Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: Analyzing patterns of progression in 285 patients†
Potential conflict of interest: Nothing to report.
Abstract
Although most cancers are considered predominantly systemic processes, this may not hold true for hepatocellular carcinoma (HCC). The literature regarding patterns of progression of HCC (local versus systemic) has been relatively sparse. Our objectives were to: (1) analyze patterns of progression in HCC patients presenting with intrahepatic disease from initial treatment until death, and (2) identify clinically relevant risk factors for the development of metastases. Over a 9-year period, 285 patients treated with transarterial locoregional therapies underwent scheduled imaging follow-up from treatment until death and were categorized by pattern of progression: (i) intrahepatic (increased tumor enhancement/size, development/progression of vascular invasion, new hepatic lesions) progression or (ii) extrahepatic (adrenal/bone/lung/lymph node) metastases. Uni/multivariate analyses assessing the risk factors for the development of metastases were performed. The median time from last scan to death was 2.4 months (interquartile range: 1.3-4.8 months). The time to development of metastases, vascular invasion, and/or new lesions was 13.8 months (confidence interval: 11.3-17.7 months). Of the 209 patients followed until death, only 50 developed extrahepatic metastases (24%). Multivariate analyses identified age <65 years (P = 0.038), alpha-fetoprotein >200 ng/mL (P = 0.04), and vascular invasion (P = 0.017) as significant predictors of metastases development. Conclusion: Knowledge of the risk factors associated with the development of metastases may help guide assessment of patient prognosis. Because 76% of patients presenting with local disease treated with locoregional therapies die without developing extrahepatic metastases, the notion of HCC as a systemic disease, as detected by imaging, may be reconsidered. (HEPATOLOGY 2011)
The incidence of hepatocellular carcinoma (HCC) is increasing worldwide.1 It is the most common primary malignancy of the liver and ranks third among cases of cancer-related mortality.2, 3 In general, cancer progression takes many forms; it may either progress in preexisting tumors or develop in new disseminated metastatic sites. In the latter scenario, the cause of death is often overwhelming inanition from systemic disease. Although most cancers (colon, breast, lung) uniformly develop into systemic processes with the burden of disseminated disease being the cause of death, HCC may be different. As opposed to these other malignancies, HCC progression is unique from an imaging and biologic pattern standpoint; it occurs most commonly locally (lesions may develop increased vascularity, grow in size, invade the portal vein, metastasize intrahepatically, and develop satellites and new nodules), or less commonly systemically (bone, adrenal, lungs).4 Furthermore, HCC is a unique condition, where the cause of death may not only be due to progressive cancer, but also cirrhosis. As a result, the competing risks of death in HCC translate into a history of disease where patients succumb to either cirrhosis or the unique pattern of cancer progression.
Although the treatment for HCC includes systemic agents, most recognize the predominantly local nature of this disease. Hence, local therapies represent the backbone of treatment strategies for HCC (resection, ablation, transplantation, embolization, radiation).5-11 One pertinent question given this treatment algorithm becomes: Is systemic progression part of the history of HCC after treatment with locoregional therapies (LRTs)? Do patients exhibit extrahepatic disease, or is this a localized malignancy that supports the rationale for local therapies? The literature regarding the imaging patterns of progression has been relatively sparse. The few studies reporting on this subject have varied substantially in their methodology, resulting in a reported incidence of HCC metastases ranging from 14%-37%.12, 13 In general, the incidence of local intrahepatic recurrence is much greater than that of distant metastases.14-16
The purpose of this study was to provide a comprehensive analysis detailing the imaging patterns of HCC progression by analyzing the incidence of intra- and extrahepatic metastases in a large cohort of patients treated with transarterial LRTs scanned on protocol at 2 to 3-month intervals until death. The findings suggest the potential to challenge the concept of HCC as a systemic disease (detectable by imaging) in patients treated with transarterial locoregional therapies and further support the rationale (and continued research/development) for local therapies. Furthermore, because patient prognosis decreases significantly once extrahepatic metastases have occurred, it is clinically relevant to identify risk factors (e.g., vascular invasion) associated with the development of extrahepatic metastases.
Abbreviations
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, 95% confidence interval; CT, computerized tomography; CTP, Child-Turcotte-Pugh; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; IQR, inter-quartile range; LRT(s), locoregional therapy(ies); MRI, magnetic resonance imaging; PET, positron emission tomography; PVT, portal venous thrombosis; UNOS, United Network for Organ Sharing; WHO, World Health Organization.
Patients and Methods
Patients treated with transarterial LRTs (chemoembolization, radioembolization) at our institution from January 2000 to December 2008 were identified from our prospectively collected imaging database. After excluding patients who underwent curative therapy (transplantation) and those with metastases at baseline, we identified 285 patients with liver-only HCC that were treated in a palliative setting with transarterial LRTs. A comprehensive imaging review by two radiologists was performed (as described below in Imaging Analysis). Our institutional criteria for LRTs include unresectable HCC (determined by transplant surgery) and bilirubin <3.0 mg/dL. This project was compliant with the Health Insurance Portability and Accountability Act and approved by the Northwestern University Institutional Review Board.
Baseline Staging and Treatment.
Diagnostic criteria for HCC included biopsy or radiographic findings as defined by guidelines.17 All patients were staged by Child-Turcotte-Pugh (CTP-liver function), United Network for Organ Sharing (UNOS-tumor size/number [T1: one lesion <2 cm, T2: one lesion 2-5 cm OR 2 or 3 lesions all ≤3 cm, T3: one lesion >5 cm OR 2 or 3 lesions with at least one >3 cm, T4a: ≥4 nodules, T4b: vascular invasion, N: nodal metastases, M: extrahepatic metastases]) and Barcelona Clinic Liver Cancer classification systems (BCLC-composite of function, tumor size/number and symptoms).17 Tumor burden was defined as the ratio of tumor:whole liver volume. Patients were categorized as having cirrhosis in the presence of irregular, nodular liver margin, varices, and/or splenomegaly. Patients were categorized as having portal hypertension if they exhibited varices, splenomegaly, or thrombocytopenia. None of the patients were deemed candidates for radiofrequency ablation (location, tumor size), surgery (location, tumor size, comorbidities, portal hypertension, liver function), or transplantation (tumor stage, vascular invasion); these considerations as well as the ultimate treatment modality were discussed at weekly HCC conference represented by medical oncology, hepatology, transplant surgery, and interventional radiology.
Imaging Analysis.
Baseline imaging scans for all 285 patients (285 initial scans) were reviewed by two board-certified radiologists (one cross-sectional imaging expert, one interventional radiologist). Following LRT, all patients were followed using contrast-enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) at 1-month and subsequently at scheduled 2 to 3-month intervals per routine standard of care. Chest CTs were obtained every 6 months.
Follow-up scans for all 285 patients (1,053 scans, mean: 3.7 follow-up scans per patient) were subsequently reviewed searching for radiographic evidence of disease progression. Using our previously published methodology, the patterns of progression were classified as intrahepatic (new liver lesions, development of portal vein thrombosis [PVT], any increase in preexisting PVT size [length, width, extent (e.g., branch to main portal trunk)] or enhancement, increased tumor size [25% increase in bidimensional measurements], any increase in enhancement of the target lesion [representing tumor growth]) or extrahepatic (new extrahepatic lesions [adrenal/bone/lung], lymph node growth to >2 cm, peritoneal carcinomatosis); identification of any of the above findings represented a disease progression endpoint.18 Patients exhibiting simultaneous intra- and extrahepatic progression were classified as extrahepatic progressors. In addition to CT/MRI, when available, bone and positron-emission tomography (PET) scans were also included in the imaging analysis (≈10% of patients). This scenario would most commonly occur in patients as part of metastatic workup prior to consideration of transplantation. Even if progression was not noted on the primary diagnostic modality (i.e., CT/MRI), results from these ancillary bone or PET scans were captured (and counted as progression endpoints) to provide the most conservative estimate of progression. In other words, a patient with a stable liver tumor but a new area of uptake on a recently obtained bone scan (from symptoms such as new bone pain) was counted as having progressed extrahepatically.
It should be noted that most clinical trials with progression endpoints (time-to-progression, progression-free survival) often discontinue imaging follow-up once progression has been documented; patients are subsequently taken off trial and only followed for survival. In this study, patient CTs and MRIs were followed even after progression on LRTs (and until death) in order to document the complete history of transarterial LRTs in HCC. This represents one of the novel concepts of the analysis.
Locoregional Treatments.
In this analysis of 285 patients, transarterial treatments received included chemoembolization or radioembolization. Chemoembolization was performed using 30 mg mitomycin, 30 mg adriamycin, and 100 mg cisplatin mixed with lipiodol, followed by transarterial embolization using 300-500 μm particles.7 Radioembolization was performed with glass microspheres with a median dose of 120 G.19, 20
Statistical Analyses.
Data were summarized using count and frequency for categorical variables. Substratification analyses by CTP and BCLC stages were also performed. Univariate (Kaplan-Meier) and multivariate analyses (Cox proportional hazards) were conducted to compare risk factors associated with the development of metastases.21, 22 The following variables were entered into the multivariate model (development of extrahepatic metastases): age, gender, PVT, Eastern Cooperative Oncology Group (ECOG) performance status, alpha-fetoprotein (AFP) >200 ng/mL, multifocal disease and tumor burden (≤25% versus 26%-100%). Factors were included in the multivariate model if P < 0.10 in the univariate analysis. Hazard ratio estimates were based on simultaneous analysis of all predicted variables.
Time-to-progression was determined using Kaplan-Meier methodology.21 Patients who died without exhibiting progression on their last imaging scan were censored as nonprogressors. P < 0.05 was considered significant. Survival analyses were performed from the date of first LRT and from the date of development of metastases.17
Results
Patient Sample
Table 1 summarizes the baseline patient demographics. The median time from last scan to death was 2.4 months (interquartile range: 1.3-4.8). Most patients were stages CTP A and BCLC B/C. Transarterial treatments included radioembolization (n = 196) and chemoembolization (n = 89).
| Total Patients N = 285 | |||||
|---|---|---|---|---|---|
| Demographics | Imaging characteristics | ||||
| Variable | Category | N (%) | Variable | Category | N (%) |
| Age (years) | <65 | 131 (46) | Portal hypertension | Present | 198 (69) |
| ≥65 | 154 (54) | Absent | 87 (31) | ||
| Ethnic group | Caucasian | 202 (72) | Ascites | Present | 48 (17) |
| Asian | 26 (9) | Absent | 237 (83) | ||
| Hispanic | 21 (7) | Cirrhosis | Present | 250 (88) | |
| African-American | 35 (12) | Absent | 35 (12) | ||
| Native-American | 1 (0) | Distribution | Solitary | 89 (31) | |
| Etiology | Alcohol | 54 (19) | Multifocal | 196 (69) | |
| Alpha-1-antitrypsin deficiency | 1 (0) | Largest index tumor size (cm) | ≤5 cm | 134 (47) | |
| Autoimmune hepatitis | 4 (1) | >5 cm | 151 (53) | ||
| Tumor burden | ≤25% | 237 (83) | |||
| Cryptogenic | 51 (18) | >25% | 48 (17) | ||
| HBV | 27 (9) | PVT | Absent | 192 (67) | |
| HBV + HCV | 3 (1) | Present | 93 (33) | ||
| HBV + hemochromatosis | 1 (0) | Laboratory values | |||
| HCV | 104 (36) | AFP (ng/mL)a | ≤200 | 168 (60) | |
| HCV + alcohol | 13 (5) | >200 | 113 (40) | ||
| HCV + hemochromatosis | 1 (0) | Bilirubin (mg/dL) | >2 | 34 (12) | |
| Hemochromatosis | 6 (2) | ≤2 | 251 (88) | ||
| Nonalcoholic steatohepatitis | 5 (2) | Stage | |||
| Primary biliary cirrhosis | 2 (1) | Child-Turcotte-Pugh | A | 149 (52) | |
| Unknown | 13 (5) | B | 130 (46) | ||
| Gender | Male | 216 (76) | C | 6 (2) | |
| Female | 69 (24) | BCLC | A | 40 (14) | |
| Method of diagnosis | Biopsy | 135 (47) | B | 73 (26) | |
| Imaging | 150 (53) | C | 166 (58) | ||
| ECOG | 0 | 156 (55) | D | 6 (2) | |
| UNOS | T1 | 4 (1) | |||
| 1 | 113 (40) | T2 | 53 (19) | ||
| T3 | 61 (21) | ||||
| 2 | 16 (5) | T4a | 74 (26) | ||
| T4b | 93 (33) | ||||
- AFP: alpha-fetoprotein, BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; PVT: portal vein thrombosis; UNOS: United Network for Organ Sharing.
- a Baseline AFP was not available in four patients.
Uni/Multivariate Analyses for Development of Extrahepatic Metastases
Uni/multivariate analyses are presented in Table 2. Age <65 (hazard ratio [HR]: 1.96, 95% confidence interval [CI]: 1.11-3.44), AFP >200 (HR: 2.47, CI: 1.37-4.46), PVT (HR: 2.82, CI: 1.34-5.94) and tumor burden >25% (HR: 2.11, CI: 0.89-4.97) were significant prognosticators of development of metastases by univariate analysis. By multivariate analysis, age (HR: 1.85, CI: 1.04-3.23), AFP >200 (HR: 1.82, CI: 1.02-3.24), and PVT (HR: 2.06, CI: 1.14-3.71) remained significant predictors.
| Univariate Analysis | Multivariate Analysisa | ||||
|---|---|---|---|---|---|
| Characteristic | Category | Hazard Ratio (CI) | P-value | Hazard Ratio (CI) | P-value |
| Age | ≥65 | 1.00 | 0.014 | 1.00 | 0.038 |
| <65 | 1.96 (1.11-3.44) | 1.85 (1.04-3.23) | |||
| Gender | Female | 1.00 | 0.691 | — | — |
| Male | 0.87 (0.44-1.75) | — | — | ||
| Ethnicity | African-American | 1.08 (0.47-2.47) | 0.855 | — | — |
| Hispanic | 0.76 (0.27-2.17) | 0.641 | — | — | |
| Asian | 0.32 (0.13-0.81) | 0.106 | — | — | |
| Caucasian | 1.00 | — | — | ||
| Hepatitis C | Negative | 1.00 | 0.163 | — | — |
| Positive | 1.47 (0.84-2.58) | — | — | ||
| Child-Turcotte-Pugh Class | A | 0.92 (0.53-1.61) | 0.920 | — | — |
| B/C | 1.00 | — | — | ||
| BCLC | A | 0.49 (0.19-1.26) | 0.14 | — | — |
| B | 0.80 (0.42-1.50) | 0.48 | — | — | |
| Cb | 1.00 | — | — | ||
| Baseline bilirubin | <2 mg/dL | 0.35 (0.14-0.87) | 0.129 | — | — |
| ≥2 mg/dL | 1.00 | — | — | ||
| Baseline albumin | >3.5 g/dL | 0.89 (0.38-2.04) | 0.764 | — | — |
| ≤3.5 g/dL | 1.00 | — | — | ||
| Ascites | Absent | 1.00 | 0.121 | — | — |
| Present | 1.71 (0.75-3.93) | — | — | ||
| PVT | Absent | 1.00 | 0.0005 | 1.00 | 0.017 |
| Present | 2.82 (1.34-5.94) | 2.06 (1.14-3.71) | |||
| ECOG | 0 | 1.00 | 0.599 | — | — |
| >0 | 1.16 (0.65-2.07) | — | — | ||
| AFP (ng/mL) | ≤200 ng/mL | 1.00 | 0.0008 | 1.00 | 0.04 |
| >200 ng/mL | 2.47 (1.37-4.46) | 1.82 (1.02-3.24) | |||
| Baseline dimension of index lesion | ≤5 cm | 1.00 | 0.422 | — | — |
| >5 cm | 1.25 (0.72-2.17) | — | — | ||
| Solitary/multifocal | Solitary | 1.00 | 0.078 | 1.00 | 0.38 |
| Multifocal | 1.77 (1.00-3.15) | 1.35 (0.69-2.63) | |||
| Tumor burden | 0-25% | 1.00 | 0.024 | 1.00 | 0.166 |
| 26-100% | 2.11 (0.89-4.97) | 1.63 (0.82 -3.23) | |||
| Treatment | Radioembolization | 1.00 | 0.8078 | — | — |
| Chemoembolization | 0.93 (0.51-1.68) | — | — | ||
- AFP: alpha-fetoprotein, BCLC: Barcelona Clinic Liver Cancer; CI: 95% confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; PVT: portal vein thrombosis.
- a Factors were included in multivariate analysis if P <.10 in univariate analysis.
- b Six BCLC D patients were included in the BCLC C group for univariate analysis.
Patterns of Progression and Metastases by Child-Turcotte-Pugh (Fig. 1)
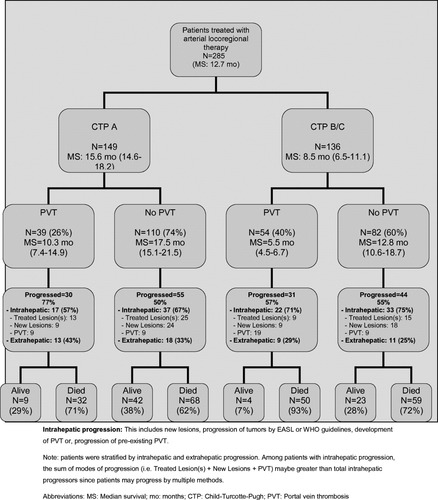
The exact incidence and pattern of metastatic disease by CTP stage and presence/absence of PVT.
CTP A. PVT was seen in 26% of CTP A patients (39/149).
Most (30/39 = 77%) of these patients progressed (intrahepatic: 17/30 = 57%; extrahepatic: 13/30 = 43%). Of the remaining 74% (110/149) without PVT, half (55/110 = 50%) progressed (intrahepatic: 37/55 = 67%, extrahepatic 18/55 = 33%). In total, 31 of 149 (21%) CTP A patients progressed extrahepatically.
CTP B/C. PVT was seen in 40% of the CTP B/C patients (54/136).
Most of these patients (31/54 = 57%) progressed (intrahepatically: 22/31 = 71%; extrahepatic: 9/31 = 29%). Of the remaining 60% (82/136) without PVT, over half (44/82 = 55%) progressed (intrahepatic: 33/44 = 75%, extrahepatic: 11/44 = 25%). In total, 20 of 136 (14.7%) CTP B/C patients progressed extrahepatically.
Patterns of Progression and Metastases by BCLC (Fig. 2)
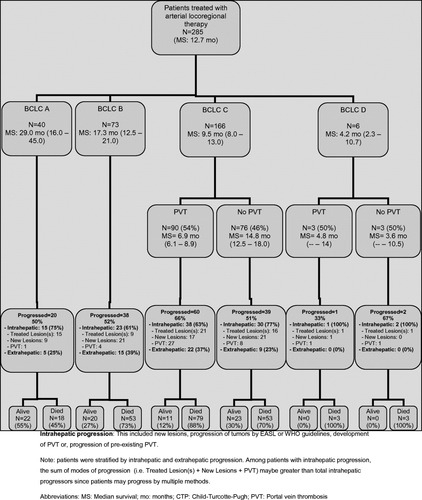
The exact incidence and pattern of metastatic disease by BCLC stage.
BCLC A.
Twenty of the 40 (50%) BCLC A patients progressed, with the majority demonstrating intrahepatic (15/20 = 75%) rather than extrahepatic progression (5/20 = 25%). In total, 5 of 40 (12.5%) BCLC A patients progressed extrahepatically.
BCLC B.
Thirty-eight of 73 (52%) BCLC B patients progressed, with the majority demonstrating intrahepatic (23/38 = 61%) rather than extrahepatic progression (15/38 = 39%). Fifteen of 73 (21%) BCLC B patients progressed extrahepatically.
BCLC C.
PVT was seen in 54% (90/166) of the BCLC C patients (90/166). Most (38/60 = 63%) of these patients progressed intrahepatically; extrahepatic progression was observed in a minority (22/60 = 37%). Of the remaining 46% (76/166) without PVT that progressed, over half (30/39 = 77%) progressed intrahepatically, whereas a minority (9/30 = 23%) progressed extrahepatically. Also of note, BCLC C patients with baseline PVT developed metastases at approximately double the rate of BCLC C patients without baseline PVT (22/90 = 24% versus 9/76 = 12%, respectively). This occurred despite significantly shorter median survival times—6.9 months for BCLC C patients with PVT versus 14.8 months for BCLC C patients without PVT. In total, 31 of 166 (19%) BCLC C patients progressed extrahepatically.
Location of Metastases.
Fifty-one (18%) patients exhibited extrahepatic disease at any time during their imaging follow-up. The patterns of metastases were: 30 (11%) lymph nodes/peritoneum, 38 (7%) lung, and five (2%) bone (patients may exhibit >1 mode of progression).
Patterns and Incidence of Progression.
Table 3 summarizes the incidence of extrahepatic metastases. Of the 285 patients, 209 had died by closure of the database. Of these, 50 (24%) developed extrahepatic disease at the time of death. Table 3 also summarizes the time-to-progression stratified by pattern (PVT, new lesion, extrahepatic metastases).
| Patterns of HCC Metastases in 209 Patients Followed to Death | ||||
|---|---|---|---|---|
| Characteristic | Child-Turcotte-Pugh N (%) | BCLC N (%) | ||
| Did not develop metastases prior to death | Total | 159 (76) | Total | 159 (76) |
| A | 70 (44) | A | 13 (8) | |
| B | 83 (52) | B | 38 (24) | |
| C | 6 (4) | C (N = 102) D (N = 6) | 108 (68) | |
| Developed metastases prior to death | Total | 50 (24) | Total | 50 (24) |
| A | 30 (60) | A | 5 (10) | |
| B | 20 (40) | B | 15 (30) | |
| C | 0 (0) | C (N = 30) D (N = 0) | 30 (60) | |
| Imaging Follow-up | Median Follow-up in Months (interquartile range) | |
|---|---|---|
| Median Time from Last Scan to Death | 2.4 (1.3-4.8) | |
| Pattern and Time-to-Progression Among 192 Patients Without Baseline PVT | ||
| Characteristic | Patients | Median TTP in Months (CI) |
| Time to metastases, PVT or new lesion | All patients (N = 192)a | 13.8 (11.3-17.7) |
| Patients that develop metastases, PVT or new lesions (N = 82) | 7.4 (6.3-10.0) | |
| Time to metastases | All patients (N = 192)a | 38.2 (34.6–) |
| Patients that develop metastases (N = 29) | 6.0 (4.4-8.3) | |
| Time to PVT | All patients (N = 192)a | Insufficient endpoints |
| Patients that develop PVT (N = 19) | 8.8 (2.8-15.1) | |
| Time to metastases or PVT | All patients (N = 192)a | 34.8 (20.2--) |
| Time to new lesion | All patients (N = 192)a | 18.2 (13.9-28.3) |
- BCLC: Barcelona Clinic Liver Cancer; CI: 95% confidence interval; PVT: portal vein thrombosis.
- a Since not all patients reach the endpoint of imaging progression, time-to-endpoint calculation was performed using Kaplan-Meier.
Survival
Table 4 reports survival outcomes. Median survival was 17.3 (15.0-21.4) months for CTP A patients who did not develop metastases and 12.0 (8.7-14.9) months for those who did (P = 0.04). Median survival was 8.8 (8.7-10.5) months for CTP B/C patients who did not develop metastases and 6.3 (3.6-8.0) months for those who did (P = 0.33) (Supporting Fig. 1). Median survival from development of metastases was 4.1 and 1.8 months in CTP A and B/C patients, respectively.
| Characteristic | Median (CI) (Months) | P-value | |||
|---|---|---|---|---|---|
| Child-Turcotte-Pugh | Based on the development of metastases | A | All patients | 15.6 (14.6-18.2) | |
| Developed extrahepatic metastases | 12.0 (8.7-14.9) | 0.004 | |||
| Did not develop extrahepatic metastases | 17.3 (15.0-21.4) | ||||
| B/C | All patients | 8.5 (6.5-11.1) | |||
| Developed extrahepatic metastases | 6.3 (3.6-8.0) | 0.33 | |||
| Did not develop extrahepatic metastases | 8.8 (8.7-10.5) | ||||
| Based on the presence/absence of baseline PVT | A | PVT present | 10.3 (7.4-14.9) | 0.001 | |
| PVT absent | 17.5 (15.1-21.5) | ||||
| B/C | PVT present | 5.5 (4.5-6.7) | <0.0001 | ||
| PVT absent | 12.8 (10.6-18.7) | ||||
| BCLC | Based on the development of metastases | A | All | 29.0 (16.0-45.0) | |
| Developed extrahepatic metastases | 11.3 (-, 31) | 0.034 | |||
| Did not develop extrahepatic metastases | 30.2 (17.5-46.0) | ||||
| B | All | 17.3 (12.5-21.0) | |||
| Developed extrahepatic metastases | 18.2 (11.2-24.3) | 0.46 | |||
| Did not develop extrahepatic metastases | 17.2 (11.3-22.0) | ||||
| Ca | All | 9.3 (7.5-12.5) | |||
| Developed extrahepatic metastases | 8.7 (7.5-14.0) | 0.116 | |||
| Did not develop extrahepatic metastases | 10.1 (9.1-14.0) | ||||
| BCLC A vs. BCLC B | BCLC A | 29.0 (16.0-45) | 0.07 | ||
| BCLC B | 17.3 (12.5-21.0) | ||||
| Based on the presence/absence of baseline PVT in BCLC Ca | PVT present | 6.9 (5.5-8.0) | 0.0002 | ||
| PVT absent | 14.7 (12.5-15.0) | ||||
- CI: 95% confidence interval; BCLC: Barcelona Clinic Liver Cancer; PVT: portal vein thrombosis.
- a Six BCLC D patients were included in the BCLC C group.
Median survival was 30.2 (17.5-46.0) months for BCLC A patients who did not develop metastases and 11.3 (-, 31.0) months for those who did (P = 0.034). Median survival was 17.2 (11.3-22.0) months for BCLC B patients who did not develop metastases and 18.2 (11.2-24.3) months for those who did (P = 0.46). Median survival was 10.1 (9.1-14.0) months for BCLC C patients who did not develop metastases and 8.7 (7.5-14.0) months for those who did (P = 0.116) (Supporting Fig. 1). Median survival from development of metastases was 4.1, 4.5, and 2.8 months in BCLC A, B, and C patients, respectively. Survival (from treatment) was significantly longer in patients who were categorized as BCLC C on the basis of ECOG rather than the presence of PVT (14.8 versus 6.9 months, P = 0.0001).
When stratified by presence/absence of baseline PVT, median survival was 10.3 months (7.4-14.9) for CTP A patients with PVT versus 17.5 months (15.1-21.5) for those without (P = 0.001). Median survival was 5.5 months (4.5-6.7) for CTP B/C patients with PVT versus 12.8 months (10.6-18.7) for those without (P < 0.001). Median survival was 6.9 months (5.5-8.0) for BCLC C patients with PVT versus 14.7 months (12.5-15.0) for those without (P = 0.0002) (Supporting Fig. 2).
Discussion
HCC is a condition where many patients present with poor prognosis, beyond potentially curative options.23 Sorafenib has been shown to extend survival in advanced disease.24 Outside of systemic therapy, treatment of metastases using LRTs is often not pursued due to the belief that in this context, local treatment will not affect overall survival.25 However, clinicians have long noted that the incidence of extrahepatic disease in HCC is low, resulting in the potentially frequent overdiagnosis of metastases from HCC. Consequently, there has been clear rationale for local therapies in the majority of patients.
The novel aspect of this study is the comprehensive imaging follow-up and progression analysis in a large cohort of patients treated with transarterial LRTs. Conservative reporting methodology and thorough imaging assessment was performed, with reporting of progression by multiple modalities.26 Although HCC is often referred to as a systemic process, it is notable that in the 209 patients followed to death following transarterial LRTs, the incidence of extrahepatic disease was 24%, with a median time from last scan to death of 2.4 months. These data lend support to the concept of HCC progression being predominantly local (intrahepatic) in patients treated with LRTs. As previously stated, the relatively few studies documenting incidence of extrahepatic metastases have produced varied rates, ranging from 14% to 37%.12, 13
A review of the literature demonstrates limited data on the pattern of HCC progression. Yang et al.25 reported that extrahepatic metastases were more common in patients with vascular invasion, intrahepatic metastases, and more advanced tumor stage. The group also divided recurrence after resection into different patterns: pattern I: intrahepatic first recurrence followed by extrahepatic metastases; pattern II: simultaneous intra/extrahepatic recurrence; and pattern III: extrahepatic disease only. The survival rate and disease-free survival rate of pattern I was greater than that of pattern II (P < 0.05). However, there was no significant difference in disease-free survival between patterns I and III or between patterns II and III. In our study the vast majority of patients demonstrated intrahepatic progression without evidence of extrahepatic progression, prohibiting comparison between groups.
In addition to studying prognostic factors (discussed below) for developing metastases, we also documented the pattern in which patients progressed. In CTP A patients, comparing those without PVT to those with PVT, the rates of overall progression were different, at 50% and 77%, respectively. Conversely, in CTP B/C patients, comparing those without PVT to those with PVT, the rate of overall progression (55% and 57%, respectively) was similar, as were percentage with intrahepatic (75% and 71%) and extrahepatic progression (25% and 29%). One factor to consider when assessing progression is the longer survival of CTP A compared to CTP B/C patients. The comparable rates of progression seen in CTP A patients in a relatively longer time versus CTP B/C patients in a relatively shorter time may be reflective of a more aggressive disease process in the latter group. Although discrepancies between progression rates of CTP A patients (±PVT) were not seen in CTP B/C patients (±PVT), it is possible that these differences may have manifest had they survived longer.
Analysis of progression by BCLC demonstrated interesting findings; 12.5% (5/40) of BCLC A patients progressed extrahepatically. In intermediate BCLC B patients, 21% (15/73) progressed extrahepatically. In BCLC C patients on the basis of ECOG, 12% (9/76) progressed extrahepatically. Finally, despite significantly shorter survival times, BCLC C patients on the basis of PVT appeared to exhibit a doubling of the incidence of extrahepatic progression (22/90 = 24%) when compared with BCLC C patients due to ECOG. These findings by BCLC classification support the observations stratified by Child-Pugh of HCC progression being predominantly local with vascular invasion representing a risk factor for extrahepatic progression. It also suggests that BCLC C patients may progress differently depending on whether the basis for advanced stage designation is ECOG 1-2 or PVT.
Substratifying prognostic factors for developing metastases allowed prognostication of patient outcomes by combining baseline and imaging characteristics.27 An analysis by Si et al.27 found that tumor grade and size were significant risk factors for metastases; however, after removing tumor grade (not always available to the clinician), their model revealed weight loss and portal vein obstruction as the only significant factors, albeit with a model with poorer fit of the data. In our cohort, multivariate analysis revealed that invasion of the portal vein was a significant predictor of development of metastases. Knowledge of this information is potentially clinically useful as it may help with assessment of patient prognosis. Multivariate analyses also demonstrated that age <65 was a significant predictor of metastases development. As with other malignancies, this result lends support to the theory of more aggressive tumor biology in younger patients. Finally, AFP >200 was also noted to result in a higher likelihood of metastases. Given the lack of accepted tumor marker treatment endpoints, we postulate that an AFP cutoff of 200 may be tested in future research as an objective treatment endpoint in HCC.
It is worthwhile to note that HCC progression may take many forms, including development of extrahepatic metastases, new intrahepatic lesions, growth of preexisting lesions, and development/growth of PVT. Factors complicating our study of HCC progression include the difficulties associated with diagnosing HCC, as well as causes of death in patients with cirrhotic livers who have concurrent new or metastatic lesions. Given that the majority of HCCs develop in patients with cirrhosis, nodular hepatic lesions may take several forms, including regenerative or neoplastic lesions.4 Furthermore, cirrhotic patients often have enlarged lymph nodes that are benign. Differentiating benign from malignant lymph node enlargement can be difficult, given that some causes of benign adenopathy (e.g., primary sclerosing cholangitis, primary biliary cirrhosis, viral hepatitis) also predispose to the development of HCC.13, 28 This is in keeping with differences in progression in other tumor types; new lymph nodes in patients with colorectal cancer are almost universally malignant. Moreover, once extrahepatic metastases develop in HCC, determining the cause of death in patients with underlying cirrhosis (and decompensated liver disease) is more challenging than in patients with other solid organ neoplasms.
Strengths and Limitations.
There are limitations to the study. First, patient selection does play a role in these findings; the observed progression patterns may be specific to transarterial LRTs. Second, because there was an interval of time from last scan to death, metastases may have developed in the interim. However, metastases are unlikely in a patient who has biologically not exhibited metastases until late in the disease, and the more advanced patients may not have survived long enough to demonstrate metastases. Third, tumor grade could not be used in the uni/multivariate analysis predicting development of metastases.27 Biopsy confirmation of metastatic disease was not possible in most cases; progression was documented by imaging as defined by HCC guidelines. Fourth, overdiagnosis of extrahepatic metastases was possible in the setting of: (1) cirrhosis-induced lymph node enlargement to >2 cm, or (2) in lesions that may have been equivocal by imaging for new HCCs. Overdiagnosis of new lesions was minimized; we used the concept of confirmatory progression when postprogression follow-up scans were available. Finally, it is possible that we underdiagnosed microscopic hematogenous disease and, because of the 6-month frequency of chest CTs, underdiagnosed lung metastases.
There are strengths to the study. First, this study had a large sample size, with mature and comprehensive follow-up (with supplementary use of PET and bone scans). Second, given the regular follow-up, a median interval time of 2.4 months from last scan to death was within reasonable limits. Such mature follow-up further supports the notion of infrequent extrahepatic metastases in HCC even late in the disease course. Third, imaging follow-up was thorough and this type of analysis is novel; patients were followed even after they had progressed on the primary LRT. Fourth, growth (expansion, elongation) of preexisting PVT was counted as progression in order to report most conservatively, even if this observation was made without other more objective evidence of progression (e.g., increased tumor size, new lesions, extrahepatic metastases). Finally, given the rapidly changing landscape and the important role systemic agents are now playing in HCC, this analysis may be the last opportunity to study outcomes and patterns of HCC progression without the confounding effect of systemic therapy.
Conclusion
Knowledge of the risk factors associated with the development of metastases (implying a dismal prognosis) may help with assessment of patient prognosis in HCC. Because most LRTs are palliative, treatment endpoints are far from clear. Identifying risk factors for the development of metastases may be the subject of future research as potential treatment endpoints. It is noteworthy that 76% of HCC patients died without developing extrahepatic disease following transarterial LRTs. The pattern of progression in HCC was most commonly intrahepatic rather than extrahepatic. Consequently, whereas certain malignancies are recognized as systemic processes (colon, breast, lung) in nearly all cases, the paradigm of HCC as a systemic disease with extrahepatic manifestation (detectable by imaging) appears to be unsupported in the majority of patients. Despite these findings, the future of HCC research should include local and systemic therapies.




