TIMP-1 deficiency leads to lethal partial hepatic ischemia and reperfusion injury†‡
Potential conflict of interest: Nothing to report.
Supported by grants from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) R01AI057832, UCLA Academic Senate, and the Pfleger Foundation (to A.J.C.). S.D. was supported in part by a doctoral fellowship from the Fundaçγo para a Ciência e Tecnologia (FCT), Portugal.
Abstract
Hepatic ischemia and reperfusion injury (IRI) remains an important challenge in clinical orthotopic liver transplantation (OLT). Tissue inhibitor of metalloproteinase-1 (TIMP-1) is the major endogenous regulator of matrix metalloproteinase-9 (MMP-9). In this study we investigated the functional significance of TIMP-1 expression in a well-established mouse model of partial liver IRI. Compared to wildtype mice, TIMP-1−/− mice showed further impaired liver function and histological preservation after IRI. Notably, TIMP-1 deficiency led to lethal liver IRI, as over 60% of the TIMP-1−/− mice died postreperfusion, whereas all TIMP-1+/+ mice recovered and survived surgery. Lack of TIMP-1 expression was accompanied by markedly high levels of MMP-9 activity, which facilitates leukocyte transmigration across vascular barriers in hepatic IRI. Indeed, TIMP-1−/− livers were characterized by massive leukocyte infiltration and by up-regulation of proinflammatory mediators, including tumor necrosis factor alpha, interferon-gamma, and inducible nitric oxide synthase post-IRI. The inability of TIMP-1−/− mice to express TIMP-1 increased the levels of active caspase-3 and depressed the expression of Bcl-2 and the phosphorylation of Akt, emphasizing an important role for TIMP-1 expression on hepatocyte survival. Using independent parameters of regeneration, 5-bromodeoxyuridine incorporation, proliferating cell nuclear antigen expression, and histone H3 phosphorylation, we provide evidence that hepatocyte progression into S phase and mitosis was impaired in TIMP-1-deficient livers after IRI. Inhibition of the cell cycle progression by TIMP-1 deficiency was linked to depressed levels of cyclins-D1 and -E and to a disrupted c-Met signaling pathway, as evidenced by reduced phosphorylated c-Met expression and elevated c-Met ectodomain shedding postliver IRI. Conclusion: These results support a critical protective function for TIMP-1 expression on promoting survival and proliferation of liver cells and on regulating leukocyte recruitment and activation in liver IRI. (HEPATOLOGY 2012;56:1074–1085)
Hepatic ischemia/reperfusion injury (IRI) occurs during trauma, shock, orthotopic liver transplantation (OLT), and other surgical procedures where the blood supply to the liver is temporarily interrupted.1 Hepatic IR-related damage is the result of various factors that include leukocyte migration, release of cytokines, and free radicals.1, 2
Leukocytes migration across endothelial and extracellular matrix (ECM) barriers is dependent on cellular adhesion-release and focal matrix degradation mechanisms.3 Although adhesion molecules are important for the successful leukocyte transmigration by providing leukocyte attachment to the endothelium, there is a growing body of evidence suggesting that matrix metalloproteinases (MMP) are critical for facilitating leukocyte movement across vascular barriers.3 In this regard, our previous studies showed an important role for leukocyte-expressed MMP-9, or gelatinase B, as a key mediator of leukocyte transmigration leading to liver injury.4
Tissue inhibitors of metalloproteinases (TIMPs) are a family of naturally occurring inhibitors of MMPs. Alterations in the MMP-TIMP balance have been linked to pathological conditions that require disruption of the basement membrane, such as tumor invasion, angiogenesis, and wound healing.5 There are at least four identified members (TIMP 1-4) in the TIMP family, varying in tissue-specific expression and in their ability to inhibit various MMPs.6 Among the different TIMPs, TIMP-1 is of particular interest; TIMP-1 is a 28.5-kDa soluble glycoprotein known to inhibit MMP-9 with high affinity, without interacting with MMP-2, or gelatinase A (the other member of the gelatinase family), as it lacks the required C-terminal MMP-2-interacting residues.7, 8
In addition to its ability to inhibit MMP activity, TIMP-1 possesses other biological activities, such as cell growth regulation, that are just beginning to be recognized and characterized.9 The specific effects of TIMPs likely depend on the cell context and on the pathological condition. Although TIMP-1 has been detected in the plasma of patients after liver transplantation,10 and in rat liver grafts after IRI,11 its role in liver IRI, or in OLT, remains to be established. Therefore, in the present study we used mice lacking TIMP-1 to examine the significance of TIMP-1 expression in hepatic IRI.
Abbreviations
ALT, alanine transaminase; AST, aspartate transaminase; BrdU, 5-bromodeoxyuridine; ECM, extracellular matrix; IFN-γ, interferon-gamma; iNOS, inducible nitric oxide synthase; IRI, ischemia and reperfusion injury; MMP-9, matrix metalloproteinase-9; OLT, orthotopic liver transplantation; PCNA, proliferating cell nuclear antigen; TIMP-1, tissue inhibitor of metalloproteinase-1; TNF-α, tumor necrosis factor alpha.
Materials and Methods
Mice and Model of Hepatic IRI.
Male TIMP-1−/− knockout (KO) mice in the C57BL/6 background (B6.129S4-Timp1tm1Pd/J) and respective TIMP-1+/+ wildtype (WT) C57BL-6 controls were obtained from the Jackson Laboratory. Hepatic IRI was performed as described.4 Briefly, arterial and portal venous blood supplies were interrupted to the cephalad lobes of the liver for 90 minutes using an atraumatic clip and mice were sacrificed after reperfusion. The animal studies were performed according to approved guidelines by the American Association of Laboratory Animal Care.
Assessment of Liver Damage.
Serum alanine transaminase (ALT) and serum aspartate transaminase (AST) levels were measured with an autoanalyzer by ANTECH Diagnostics (Los Angeles, CA), as described.4 Liver specimens were fixed with a 10% buffered formalin solution, embedded in paraffin, and processed for hematoxylin and eosin (H&E) staining; to determine the percentage of necrotic area, 10 random sections per slide were evaluated in duplicate using National Institutes of Health (NIH) Image-J.
Immunohistochemistry.
Immunostaining was performed in cryostat sections as described.4, 11 Mac-1 (M1/70) and Ly-6G (1A8), from BD Biosciences, TIMP-1 (Ab86482; Abcam), MMP-9 (AF909; R&D Systems), and cleaved-caspase-3 (ASP175; Cell Signaling) antibodies were used at optimal dilutions. Sections were blindly evaluated by counting 10 high-powered fields (HPFs)/section in triplicate. Dual/triple staining was detected by immunofluorescence with Alexa Fluor 594-red antigoat immunoglobulin G (IgG) (H+L) (Molecular Probes), and Texas Red antirat IgG (H+L) antibodies (Vector Laboratories). Alexa Fluor 488 phalloidin (Molecular Probes) and Vectashield mounting media with DAPI (Vector Laboratories) were used for F-actin and nuclear staining, respectively. Slides were analyzed using a Leica Confocal Microscope (UCLA Brain Research Institute).
Parameters of Regeneration.
Mice were injected intraperitoneally with 50 mg/kg of 5-bromodeoxyuridine (BrdU) (Sigma) 2 hours prior to liver harvest as described.12 BrdU incorporation, proliferating cell nuclear antigen (PCNA), and phosphorylated histone H3 were detected by immunohistochemistry in paraffin sections using anti-BrdU (Bu20a; Neomarkers), anti-PCNA (PC-10; Neomarkers), and anti-pH3 (Ser10; Cell Signaling) antibodies. Proliferation indexes were determined in triplicate and quantified under light microscopy by counting 10, randomly chosen, HPFs/section. Data are expressed as the percentage of BrdU, PCNA, or pH3 stained hepatocytes per total number of hepatocytes.
Myeloperoxidase (MPO) Assay.
MPO activity was evaluated in frozen tissue homogenized in an iced solution of 0.5% hexadecyltrimethyl-ammonium and 50 mmol/L of potassium phosphate buffer solution.4 After centrifugation the supernatants were mixed in a solution of hydrogen peroxide-sodium acetate and tetramethyl benzidine (Sigma). The quantity of enzyme degrading 1 μmol/L of peroxide/min at 25°C/g of tissue was defined as 1U of MPO activity.
Western Blot and Zymography Analysis.
Western blots and Zymography were performed as described.4, 11 Proteins (40 μg/sample) in sodium dodecyl sulfate (SDS)-loading buffer were electrophoresed through 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes. Membranes were incubated with specific antibodies against cleaved caspase-3 (ASP175), phospho-AKT (D9E; C31E5E), AKT (C67E7), phospho-c-Met (D26 and 130H2), c-Met (25H2) (Cell Signaling), Bcl-2 (Abcam), and cyclin D1 (BD Biosciences). After development, membranes were stripped and reblotted with antiactin antibody (Santa Cruz).
Gelatinolytic activity was detected in liver extracts (100 μg) by 10% SDS-PAGE contained 1 mg/mL of gelatin (Invitrogen) under nonreducing conditions. After incubation in development buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, and 0.02% NaN3, pH 7.5), gels were stained with Coomassie brilliant blue R-250 (Bio-Rad) and destained with methanol/acetic acid/water (20:10:70). Prestained molecular weight markers (Bio-Rad) and MMP-9 (BIOMOL International) served as standards. Relative quantities of protein were determined using a densitometer (NIH Image J software).
RNA Extraction and Reverse Transcriptase Polymerase Chain Reaction (PCR).
RNA was extracted from livers with Trizol (Life Technologies) as described.4 Reverse transcription was performed using 5 μg of total RNA in a first-strand complementary DNA (cDNA) synthesis reaction with SuperScript III RNaseH Reverse Transcriptase (Life Technologies) as recommended by the manufacturer. The cDNA product was amplified by PCR using primers specific for each target cDNA.
Data Analysis.
Data in the text and figures are expressed as mean ± standard deviation. Statistical comparisons between groups of normally distributed data were performed with Student's t test using statistical package SPSS (Chicago, IL). Kaplan-Meier analysis was used to determine statistical significance of the differences in mouse survival. P < 0.05 was considered statistically significant.
Results
Time Course of TIMP-1 Expression in Wildtype Livers Post-IRI.
TIMP-1 messenger RNA (mRNA) was almost undetectable in naive livers and it was significantly up-regulated in TIMP-1+/+ livers from 3 hours to 7 days postreperfusion (Fig. 1A). TIMP-1 protein expression was mildly detected in TIMP-1+/+ naive livers and it was markedly increased in livers after 6 hours of reperfusion, particularly at 24 hours and 48 hours post-IRI (Fig. 1B). Immunofluorescence analysis showed TIMP-1 staining in the surviving parenchyma predominantly around the portal triads of wildtype livers (Fig. 1C); TIMP-1+ staining was mostly detected in cells along hepatic sinusoids, likely hepatic stellate cells (HSCs), and in scattered hepatocytes. In vitro studies have linked TIMP-1 production to HSC and to hepatocytes.13 Conversely, TIMP-1 staining was absent in TIMP-1−/− livers after IRI (Fig. 1C).
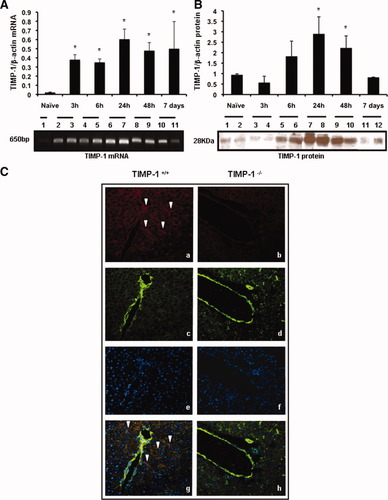
Time course of TIMP-1 expression in TIMP-1+/+ livers post-IRI. TIMP-1 mRNA expression (A) was almost absent in naive WT livers (lane 1) and it was significantly up-regulated at 3 hours (lanes 2 and 3), 6 hours (lanes 4 and 5), 24 hours (lanes 6 and 7), 48 hours (lanes 8 and 9), and 7 days (lanes 10 and 11) after liver IRI. TIMP-1 protein (B) was mildly expressed in naive WT livers (lanes 1 and 2) and in livers at 3 hours (lanes 3 and 4) and 7 days (lanes 10 and 11) post-IRI; however, it was markedly increased in livers at 6 hours (lanes 5 and 6), 24 hours (lanes 7 and 8), and 48 hours (lanes 9 and 10) after IRI. (C) Representative immunofluorescence staining in TIMP-1+/+ (a,c,e,g) and TIMP-1−/− (b,d,f,h) livers at 6 hours post-IRI; TIMP-1 in red (a,b; Alexa Fluor 594), F-actin in green (c,d; Alexa Fluor 488 phalloidin), nuclear stain in blue (e,f; Dapi), and staining overlay (g,h); TIMP-1-positive staining was mostly detected in the surviving parenchyma surrounding the vasculature of WT livers post-IRI, whereas TIMP-1 staining was undetectable in the TIMP-1−/− livers (arrows denote TIMP-1 staining; n = 4/group; *P < 0.05 relative to naive livers).
TIMP-1-Deficient Mice Had Reduced Survival Rate After Hepatic IRI.
To test the significance of TIMP-1 expression in liver IRI, our experiments included TIMP-1-deficient and respective wildtype (TIMP-1+/+) control mice. The model of partial liver IRI is nonlethal14; regardless of the significant liver damage detected during the first few days of hepatic IRI, virtually every animal survives after reperfusion. Notably, TIMP-1 deficiency resulted in an unanticipated reduced survival rate post-IRI (37% versus 100%; P < 0.05). Only three out of the eight TIMP-1−/− mice survived after reperfusion, whereas all eight TIMP-1+/+ WT animals recovered from injury and survived up to 7 days post-IRI (Fig. 2). TIMP-1−/− mice failed to recover from the injury and succumbed between the second and fourth day post-IRI. Therefore, these results indicate an important role for TIMP-1 expression in hepatic IRI.
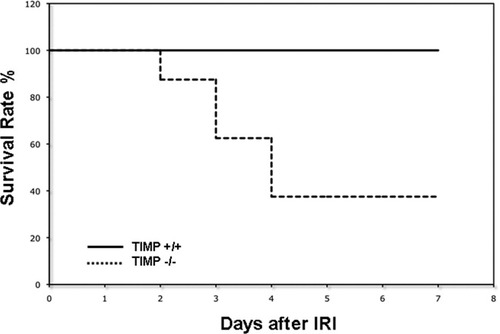
Mouse survival after liver IRI. TIMP-1−/−-deficient mice (dotted line) showed a significantly reduced survival rate at 7 days post-IRI as compared with TIMP-1+/+ mice (solid line); TIMP-1−/− animal survival was 37% versus 100% in the respective controls (n = 8/group; P < 0.05).
Liver Damage Was Increased in TIMP-1-Deficient Mice After IRI.
There were no detectable differences in liver histology and transaminase levels between naive TIMP-1−/− and naive WT mice. WT livers were characterized by significant sinusoidal congestion and extensive necrosis after reperfusion; however, TIMP-1 deficiency was associated with further lobular architecture disruption at 6 hours, 48 hours, and 7 days post-IRI (Fig. 3A). Indeed, TIMP-1−/− mice demonstrated 2 to 3-fold higher levels of hepatocellular necrosis (P < 0.05) when compared with TIMP-1+/+ mice at 48 hours post-IRI (Fig. 3B). TIMP-1−/− mice that survived surgery showed improved liver histology at 7 days post-IRI; however, levels of liver necrosis were still higher in these mice when compared to respective WT controls (Fig. 3A,B). The serum transaminase levels (U/L) were significantly increased in TIMP-1 mice at 6 hours (sAST: 30,040 ± 12,104 versus 16,033 ± 6,598, P < 0.05; sALT: 40,660 ± 21,970 versus 18,148 ± 8,727, P < 0.05), 48 hours (sAST: 3,290 ± 2,170 versus 197.75 ± 82.44, P < 0.05; sALT: 6,720 ± 5,298 versus 571.25 ± 348.9, P < 0.05), and 7 days (sAST: 1,909 ± 155 versus 1,472 ± 62, P < 0.05; sALT: 254 ± 88 versus 119 ± 42, P < 0.05) post-IRI (Fig. 3C). Altogether, these data emphasize the concept that TIMP-1 has a protective function in hepatic IRI.
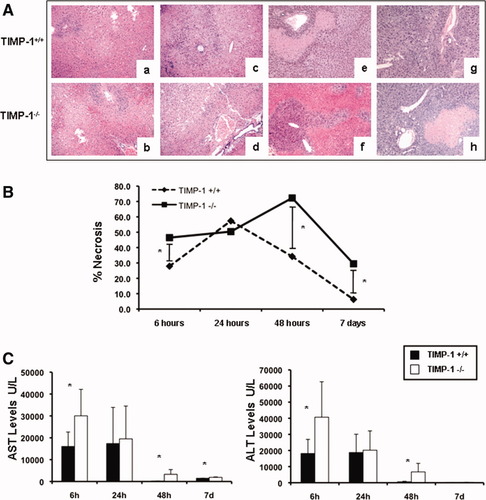
Liver histological preservation and serum transaminases in TIMP-1−/− and TIMP-1+/+ mice. Representative H&E staining (A) of TIMP-1+/+ (a,c,e,g) and TIMP-1−/− (b,d,f,h) livers at 6 hours (a,b), 24 hours (c,d), 48 hours (e,f), and 7 days (g,h) post-I/R injury; TIMP-1 deficiency was associated with further disruption of lobular architecture as compared with TIMP-1+/+ livers, particularly at 6 hours, 48 hours post-IRI, and 7 days. The percentage of hepatocellular necrosis (B) was increased 2 to 3-fold in the TIMP-1−/− livers at 48 hours after IRI. sAST and sALT levels (C) were measured in blood samples retrieved after IRI; transaminase levels were significantly increased in TIMP-1−/− mice at 6 hours, 48 hours, and 7 days post IRI as compared with respective TIMP-1+/+ controls (n = 4-6 mice/group *P < 0.05).
MMP-9 Expression and Activity Was Up-regulated in TIMP-1-Deficient Livers After IRI.
TIMP-1−/− mice showed significantly up-regulated MMP-9/β-actin mRNA expression at 6 hours (0.44 ± 0.17 versus 0.20 ± 0.11; P < 0.05), 48 hours (0.53 ± 0.15 versus 0.29 ± 0.07; P < 0.05), and 7 days (0.48 ± 0.13 versus 0.19 ± 0.14; P < 0.05) after IRI (Fig. 4A). Moreover, zymography analysis showed that MMP-9 activity was almost undetected in naive livers and highly expressed in TIMP-1−/− and WT livers post-IRI; however, MMP-9 activity was markedly up-regulated in the livers of TIMP-1−/− mice after 6 hours (P < 0.05) and 48 hours (P < 0.05) of reperfusion as compared to controls (Fig. 4B). Indeed, the MMP-9 activity increase observed in the TIMP-1−/− mice was over 4-fold that obtained in the control animals at 48 hours post-IRI (Fig. 4C). Finally, MMP-9+ leukocytes were present in significantly higher numbers in TIMP-1−/− livers at 6 hours (52 ± 3 versus 35 ± 14; P < 0.05), 48 hours (123 ± 13 versus 87 ± 12; P < 0.05), and 7 days (32 ± 4 versus 15 ± 3; P < 0.05) post-IRI (Fig. 4D,E). Thus, TIMP-1 deficiency is correlated with increased levels of MMP-9 expression/activity in hepatic IRI.
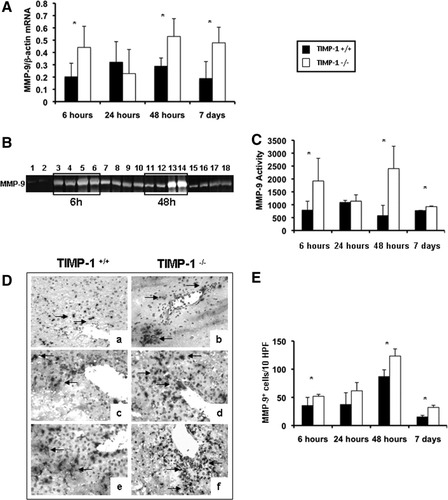
MMP-9 expression and activity in TIMP-1−/− and TIMP-1+/+ mice. MMP-9 mRNA expression (A), as detected by RT-PCR analysis, was significantly up-regulated in TIMP-1−/− mice at 6 hours, 48 hours, and 7 days after IR injury as compared to the respective WT controls. MMP-9 activity (B,C), analyzed by zymography in TIMP-1+/+ (lanes 1, 3, 4, 7, 8, 11, 12, 15, and 16) and TIMP-1−/− (lanes 2, 5, 6, 9, 10, 13, 14, 17, and 18) livers; MMP-9 activity was almost absent in naive livers of TIMP-1+/+ (lane 1) and TIMP-1−/− (lane 2) mice and highly detectable in TIMP-1+/+ and TIMP-1−/− livers at 6 hours (lanes 3-6), 24 hours (lanes 7-10), 48 hours (lanes 11-14), and 7 days (lanes 15-18) post-IRI; however, compared to controls, MMP-9 activity was markedly up-regulated in TIMP-1−/− livers at 6 hours, 48 hours, and 7 days after IRI. MMP-9+ cells (D,E) in WT controls (a,c,e) and TIMP-1−/− livers (b,d,f) at 6 hours (a,b), 24 hours (c,d), and 48 hours (e,f) post-IRI; MMP-9+ cells were detected in significantly higher numbers in TIMP-1−/− livers, particularly at 6 hours, 48 hours, and 7 days postreperfusion (n = 4-5/group; *P < 0.05).
Deficiency in TIMP-1 Enhanced MPO Activity and Leukocyte Accumulation/Activation in Hepatic IRI.
MPO activity (U/g) was increased in TIMP-1−/− livers (9.5 ± 2.1 versus 4.7 ± 0.07; P < 0.05) at 6 hours postreperfusion as compared with TIMP-1+/+ controls (Fig. 5A). MPO activity was comparable in both TIMP-1−/− and control livers at 24 hours post-IRI. However, MPO activity in TIMP-1−/− livers increased again over controls at 48 hours (12.8 ± 4.9 versus 5.1 ± 2.6; P < 0.05) and 7 days (5.4 ± 2.0 versus 1.8 ± 0.8; P < 0.05) post-IRI (Fig. 5A). MPO activity correlated with Ly-6G+ cell numbers; Ly-6G neutrophils were increased in the absence of TIMP-1 at 6 hours (73 ± 2 versus 39 ± 10; P < 0.05), 48 hours (123 ± 13 versus 88 ± 12; P < 0.05), and 7 days (37 ± 9 versus 20 ± 8; P < 0.05) post-IRI (Fig. 5B,D). Moreover, TIMP-1 deficiency also caused a substantial increase of infiltrating Mac-1 macrophages at 6 hours (67 ± 3 versus 37 ± 10; P < 0.05), 24 hours (73 ± 2 versus 41 ± 8; P < 0.05), 48 hours (154 ± 34 versus 101 ± 15; P < 0.05), and 7 days (64 ± 19 versus 30 ± 5; P < 0.05) post-IRI (Fig. 5C,D). The extent of leukocyte infiltration correlated with proinflammatory cytokine expression; tumor necrosis factor alpha (TNF-α) (0.66 ± 0.15 versus 0.37 ± 0.28; P < 0.05), interleukin (IL)-1β (1.08 ± 0.29 versus 0.75 ± 0.24 P < 0.05), and interferon-gamma (IFN-γ) (1.08 ± 0.29 versus 0.75 ± 0.24; P < 0.05) were significantly up-regulated in TIMP-1−/− livers at 6 hours post-IRI (Fig 5E). TIMP-1−/− livers at 48 hours (IL-1β: 0.21 ± 0.04 versus 0.10 ± 0.02; P < 0.05) and 7 days (IL-1β: 0.20 ± 0.04 versus 0.14 ± 0.03 and TNF-α: 0.32 ± 0.07 versus 0.21 ± 0.04; P < 0.05) post-IRI were also characterized by significantly increased proinflammatory cytokine expression. Further, inducible nitric oxide synthase (iNOS) expression, which associates with liver injury,15 showed an ≈2.5-fold increase (P < 0.05) in 6-hour TIMP-1−/− livers. In contrast, IL-10, well known for its protective role in hepatic IRI,16 was down-regulated in TIMP-1−/− livers at 48 hours (0.26 ± 0.13 versus 0.65 ± 0.14; P < 0.05) and 7 days (0.43 ± 0.21 versus 0.82 ± 0.14; P < 0.05) post-IRI.
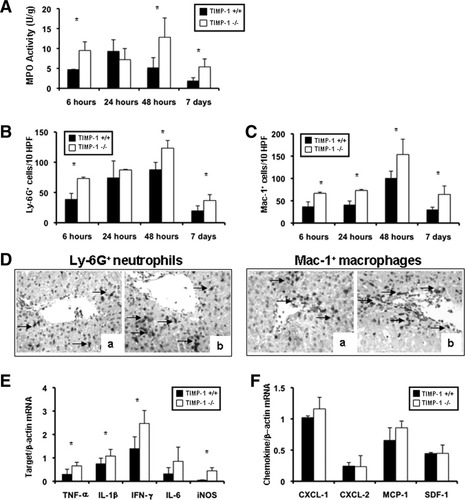
Intrahepatic MPO enzyme activity and leukocyte infiltration/activation in TIMP-1−/− and TIMP-1+/+ mice. MPO enzymatic activity (A), an index of neutrophil infiltration, was markedly up-regulated in TIMP-1−/− livers when compared to WT controls at 6 hours, 48 hours, and 7 days after IRI. Ly-6G+ neutrophil infiltration (B) was significantly increased in livers of TIMP-1−/− mice at 6 hours, 48 hours, and 7 days post-IRI. Mac-1+ macrophages (C) were detected in higher numbers in livers of TIMP-1−/− mice at 6 hours, 24 hours, 48 hours, and 7 days post-IRI. (D) Immunostaining of Ly-6G neutrophils (left) and Mac-1 macrophages (right) in TIMP-1+/+ livers (a) and TIMP-1−/− (b) at 6 hours after IRI. Proinflammatory mediators (E) in TIMP-1+/+ and TIMP-1−/− livers; TNF-α, IL-1β, IFN-γ, and iNOS mRNA levels were significantly up-regulated in TIMP-1−/− deficient livers at 6 hours post-IRI as compared to respective controls. Chemokine gene evaluation (F) showed comparable expressions of CXCL-1, CXCL-2, MCP-1, and SDF-1 in TIMP-1+/+ and TIMP-1−/− livers after reperfusion (n = 4-5/group; *P < 0.05).
TIMP-1 Deficiency Did Not Alter the Expression of Major Chemokines in Hepatic IRI.
To determine whether TIMP-1 deficiency affects chemokine expression, we assessed major cell activating chemokines linked to liver IRI (Fig. 5F). CXCL-1 (1.16 ± 0.19 versus 1.02 ± 0.03) and CXCL-2 (0.24 ± 0.18 versus 0.24 ± 0.06) were comparably expressed in both TIMP-1−/− and wildtype livers at 6 hours post-IRI. Moreover, TIMP-1−/− and WT livers also expressed similar levels of MCP-1 (0.86 ± 0.11 versus 0.66 ± 0.20) and SDF-1 (0.45 ± 0.13 versus 0.45 ± 0.02) 6 hours postreperfusion. The expression levels of these chemokines were also comparable in TIMP-1−/− and WT livers at 24 hours, 48 hours, and 7 days post-IRI (data not shown).
Deficiency in TIMP-1 Impaired Liver Regeneration After IRI.
To determine whether TIMP-1 deficiency interferes with cell proliferation, the percentage of cells in S phase, the BrdU and PCNA labeling indexes, and the percentage of phosphorylated histone H3 (P-H3)-positive cells, the mitotic index (MI), were evaluated after liver IRI. BrdU (0.53 ± 0.11 versus 1.70 ± 0.13; P < 0.05), PCNA (0.51 ± 0.46 versus 5.02 ± 2.98; P < 0.05), and MI (0.50 ± 0.46 versus 2.96 ± 1.67) indexes were modestly detected at 24 hours post-IRI, with decreased proliferation indexes in the TIMP-1−/− livers when compared to controls. Although BrdU (0.92 ± 0.11 versus 6.46 ± 0.24; P < 0.05), PCNA (2.65 ± 0.33 versus 26.96 ± 2.74; P < 0.05), and MI (1.87 ± 1.71 versus 10.74 ± 1.82; P < 0.05) indexes were still almost negligible in TIMP-1−/− livers at 48 hours post-IRI, they were significantly increased in TIMP-1+/+ controls (Fig. 6A-C). Several TIMP-1−/− animals died between the second and fourth day post-IRI; nonetheless, TIMP-1−/− mice that survived surgery exhibited some evidence of delayed liver regeneration, as the MI (7.16 ± 2.47 versus 3.39 ± 1.17) was enhanced in these animals at 7 days post-IRI. Moreover, cyclin D1, a regulator of the G1-to-S phase transition,17 and cyclin E, also necessary for entry into S phase,18 were down-regulated at mRNA levels in TIMP-1−/− livers (cyclin D1: 0.21 ± 0.04 versus 0.53 ± 0.11; P < 0.05; cyclin E: 0.44 ± 0.32 versus 1.18 ± 0.42; P < 0.05) at 48 hours post-reperfusion (Fig 6D). Cyclin D1 was almost absent in TIMP-1−/− livers at the protein level (0.20 ± 0.26 versus 1.19 ± 0.25; P < 0.05), contrasting with an almost 6-fold increased expression detected in WT livers at 48 hours post-IRI (Fig. 6E). c-Met-HGF interactions result in c-Met phosphorylation, which is the central stimulus for the G1-S progression of hepatocytes.19 The inability of TIMP-1−/− mice to express TIMP-1 led to markedly decreased HGF/c-Met signaling, as evidenced by the markedly reduced levels of phosphorylated c-Met (0.05 ± 0.07 versus 0.35 ± 0.20; P < 0.05) in their livers at 48 hours post-IRI (Fig. 7A). Further, c-Met ectodomain shedding, a process by which proteins are proteolytically released from the cell surface, negatively regulates c-Met signaling.20 In our settings, the absence of TIMP-1 resulted in significantly enhanced c-Met ectodomain shedding in liver IRI (Fig. 7B). Therefore, these results evidence that loss of TIMP-1 interferes with liver regeneration after IRI.
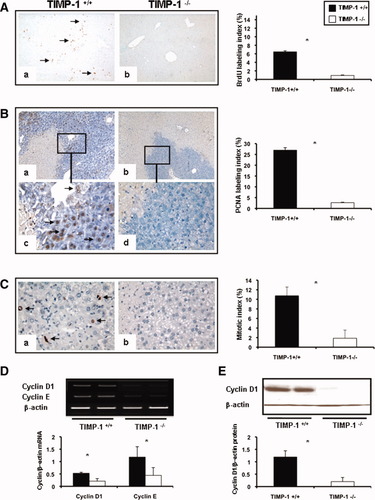
Expression of hepatic regenerative markers in TIMP-1−/− and TIMP-1+/+ mice. Hepatocyte BrdU incorporation (A), PCNA labeling (B), and phosphorylated histone H3-positive cells (C) in TIMP-1+/+ (a,c) and TIMP-1−/− (b,d) livers at 48 hours post-IRI; PCNA staining (c,d) is shown at higher magnification to better illustrate positive (c) and virtually negative (d) PCNA hepatocyte-labeling in the surviving parenchyma of TIMP-1+/+ and TIMP-1−/− livers, respectively. TIMP-1−/− livers showed markedly diminished BrdU, PCNA, and mitotic labeling indexes as compared to controls. The densitometric ratios of cyclin D1/β-actin and cyclin E/β-actin mRNA (D) were significantly depressed in TIMP-1−/− livers at 48 hours post-IRI. Cyclin D1 at the protein level (E) was also profoundly depressed in TIMP-1−/− livers at 48 hours post-IRI (n = 4-5/group; *P < 0.05).
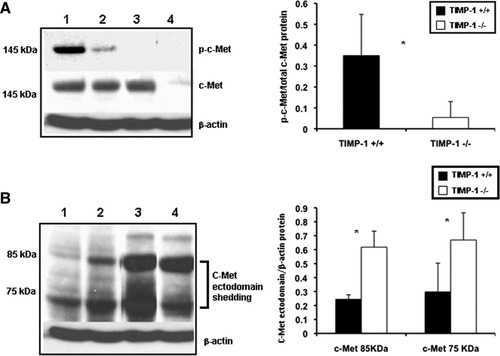
cMet phosphorylation and c-Met ectodomain shedding in TIMP-1−/− and TIMP-1+/+ livers. c-Met, the high-affinity tyrosine kinase receptor for hepatocyte growth factor, was readily phosphorylated in TIMP-1+/+ WT livers (lanes 1 and 2), contrasting with the almost lack of c-Met phosphorylation detected in TIMP-1−/− livers (lanes 3 and 4) post-IRI; the densitometric phospho-c-Met/c-Met ratio was decreased several-fold in the TIMP-1−/− livers at 48 hours post-IRI as compared to respective WT controls. Moreover, the c-Met ectodomain fragments 85 kDa and 75 kDa (B) were particularly elevated in TIMP-1−/− livers (lanes 3 and 4) at 48 hours post-IRI when compared with respective matched WT controls (lanes 1 and 2), (n = 4-5/group; *P < 0.05).
Lack of TIMP-1 Exacerbates Caspase-3 Activation in Liver IRI.
Caspase-3 is expressed in tissues as an inactive 32-kDa precursor, which is cleaved to generate a 17-kDa mature active form during apoptosis.21 The active caspase-3 was absent in naive livers and increased in TIMP-1−/− and WT livers at 6 hours postreperfusion; however, 17 kDa caspase-3 expression was significantly higher (0.55 ± 0.22 versus 0.12 ± 0.08; P < 0.05) in the livers of TIMP-1−/− mice as compared to controls. Notably, the active 17 kDa caspase-3 was particularly increased in livers of mice deficient in TIMP-1 (1.79 ± 0.24 versus 0.27 ± 0.16; P < 0.05) at 48 hours, preceding TIMP-1−/− mouse death post-IRI (Fig. 8A). Immunofluorescence analysis of cleaved-17 kDa caspase-3 indicated that, whereas the active form of capase-3 was minimally expressed in scattered cells in WT livers at 48 hours post-IRI, it was readily detected in the TIMP-1-deficient livers in the still surviving tissue areas (Fig. 8B,C). Moreover, Bcl-2, a known inhibitor of cell death, was almost absent in the TIMP-1−/− livers at 48 hours post-IRI (0.13 ± 0.08 versus 0.69 ± 0.19; P < 0.05) (Fig. 8A). Finally, phosphorylation of Akt, a 57-kD protein-serine/threonine kinase with prosurvival-associated functions,22 was depressed in TIMP-1−/− livers (0.10 ± 0.07 versus 0.44 ± 0.30; P < 0.05) at 48 hours post-IRI (Fig. 8A). At 7 days post-IRI, Bcl-2 was still reduced (≈0.6-fold; P < 0.05) in TIMP-1−/− livers compared to controls. Hence, these results support a major protective role for TIMP-1 expression in hepatic IRI.
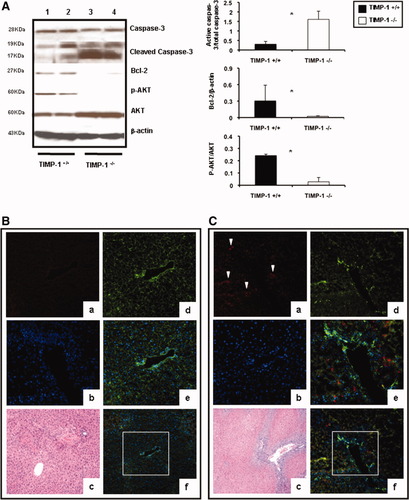
Apoptotic and prosurvival markers in livers of TIMP-1−/− and TIMP-1+/+ mice. Caspase-3, Bcl2, and Akt expressions (A) in WT (lanes 1 and 2) and TIMP-1−/− (lanes 3 and 4) livers at 48 hours post-IRI; the densitometric ratio of active caspase-3/total caspase-3 was significantly increased in TIMP-1−/− livers at 48 hours post-IRI, whereas the densitometric ratios of Bcl-2/β-actin and pAkt/Akt were markedly reduced in these livers. Representative triple immunofluorescence in TIMP-1+/+ (B) and TIMP-1−/− (C) livers of cleaved caspase-3 in red (a, Alexa Fluor 594), F-actin in green (d, Alexa Fluor 488 phalloidin), nuclear stain in blue (b, Dapi), and staining overlay (e,f); H&E staining (c). Active caspase-3 was predominantly detected in the still surviving tissue adjacent to the large vessels and surrounded by extensive areas of necrosis in TIMP-1−/− livers at 48 hours post-IRI (n = 4-5/group; *P < 0.05).
Discussion
The understanding of the functions of TIMPs in liver IRI has the potential to contribute to the development of novel therapeutic approaches to prevent hepatic IRI and, consequently, to improve the outcome of liver transplantation. In this study we investigated the functional significance of TIMP-1 expression in a well-established 90-minute mouse model of partial liver warm IRI.4
Interactions between ECM components and cell adhesion receptors regulate leukocyte functions; therefore, it is not unanticipated that enzymatic degradation of ECM can alter leukocyte behaviors.23 Indeed, cells employ proteolytic enzymes, particularly MMPs, to control the ECM turnover, to release growth factors, and to migrate across ECM.24 There is a growing body of evidence supporting key functions for MMP expression in the pathogenesis of liver diseases.3, 25, 26 In this regard, we have previously shown that MMP-9 regulates leukocyte recruitment and contributes to hepatic IRI.4 Although TIMP-1 can inhibit a broad range of MMPs, it is particularly potent for MMP-9.27 However, compared to MMP-9, the role of its natural inhibitor, TIMP-1, is virtually unknown in liver IRI. TIMP-1 expression is very low in naive livers and it is induced after liver IRI; however, it is still insufficient to prevent an elevated MMP activity in liver IRI.11 In the present study we show that TIMP-1 deficiency resulted in further exaggerated up-regulation of MMP-9 activity and, more strikingly, it led to a poor survival rate after reperfusion. This is particularly interesting in that the model of partial liver IRI is nonlethal.14 Indeed, all TIMP-1+/+ mice survived hepatic IRI despite the significant damage detected in the livers after reperfusion; in contrast, only three out of eight TIMP-1−/− mice survived more than 4 days after liver IRI. In general, TIMP-1−/− mice showed additional impairment of liver function and more severe lesions, which likely led to their death between the second and fourth day postreperfusion.
Although infiltrating leukocytes are recognized as mediators of hepatic IRI,3, 28 the mechanisms involved in their recruitment to sites of inflammatory stimulation in liver are still far from being understood. TIMP-1−/− livers showed massive leukocyte accumulation post-IRI. This latter feature, together with the findings that MMP-9 enzymatic activity was significantly increased in the TIMP-1−/− livers, strongly support an important regulatory role for TIMP-1 on leukocyte recruitment in hepatic IRI. Local concentrations of TIMP-1 are important for regulating MMP-9 activity in vivo,29 and TIMP-1 has also been implicated in leukocyte infiltration into the damaged brain.30 In addition to amplified leukocyte migration, TIMP-1-deficient mice showed significantly increased levels of proinflammatory mediators after liver injury. IFN-γ and iNOS, which have been linked to tissue injury, including hepatic injury,15, 31 were markedly up-regulated in the TIMP-1−/− livers post-IRI. Moreover, TNF-α, whose expression is often associated with neutrophil infiltration and liver damage,32 was also significantly increased in the TIMP-1 livers after reperfusion.
Impaired liver regeneration/repair is one of the most frequent features in acute liver failure. Adult hepatocytes, which make up to 80% of hepatic cells, are long-lived and normally do not undergo cell division; however, they maintain the ability to proliferate in response to injury.33 Using three independent parameters of regeneration (BrdU, PCNA, and MIs), we provide evidence that hepatocyte progression into S phase and mitosis was disrupted in TIMP-1-deficient mice during the first 48 hours post-IRI. Cyclins D1 and E, which are necessary for entry into S phase,17, 18 were profoundly depressed in the TIMP-1-deficient livers post-IRI. It is known that inhibition of cyclin D1 leads to growth arrest and to impaired hepatic regeneration.34 It is perhaps important to stress that the role of TIMP-1 in liver regeneration may depend on the type of injury, as TIMP-1 can negatively affect regeneration after substantial hepatic resection.35
Our results agree with previous findings indicating that TIMP-1 has a growth-promoting activity in a broad variety of cells,9, 36, 37 including in hepatocytes,38 and that TIMP-1 can stimulate the HGF/cMet pathway by inhibiting MMP-mediated c-Met shedding.39 Activation of the HGF/cMet signaling pathway requires phosphorylation of c-Met, which is needed for efficient liver regeneration.40 In our settings, the inability of TIMP-1−/− mice to express TIMP-1 led to virtually undetectable phosphorylated c-Met levels after liver reperfusion. Further, TIMP-1 deficiency resulted in increased proteolytic cMet ectodomain shedding, which may account in part for the reduced levels of phosphorylated c-Met postliver IRI; soluble c-Met shed ectodomains act as decoy receptors by interfering with HGF binding to c-Met.20 Therefore, our work strongly supports the view that TIMP-1−/− livers have an impaired capability to regenerate after IRI.
In addition to impaired liver regeneration, cell death by necrosis, apoptosis, or necroapoptosis is a prominent feature of liver IRI.14, 41 The expression of TIMP-1 was detected in the surviving parenchyma of WT mice after the ischemic insult, suggesting a potential role for TIMP-1 in conferring resistance to cell death. Indeed, TIMP-1−/−-deficient livers exhibited increased liver necrosis, particularly at 48 hours post-IRI. Moreover, caspase-3 activation, the executor of apoptosis,42 was significantly increased in TIMP-1−/− livers as compared with control littermates after IRI, and it was accompanied by a decrease in Bcl-2 expression. Although morphologic alterations of apoptosis are mostly mediated by caspases,42 Bcl-2 is an integral membrane antiapoptotic protein expressed even in healthy cells.43 In this regard, it has been reported that TIMP-1 can inhibit apoptosis in a wide variety of cell types, including stellate cells,44 B cells,45 epithelial cells,46 and mesangial cells47 through MMP-dependent and -independent mechanisms. Moreover, it has also been shown that exogenous TIMP-1 confers resistance against apoptosis in isolated endothelial cells by way of activation of the PI-3/Akt signaling pathway.48 Akt is a 57-kD protein-serine/threonine kinase with prosurvival functions.22 In our settings, lack of TIMP-1 expression resulted in almost completely depleted Akt phosphorylation, without changing total Akt protein levels, suggesting that TIMP-1 activates the Akt signaling pathway in hepatic IRI. TIMP-1 inhibition of cell death can also be mediated by way of its regulatory role on MMP enzymatic activity. The ECM proteolysis mediated by MMPs can lead to detachment of liver cells, resulting in apoptosis by a phenomenon called “anoikis.”49 Indeed, we have previously shown that MMP-9, in addition to facilitating leukocyte infiltration in livers after IRI, induces hepatocyte apoptosis after IRI.15
In summary, these studies demonstrate an important protective role for TIMP-1 expression in liver IRI. Overall, we show that TIMP-1 has relevant functions in promoting cell survival and proliferation of liver cells and on regulating leukocyte recruitment and activation in liver IRI. The inability of TIMP-1−/− mice to express TIMP-1 resulted in enhanced liver damage and in lethal hepatic IRI. Moreover, our data provide the rationale for studies, currently under development in our laboratory, aimed at efficiently overexpressing TIMP-1 in vivo as a potential therapeutic approach to improve hepatic IRI.




