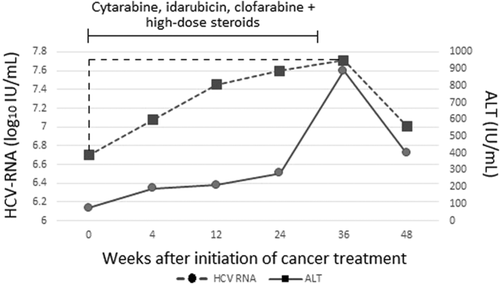
Ten (43%) of the 23 patients with HCVr also had hepatitis flare (Fig. 1). None of these 10 patients was hepatitis B surface antigen–positive. Two patients were positive for hepatitis B core antibody, but both of these patients had undetectable HBV DNA. All 10 patients were also negative for human immunodeficiency virus, immunoglobulin M antibodies to hepatitis A and hepatitis E, and cytomegalovirus DNA and herpes simplex virus DNA. None of these 10 patients had other causes of hepatitis flare, such as a new malignancy infiltrating the liver, recent history of sepsis or parenteral nutrition, sinusoidal obstruction syndrome, or graft-versus-host disease. Finally one patient received radiotherapy for gastroesophageal junction adenocarcinoma that might have caused radiation-induced hepatic injury, however ALT decreased rapidly after decreasing dose of chemotherapy thus making the diagnosis of radiation-induced hepatic injury less likely. Compared with the 13 patients without hepatitis flare, the 10 patients with hepatitis flare were more likely to have HCV genotype 2 infection (40% versus 0%, P = 0.02), rs12979860 genotype CC (63% versus 22%; P = 0.15) and higher baseline ALT (median [IQR], 87 [71-120] versus 56 [29-75] IU/mL; P = 0.004) and were less likely to be black (0% versus 38%; P = 0.04) (Supporting Table S1). Representative changes in ALT and HCV-RNA in a patient with HCVr and hepatitis flare are shown in Figure 3.
CORRELATION BETWEEN CHANGES IN HCV-RNA AND ALT
Changes in HCV-RNA and ALT were moderately correlated (r = 0.47; P < 0.0001) (Supporting Fig. S1). Compared with patients without HCVr, patients with HCVr were more likely to have hepatitis flare (43% versus 16%; P = 0.006), higher peak ALT values (median [IQR] = 152 [111-395] versus 74 [52-126] IU/mL; P < 0.0001), and greater increases in median ALT from baseline (78 [62-263] versus 22 [9-52] IU/mL; P < 0.0001) (Table 4). In patients with HCVr, serum HCV-RNA and ALT levels decreased after stopping cancer treatment.
CLINICAL OUTCOMES
Fourteen patients (14%) required cancer treatment discontinuation and/or dose reduction due to hepatitis flare: six of 23 (26%) patients with HCVr and 8 of 77 (10%) patients without HCVr (P = 0.08). Among the 13 patients with cirrhosis, five patients (38%) had decompensation after starting cancer treatment. Two patients (2%) met the criteria for acute liver failure; but neither had HCVr. One patient had compensated cirrhosis (Child-Pugh class A) and cholangiocarcinoma without liver involvement; this patient received three cycles of gemcitabine plus cisplatin. The second patient did not have cirrhosis; this patient had myelodysplastic syndrome treated with single-agent decitabine complicated by pancreatitis and septic shock. Both patients had a decrease of HCV-RNA after the start of chemotherapy. Liver decompensation was attributed to drug-induced liver injury in the first patient and drug-induced liver injury, sepsis, and shock in the second patient.
Eight patients (8%) died within 36 weeks of starting cancer treatment, 3 of 23 (13%) patients with HCVr and 5 of 77 (6%) patients without HCVr (P = 0.38). None of the three patients with HCVr had liver failure. Two of these three patients died of progressive cancer, and one died of progressive cancer and bacterial/fungal infection.
Discussion
This prospective study examined the incidence, predictors, and clinical significance of HCVr in cancer patients receiving cancer treatment. We found that 23% of patients experienced HCVr and 43% of patients with HCVr also had hepatitis flare. The use of rituximab, high-dose steroids, bendamustine, and the purine analogs fludarabine or cladribine were associated with the development of HCVr, the risk being highest with rituximab or high-dose steroids. Unlike HBV reactivation, HCVr seemed to have an indolent course. None of the patients with HCVr had liver failure or died of a liver-related cause; however, the oncologic consequences of HCVr remain to be determined, because it can affect the cancer treatment plan.
Most previous studies defined HCVr on the basis of abnormal ALT level without HCV-RNA data.12, 20-22 Those studies left open the question of whether the increase in ALT level was related to increase in HCV replication. In contrast, in our present analysis, we included only patients who had HCV-RNA measured. The HCVr incidence of 23% in the current study was lower than the incidence of 36% in our previous retrospective study.11 However, our current and previous studies differed with respect to characteristics of the patients and tumors included and frequency of HCV-RNA monitoring. Therefore, the true incidence of HCVr remains to be confirmed.
Approximately 50% of cases of HCVr in the current study were not accompanied by a significant increase in ALT and would have been missed without prospective monitoring of HCV-RNA levels. Fortunately, HCVr appeared to have a benign hepatic course, in agreement with the result of our prior retrospective study.11 Nevertheless, larger studies are needed to confirm that HCVr does not result in liver-related outcomes. This is especially important, because among hematopoietic cell transplant recipients and liver transplant recipients, immunosuppression has been clearly documented to result in accelerated progression of chronic liver disease and severe hepatocellular injury or fibrosing cholestatic hepatitis C in a small percentage of patients.16, 23, 24
Our study also showed that HCVr necessitated discontinuation or dose reduction of potentially lifesaving chemotherapy in 26% of cases, which could negatively impact patients' oncologic outcomes. Oncologist may hesitate to enroll HCV-infected patients into clinical trials due to the potential impact on cancer treatment plan. However, with the availability of multiple direct-acting antiviral agents that are safe and can lead to virologic cure in >90% of infected cancer patients with a 8-week or 12-week courses of treatment, a case can be made to treat HCV first or simultaneous with cancer treatment particularly in patients with underlying advanced liver disease or are anticipated to receive regimens associated with HCVr such as rituximab or high-dose steroids.25, 26 Alternately, HCV-infected patients can be closely monitored and antiviral treatment initiated after HCVr is detected. Prospective studies to test these approaches are warranted. Additional studies with longer follow-up are also needed to delineate the oncologic consequences of cancer treatment modifications in response to hepatitis flare associated with HCVr.
Only the use of rituximab and high-dose steroids were significantly associated with high risk of developing HCVr. Rituximab as a single agent or in combination with steroids or other chemotherapeutic agents is the drug associated with the highest risk of HBV reactivation, in persons with chronic or past HBV infection.27 Rituximab had also been described to be an important cause of HCVr.28, 29 Rituximab-based therapy causes a profound depletion of B cells and a marked reduction of T cells, mainly CD4+ cells, and has been reported to cause reactivation of other viruses, including cytomegalovirus and herpes simplex virus.30, 31 In agreement with our previous retrospective study,11 our current study confirmed that rituximab was associated with a high incidence of HCVr, more so when used in combination with high-dose steroids. Steroids are known to cause a rapid depletion and apoptosis of circulating T cells. Previous studies revealed that steroids stimulate HCV replication in vitro,32 and there are clinical reports that patients treated with high-dose steroids had an increase in HCV-RNA levels.32, 33
Lymphopenia was associated with HCVr in univariate but not in multivariate analysis, likely due to the small sample size. Bendamustine and selected purine analogs were also associated with HCVr on univariate analysis. Bendamustine causes a decline in CD4+ lymphocyte count that lasts for many months.34 Reactivation of cytomegalovirus or HBV, but not of HCV, has been described with use of bendamustine.35, 36 Purine analogs are also well known to induce profound and prolonged lymphopenia.37 Strong CD4+ T-cell responses are associated with spontaneous HCV elimination.38, 39 In addition, CD8+ depletion amplifies HCV replication.40 HCV-infected patients receiving chemotherapy regimens with expected severe or prolonged lymphopenia might need closer observation for early detection of HCVr and hepatitis flare.
In the era of targeted therapy, our understanding of the effects of targeted agents on HCV replication is limited.41 We found episodes of HCVr in patients receiving ruxolitinib, alemtuzumab, ofatumumab, ibrutinib, and bortezomib; however, except in the patient treated with ruxolitinib, the targeted therapies were given along with rituximab or high-dose steroids, which precluded conclusions about the direct effect of targeted agents on HCV replication. Ruxolitinib is an oral Janus kinase (JAK) inhibitor. JAK-STAT signaling is essential for antiviral immunity.42 Severe HBV reactivation has been reported with ruxolitinib therapy43; however, to our knowledge, ours is the first report of HCVr after ruxolitinib.
Certain chemotherapeutic agents are known to cause drug-induced liver injury, and caution is recommended with their use in patients with preexisting liver fibrosis.44 For instance, significant elevation of serum transaminases has been reported in patients receiving anthracyclines (40%), taxanes (50%), vinca alkaloids (5%-10%), methotrexate (15%-50%), erlotinib (10%), pazopanib (50%), and ruxolitinib (25%).45 Apart from ruxolitinib, none of these drugs was found to be associated with HCVr in our study. Moreover, among the patients who had a liver biopsy, we observed a lower rate of HCVr in patients with advanced fibrosis stage, possibly because these patients were exposed to less immunosuppressive chemotherapy. This anomaly may also be related to spurious results due to the small number of patients.
All patients with HCVr who had HCV genotype 2 also had hepatitis flare. Previous studies have shown that patients with HCV genotype 2 are at higher risk of hepatitis flare than patients with other HCV genotypes.46, 47 We also found that hepatitis flare was more likely to occur in patients with HCVr having rs12979860 genotype CC. A strong association of rs12979860 genotype CC with hepatic necroinflammation and increase in ALT level has been reported,46, 48 suggesting that patients with genotype CC have a more robust immunologic response that leads to increased hepatic inflammation.48 These factors associated with hepatitis flare may be clinically significant; however, because of the small number of such patients, no definite conclusions can be drawn.
Our study has several limitations. First, patients had different types of cancer, and most patients received combinations of cancer treatment. This heterogeneity could affect the analysis of chemotherapy regimens (e.g., some regimens were only used for hematologic cancers), and limit our understanding of the impact of each agent. Second, the study was conducted at a tertiary cancer center, where many patients received multiple lines of chemotherapy and salvage chemotherapy. Therefore, our results may not be generalizable to cancer patients in the community. Third, not all patients had HCV-RNA tested per protocol, and transient increase in HCV-RNA level might have been missed, resulting in underestimation of the incidence of HCVr. This study was observational in nature; HCV-RNA was measured during the routine follow-up of patients in the HCV clinic, irrespective of the administration of cancer treatment, preventing us from studying the specific correlation between HCV-RNA levels and chemotherapy for all patients. Fourth, the duration of follow-up was short, and thus any case of late HCVr would have been missed. HBV reactivation associated with rituximab has been reported to occur up to 18 months after the end of chemotherapy.49 Fifth, having a control group of HCV-uninfected patients receiving chemotherapy would help in determining whether hepatitis flares are more common in HCV-infected patients. Sixth, 25 (25%) of patients in this study were positive for hepatitis B core antibody and were at risk of HBV reactivation as potential explanation for hepatitis flare; however, all 21 patients who had HBV DNA checked before or during chemotherapy had undetectable HBV DNA. None of the four patients without HBV DNA measured had hepatitis flare during chemotherapy, received rituximab, or underwent hematopoietic cell transplant. Finally, not all patients receiving chemotherapy at our institution were screened for HCV, and not all patients with HCV infection were referred to the HCV clinic; therefore, our study population may not be representative of all HCV-infected patients.
In conclusion, HCVr occurred in 23% of patients with chronic HCV receiving cancer treatment and most had an unremarkable clinical course. Therefore, the potential risk of developing HCVr should not be considered a contraindication for chemotherapy. Our findings should help HCV-infected cancer patients have a wide access to many types of chemotherapy, with close monitoring while receiving regimens associated with HCVr. Although hepatitis flares were observed, no patient had liver failure or died of a liver-related cause; however, hepatitis flare caused modification of cancer treatment, and such modification may have a negative effect on oncologic outcomes. Additional studies in larger patient populations with longer duration of follow-up are needed to fully delineate the incidence of HCVr, its predictors, and clinical consequences in cancer care. Given the availability of highly effective (90%-95% cure rates in most series including patients with malignancies),25 well-tolerated, and short (8-12 weeks) courses of direct-acting antiviral combination therapies, HCV treatment can be initiated before or simultaneously with chemotherapy to optimize outcomes by avoiding changes in cancer treatment plan.
Acknowledgment
We thank Stephanie Deming from the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance.




