Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism†‡
Potential conflict of interest: Nothing to report.
This work was supported by the German Research Foundation (through grant SFB704 to Percy A. Knolle and Christian Kurts and through grant SFBTR57 to Percy A. Knolle, Christian Kurts, and Sören V. Siegmund), the Helmholtz Alliance (through a grant to Percy A. Knolle), the Virtual Liver Network (through a grant to Percy A. Knolle), the German National Academic Foundation (through a Ph.D. fellowship to Frank A. Schildberg), and BONFOR (through an institutional grant to Frank A. Schildberg).
Abstract
The liver has a role in T cell tolerance induction, which is mainly achieved through the functions of tolerogenic hepatic antigen-presenting cells (APCs) and regulatory T cells. Hepatic stellate cells (HSCs) are known to have various immune functions, which range from immunogenic antigen presentation to the induction of T cell apoptosis. Here we report a novel role for stellate cells in vetoing the priming of naive CD8 T cells. Murine and human HSCs and stromal cells (but not hepatocytes) prevented the activation of naive T cells by dendritic cells, artificial APCs, and phorbol 12-myristate 13-acetate/ionomycin by a cell contact–dependent mechanism. The veto function for inhibiting T cell activation was directly correlated with the activation state of HSCs and was most pronounced in HSCs from fibrotic livers. Mechanistically, high expression levels of CD54 simultaneously restricted the expression of interleukin-2 (IL-2) receptor and IL-2 in T cells, and this was responsible for the inhibitory effect because exogenous IL-2 overcame the HSC veto function. Conclusion: Our results demonstrate a novel function of HSCs in the local skewing of immune responses in the liver through the prevention of local stimulation of naive T cells. These results not only indicate a beneficial role in hepatic fibrosis, for which increased CD54 expression on HSCs could attenuate further T cell activation, but also identify IL-2 as a key cytokine in mediating local T cell immunity to overcome hepatic tolerance. (HEPATOLOGY 2011;)
Immune responses in the liver favor the induction of tolerance rather than immunity. Hepatic tolerance was initially recognized through the acceptance of liver allografts in a number of animal models1-4 and through the induction of antigen-specific tolerance after the oral or portal vein administration of antigens.5-7 Tolerogenic hepatic antigen-presenting cells (APCs) such as hepatocytes, dendritic cells (DCs), Kupffer cells, and liver sinusoidal endothelial cells (LSECs) are key to understanding the induction of hepatic T cell tolerance by major histocompatibility complex (MHC)–restricted interactions.8 However, non–MHC-restricted mechanisms also contribute to hepatic immune tolerance because LSECs veto the APC function of DCs in stimulating CD8+ T cells and thus prevent the initiation of antigen-specific T cell immunity.9
Hepatic stellate cells (HSCs) are pericytes situated in the space of Disse between LSECs and hepatocytes, and they form a sinusoidal cellular network that actively controls blood flow through the contraction of sinusoidal vessels.10 In their quiescent state, they are the main vitamin A–storing cell population of the body and contribute to the production and degradation of extracellular matrix.10 Persistent inflammation leads to the activation of HSCs and their transformation into myofibroblasts, which promote fibrosis by increasing the deposition of extracellular matrix and decreasing its degradation.11 In general, HSCs actively shape local hepatic immune regulation through the release of soluble mediators with immune function and through the promotion of lymphocyte chemotaxis and adherence.12 HSCs have potent innate immune functions13 and, by executing these innate immune functions, promote hepatic fibrogenesis.14 HSCs can contribute to the local induction of T cell immunity by antigen presentation to CD4 and CD8 T cells.15 However, these cells have also been reported to eliminate alloantigen-specific T cells during mixed lymphocyte reactions,16 to suppress DCs through interleukin-10 (IL-10),17 and to protect hepatic islet allografts from T cell–mediated rejection.18 Furthermore, they can expand regulatory T cells (Tregs) in an IL-2–dependent manner.19
Here we examine whether HSCs control the development of T cell immunity in a non–MHC-restricted fashion. We provide evidence that HSCs directly interact with T cells in a CD54-dependent fashion as a third-party inhibitory cell population.
Abbreviations
α-SMA, α-smooth muscle actin; Ad, adenovirus; APC, antigen-presenting cell; CCL4, carbon tetrachloride; CFSE, carboxyfluorescein succinimidyl ester; d, day; DC, dendritic cell; DI, division index; ELISA, enzyme-linked immunosorbent assay; GFAP, glial fibrillary acidic protein; HSC, hepatic stellate cell; HSC-CM, hepatic stellate cell–conditioned medium; IFN-γ, interferon-γ; Ig, immunoglobulin; IL, interleukin; LFA-1, lymphocyte function-associated antigen 1; LPS, lipopolysaccharide; LSEC, liver sinusoidal endothelial cell; MHC, major histocompatibility complex; MOI, multiplicity of infection; OVA, ovalbumin; NS, not significant; PCR, polymerase chain reaction; pGFP, green fluorescent protein plasmid; PMA, phorbol 12-myristate 13-acetate; TCR, T cell receptor; TGF-β, transforming growth factor β; Treg, regulatory T cell.
Materials and Methods
Mice and Materials.
All animal experiments were performed in accordance with German legislation governing animal studies and the Principles of Laboratory Animal Care guidelines (National Institutes of Health publication 85-23, 1996 revision). C57BL/6J, CD54−/−, BALB/c, and B6.C-H-2bm1 mice (bearing a point mutation in H-2Kb preventing the presentation of SIINFEKL), H-2KbSIINFEKL–restricted T cell receptor (TCR)–transgenic animals (OT-1), and H-2Kb–restricted Des-TCR mice were bred and maintained under specific pathogen-free conditions according to the guidelines of the Federation of Laboratory Animal Science Associations. Liver fibrosis was induced by intraperitoneal injections of carbon tetrachloride (CCL4; 0.5 μL/g of body weight) dissolved in an equal volume of sterile mineral oil twice per week for 6 weeks. Antibodies and reagents for flow cytometry were purchased from BD Bioscience (Heidelberg, Germany) or eBioscience (San Diego, CA). Quantitative enzyme-linked immunosorbent assays (ELISAs) were acquired from BD Biosciences. Antibodies for western blotting were purchased from Abcam (Cambridge, MA), Santa Cruz Biotechnology (Santa Cruz, CA), and Sigma (Munich, Germany).
Cell Isolation Procedures.
Primary HSCs were isolated by two-step pronase-collagenase perfusion as described previously,20 and they were cultured in Dulbecco's modified Eagle's medium/10% fetal bovine serum. Primary murine hepatocytes were isolated and cultured as described previously.21 Splenic DCs were prepared as reported previously.9 CD8+ T cells were isolated by immunomagnetic separation. Human T cells were isolated from whole blood with anti-CD8 antibody–labeled magnetic cell sorting microbeads.
T Cell Stimulation Assays.
CD8+ T cells were cocultured with splenic DCs or αCD3/28-labeled beads (Invitrogen, Karlsruhe, Germany) in the presence or absence of HSCs or the human HSC cell line LX-2 for 3 days. Cells were cocultured with a T cell/DC/HSC ratio of 40/1/4, a T cell/bead/HSC ratio of 10/4/1, or a T cell/bead/LX-2 ratio of 10/4/2.5. Proliferation was assessed with carboxyfluorescein succinimidyl ester (CFSE) dilution in Hoechst-33258− CD8+ cells by flow cytometry and quantified with FlowJo software. DCs were pulsed with grade VII ovalbumin (OVA; 1 mg/mL; Sigma) or SIINFEKL (OVA257-264; Pineda, Berlin, Germany) at 1 μM for 30 minutes. For Transwell experiments, 24-well Transwell inserts with 0.4-μm pores (Greiner, Frickenhausen, Germany) were used.
Flow Cytometry.
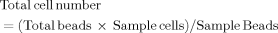 ()
()The number of surface molecules expressed per cell was quantified with QuantiBRITE PE (BD Biosciences).
Real-Time Reverse-Transcription Polymerase Chain Reaction (PCR).
RNA was extracted, transcribed into complementary DNA, and subsequently analyzed with the gene expression assay for α-smooth muscle actin (α-SMA; #Mm00725412_s1). The PCR reaction was performed with a universal PCR master mix through the amplification of 10 ng of complementary DNA for 40 cycles (95°C for 15 seconds and 60°C for 1 minute) on an ABI-Prism 7900HT. Gene expression was normalized to 18s. Reagents were obtained from Applied Biosystems (Darmstadt, Germany).
Statistical Analysis.
The results are expressed as means and standard errors of the mean. The statistical analysis was performed with an unpaired, two-tailed Student t test, and P values < 0.05 were considered significant.
Results
Murine and Human HSCs Veto the Priming of Naive CD8 T Cells.
We investigated the role of HSCs in controlling the stimulation of naive CD8 T cells by other APCs. To this end, we incubated murine HSCs isolated from mice bearing a point mutation in the H-2Kb molecule (which prevents the binding of peptides derived from the model antigen OVA) with H-2Kb–restricted, OVA-specific naive CD8 T cells (OT-1) and DCs from wild-type mice that were capable of presenting the OVA-derived peptides on H-2Kb. Coculturing antigen-presenting DCs solely with OT-1 cells resulted in strong T cell activation, proliferation, and expansion, whereas HSCs alone did not exert any stimulatory function because of their dysfunctional MHC-I molecule expression (Fig. 1A). Importantly, coculturing HSCs together with DCs and OT-1 T cells strongly impaired DC-mediated T cell activation (Fig. 1A). Antigen processing in DCs was not affected by HSCs because HSCs also prevented T cell proliferation by peptide-loaded DCs or DCs presenting endogenous peptides (Supporting Fig. 1). To investigate whether HSCs acted on DCs to impair their APC function or acted directly on T cells to prevent their activation, we replaced DCs with artificial APCs, that is, αCD3/CD28-coated microbeads that directly elicited T cell activation. HSCs also prevented the proliferation and expansion of naive T cells under these conditions (Fig. 1B), and this indicated a direct action on T cells. We confirmed the inhibitory effect on T cell proliferation with the help of the marker Ki-67, which was not up-regulated in cocultures of αCD3/CD28-stimulated T cells with HSCs (Fig. 1C). This veto function of HSCs was not restricted to a particular genetic background because HSCs from H-2d mice also impaired αCD3/CD28-induced T cell proliferation (Supporting Fig. 2). The lack of proliferation was not due to an increased rate of apoptosis because HSCs did not cause apoptotic T cell death (Fig. 1D). However, the HSC veto function was restricted to naive T cells because the stimulation of already activated T cells was not affected at all by HSCs (Fig. 1E). We extended our study to human cells with the HSC cell line LX-2. Clearly, the veto function for the inhibition of T cell activation was also valid for human HSCs in the presence of human or murine T cells (Fig. 1F and Supporting Fig. 2); this confirms that primary human HSCs also impede the TCR-driven proliferation of human naive CD8+ T cells.22 These results demonstrate the species-independent ability of HSCs to control T cell function.
HSCs prevent the proliferation of naive T cells stimulated by DCs and αCD3/CD28 beads. (A) CFSE-labeled, naive, H-2Kb–restricted OT-1 CD8+ T cells were cocultured with OVA-pulsed (1 mg/mL) H-2Kb+ splenic DCs with or without H-2bm1 HSCs. T cell proliferation was determined 3 days later by CFSE dilution. (B) T cell proliferation after stimulation with αCD3/28 beads with or without HSCs. (C) Intracellular staining for Ki-67 in T cells from panel B. Shaded areas represent unstimulated naive T cells. (D) Annexin V staining in T cells. (E) Expansion of already activated CD8+ T cells after αCD3/28 bead stimulation with or without HSCs. (F) T cell proliferation after incubation with αCD3/28 beads with or without LX-2. Abbreviations: DI, division index; NS, not significant.
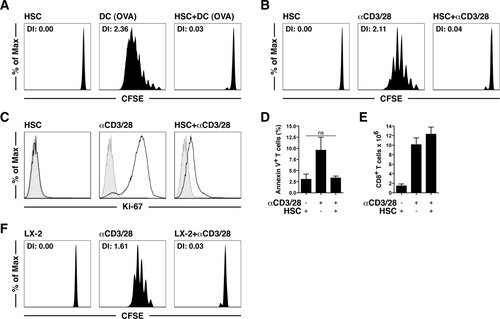
HSCs reduced the up-regulation of the activation markers CD25 und CD44 and inhibited the shedding of CD62L in αCD3/CD28-stimulated T cells (Fig. 2A). However, the activation marker CD69 was similarly up-regulated on T cells in the presence of HSCs (Fig. 2A). The release of cytokines from bead-stimulated T cells was impaired but was not completely suppressed by HSCs (Fig. 2B), and this indicated that T cells underwent an initial activation that was subsequently curtailed by the HSC veto function. Similarly, a phorbol 12-myristate 13-acetate (PMA)/ionomycin treatment, which acted downstream of TCR signaling, did not induce T cell proliferation in the presence of HSCs (Fig. 2C). PMA/ionomycin-stimulated T cells also produced less IL-2 and interferon-γ (IFN-γ) at the single-cell level in the presence of HSCs (Fig. 2D). Collectively, these results demonstrate a novel function of HSCs as inhibitory third-party cells in directly controlling T cell proliferation and cytokine expression.
Phenotypic analysis of T cells affected by the HSC veto function. (A) Naive CD8+ T cells were cultured with αCD3/28 beads in the presence (solid lines) or absence (dashed lines) of HSCs and were analyzed after 24 hours by flow cytometry. Shaded areas represent unstimulated T cells. (B) ELISA-determined expression of IL-2 and IFN-γ in the cells from panel A. (C) Proliferation of naive T cells after PMA/ionomycin (5 ng/mL, 1 μM) with or without HSCs. (D) Intracellular expression of IL-2 in the cells from panel C. ***P < 0.001. Abbreviation: DI, division index.
Specificity of the HSC Veto Function.
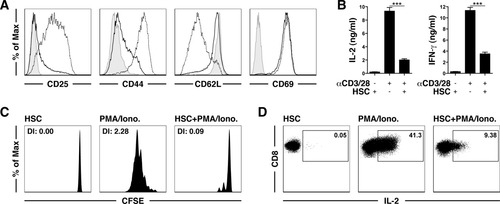
We wondered whether inhibition of T cell proliferation is a common feature of all cells in the liver, so we examined whether hepatocytes also function as third-party inhibitory cells. The murine hepatocyte cell line αML failed to control αCD3/CD28-induced T cell proliferation (Fig. 3A). Similarly, primary murine hepatocytes also did not influence T cell proliferation (Fig. 3B). Notably, primary kidney fibroblasts showed a similar veto effect for T cell proliferation (Supporting Fig. 3), and this indicates that stromal cells in different organs may fulfill similar functions. Taken together, our results reveal that the veto function is not a general feature of all liver-resident cells.
Hepatocytes do not have a third-party inhibitory function and do not influence the HSC veto function. (A) αML and (B) primary murine hepatocytes were incubated with T cells with or without αCD3/28 beads, and cell proliferation was determined. (C) Proliferation of αCD3/28 bead–stimulated T cells cultured in a Transwell assay system. (D) Proliferation of αCD3/28 bead–stimulated T cells cocultured with HSCs on a Matrigel matrix with or without αCD3/28 beads. Abbreviation: DI, division index.
Because hepatocytes influence HSC differentiation,23 we next investigated whether they modulate the inhibitory function of HSCs. To this end, we incubated hepatocytes with HSCs in a Transwell system and then investigated the regulatory HSC function. There was no attenuation of the HSC veto effect in the presence of hepatocytes (Fig. 3C). However, this pertained only to the relevance of soluble mediators because hepatocytes were separated from HSCs by the Transwell system and did not formally exclude a contribution of direct hepatocyte-HSC contact. To study whether differentiating signals from extracellular matrix might influence the veto function of HSCs, we incubated HSCs with Matrigel, which contains laminin, collagen type IV, and entactin. These environmental signals, however, did not attenuate the veto function of HSCs in αCD3/CD28-induced T cell proliferation (Fig. 3D).
The Veto Function Directly Correlates With HSC Activation.
HSCs are activated with time during in vitro culturing on plastic. This led us to investigate whether the veto function of HSCs correlates with their activation status. We were surprised to find that freshly isolated HSCs had little third-party inhibitory function in T cell proliferation (Fig. 4A). In vitro culturing over several days, however, was accompanied by an increase in the inhibitory function in T cell proliferation, which was most prominent on day 7 after isolation (Fig. 4A), and reduced cytokine release per T cell (Fig. 4B). The activation status of HSCs was confirmed by the determination of the expression of the marker α-SMA at the messenger RNA and protein levels (Fig. 4C,D). We isolated HSCs from fibrotic livers in order to formally demonstrate that HSCs act as veto cells in vivo after appropriate activation. These in vivo activated HSCs showed a strong inhibitory effect on T cell proliferation (Fig. 4E). These findings suggest that stellate cell activation is required to gain the function of third-party inhibitory cells and is operative during liver fibrosis.
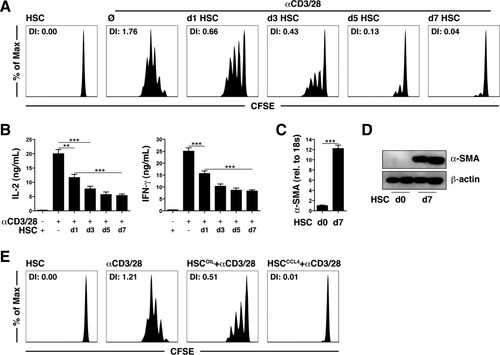
The inhibition of T cell proliferation correlates with the activation of HSCs. (A,B) T cell proliferation and cytokine expression after contact with HSCs at different times after in vitro culturing. (C,D) Relative gene expression levels of α-SMA according to real-time PCR and immunoblotting. (E) T cell proliferation after contact with HSCs isolated from the fibrotic livers of CCL4-treated mice. **P < 0.01 and ***P < 0.001. Abbreviations: d, day; DI, division index.
We next investigated whether the veto function of HSCs depends on soluble mediators or physical interactions with T cells. To this end, we incubated T cells with a conditioned medium from activated HSCs and then determined αCD3/CD28-induced T cell proliferation. Under these conditions, we did not observe any veto effect (Fig. 5A). Using a Transwell system, we found that HSCs required physical contact with T cells to exert their inhibitory effect (Fig. 5B). Also, antibody-mediated neutralization experiments showed no contribution of IL-6, IL-10, or transforming growth factor β (TGF-β) to the HSC veto effect (Supporting Fig. 4). Furthermore, HSCs needed to be viable to have veto function, and glutaraldehyde-fixed HSCs failed to have any effect on T cell proliferation (Fig. 5C). This suggests that a reciprocal interaction between HSCs and T cells is required for the veto function.
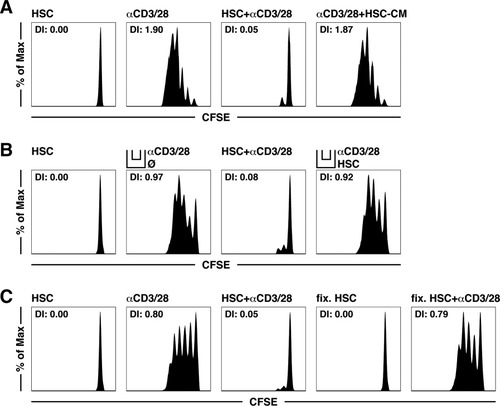
Physical contact with living HSCs is required for the inhibition of T cell proliferation. Proliferation of αCD3/28-stimulated T cells cultured (A) with a 1/1 dilution of an HSC-CM with or without αCD3/28 beads, (B) in Transwells with HSCs with or without αCD3/28 beads, or (C) with glutaraldehyde-fixed HSCs. Abbreviations: DI, division index; HSC-CM, hepatic stellate cell–conditioned medium.
CD54 Expression Is Critical for the HSC-Mediated Effector Function.
The requirement for physical interactions led us to investigate the involvement of the adhesion molecule CD54 in the inhibitory function of HSCs. CD54 is critical for mediating interactions with T cells and is dynamically regulated during these interactions.24 We observed that CD54 was up-regulated on HSCs upon contact with αCD3/CD28-stimulated T cells (Fig. 6A). To demonstrate that CD54 was involved in the veto effect, we employed HSCs from CD54 knockout animals or blocked CD54 with specific antibodies. In both situations, we observed an abrogation of the third-party inhibitory effect of HSCs on T cell proliferation (Fig. 6B) and cytokine expression (Fig. 6C). There was no difference between CD54+/+ and CD54−/− HSCs with respect to the acquisition of an activated phenotype (Fig. 6D); this confirms that CD54 expression is the critical parameter for the HSC-mediated veto function. Another adhesion molecule, CD106, which is constitutively expressed on HSCs,13 contributed in a minor way to the HSC veto effect (Supporting Fig. 5). These results raised the question whether the CD54 expression levels directly correlated with the veto function. Quantifying the absolute numbers of CD54 molecules per cell by flow cytometry with a well-established bead-based calibration method, we observed that activated HSCs on day 7 after isolation expressed twice as many CD54 molecules in comparison with freshly isolated HSCs (Fig. 6E), and this directly correlated with their veto function (Fig. 4A). As expected, primary murine hepatocytes as well as the hepatocyte cell line αML had lower CD54 expression levels on a per cell basis in comparison with primary murine HSCs (Fig. 6E), and they consequently lacked the veto function (Fig. 3A,B). To demonstrate that the CD54 expression levels were critical for third-party inhibition, we increased CD54 expression in αML by transfection. Figure 6F shows that CD54-transfected αML gained some inhibitory ability with respect to αCD3/CD28-driven T cell proliferation. This small increase in the inhibitory capacity may have been due to the relatively small increase in CD54 expression levels after transfection (Supporting Fig. 6). Taken together, these data provide evidence for a central role of high CD54 expression levels in mediating the third-party inhibitory function of HSCs.
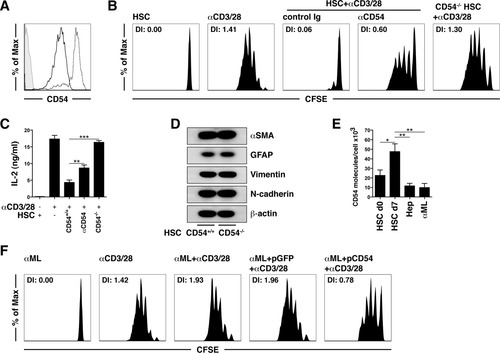
The HSC veto function is CD54-dependent. (A) CD54 expression levels of HSCs with or without αCD3/28-stimulated T cells. (B) αCD3/28-induced T cell proliferation after incubation with wild-type HSCs in the presence of αCD54 (30 μg/mL) or CD54−/− HSCs. (C) T cell cytokine expression. (D) Western blots of α-SMA, GFAP, vimentin, and N-cadherin from wild-type and CD54−/− HSCs. (E) Absolute number of CD54 molecules per cell determined by flow cytometry with QuantiBRITE PE. (F) αCD3/28-induced T cell proliferation after incubation with green fluorescent protein–transfected or CD54-transfected αML cells. *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviations: d, day; DI, division index; GFAP, glial fibrillary acidic protein; Ig, immunoglobulin; pGFP, green fluorescent protein plasmid.
The HSC Veto Function Resists Proinflammatory Stimuli but Is Reversed by Exogenous IL-2.
Next we evaluated the robustness of the HSC veto function. HSCs maintained their inhibitory function in T cell proliferation, even in the presence of the proinflammatory cytokine IFN-γ or after direct stimulation with the toll-like receptor 4 ligand lipopolysaccharide (LPS; Fig. 7A). Additionally, the infection of HSCs with an adenovirus, which has been shown to be a fairly efficient process in vitro,13 did not modify their inhibitory function (Fig. 7B). These results not only reveal that the veto function is extremely robust but also are consistent with our observation of the crucial role of CD54 because these stimuli are all known to increase the expression levels of CD54. Because the inhibition of T cell proliferation is similar to the induction of anergy by tolerogenic APCs, we tested whether IL-2, which is known to break anergy,25 could overcome the third-party inhibitory function of HSCs. Indeed, exogenous IL-2 antagonized in a dose-dependent fashion the veto function of HSCs in T cell proliferation (Fig. 7C), which seemingly acted on CD25 expressed only at low levels on αCD3/CD28-stimulated T cells in a coculture with HSCs (Fig. 2A). Mechanistically, CD54 expression on HSCs influenced the T cell expression levels of CD25 because bead-activated T cells from cocultures with CD54−/− HSCs had much higher CD25 surface expression levels than those T cells in contact with CD54-expressing HSCs (Fig. 7D). These results indicate that CD54 on third-party inhibitory cells such as HSCs prevents auto-costimulation by T cells through IL-2 by keeping CD25 expression at low levels. In Fig. 8, we illustrate the main molecular mechanisms that determine the HSC veto function.
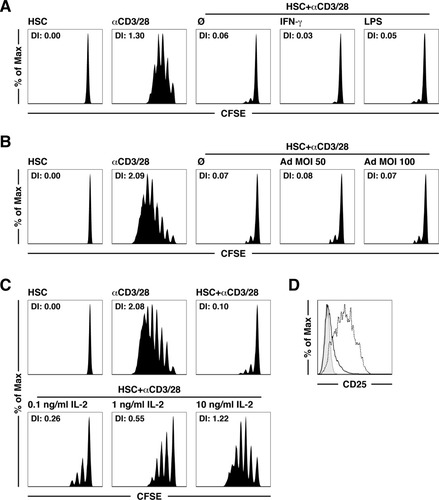
The HSC veto function resists proinflammatory stimuli but is reversed by IL-2. αCD3/28-induced T cell proliferation after incubation with HSCs (A) stimulated with IFN-γ (50 U/mL) or LPS (10 ng/mL), (B) infected with an Ad, or (C) in the presence of exogenous recombinant IL-2 (0.1-10 ng/mL). (D) CD25 expression levels of αCD3/28-stimulated T cells cultured with wild-type (solid lines) or CD54−/− HSCs (dashed lines). Unstimulated T cells served as controls (shaded area). Abbreviations: Ad, adenovirus; DI, division index; MOI, multiplicity of infection.
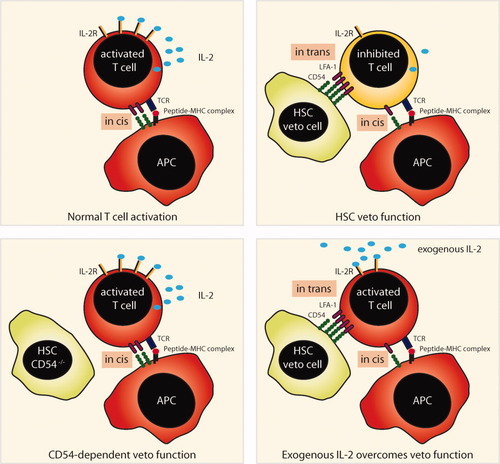
Schematic illustration of the molecular mechanisms underlying the HSC-mediated veto function inhibiting naive T cell activation.
Discussion
Many hepatic cell populations contribute to the induction of T cell tolerance rather than immunity in the liver by mechanisms such as clonal deletion, anergy induction, Treg generation/expansion, and liver DC function incapacitation.8 Here we report that HSCs employ a novel mechanism efficiently preventing immunogenic CD8 T cell priming through direct interference with T cell activation.
Earlier observations showed that HSCs inhibited allospecific T cell responses in a mixed lymphocyte culture through the B7-H1–mediated induction of T cell apoptosis16 and thus identified HSCs as gatekeepers of hepatic parenchymal tissue. Further molecular mechanisms underlying impaired CD8 T cell responses were, however, not evaluated in this study. Here we report that HSCs directly interfere with naive CD8 T cell activation with artificial APCs, that is, microbeads coated with stimulatory αCD3/CD28 antibodies. We now show that this inhibition of CD8 T cell activation depends strictly on CD54 and not on B7-H1 (not shown). CD54 is known as a potent proinflammatory molecule that mediates the adhesion of leukocytes under steady-state and inflammatory conditions.24 How can CD54 then act as a potent immune-inhibitory molecule? Several explanations are possible. First, CD54 expression on HSCs acting as third-party veto cells may lead to the redistribution of its ligand lymphocyte function-associated antigen 1 (LFA-1), which is important for the transmission of TCR signals,26 away from the TCR interacting with peptide-loaded MHC molecules on the APCs. This would ultimately lead to a failure of the T cells to become activated. This assumption is supported by the following observations: T cells undergo a weak initial stimulation, which is indicated by the up-regulation of CD69 and the release of small amounts of cytokines, and T cells ultimately are not sufficiently stimulated to enter the cell cycle or a differentiation program to become effector T cells. Second, establishing a close interaction between HSCs and T cells through CD54 may allow mediators with short-range activity to exert a regulatory function. However, we did not find evidence for the involvement of classic immune-regulatory molecules such as IL-6, IL-10, TGF-β, and retinoic acid (not shown). Yet, close physical interactions may also allow for the exchange of regulatory molecules through nanopores or exosomes, as recently described for Tregs in the suppression of DC function.27 A common feature of all these attempts to explain the immune-regulatory function of CD54 is that it is not expressed on the same cell presenting the antigen. In other words, CD54 expression in trans seems to have immune-regulatory effects, whereas CD54 expression in cis promotes the development of T cell immunity. This dichotomy can explain the apparently contradictory functions of CD54 in promoting inflammation and T cell immunity and impeding T cell activation.
The third-party veto function of HSCs portrayed here represents a novel form of immune regulation that has not been described so far. It is clearly distinct from the clonal deletion of already activated T cells reported previously for HSCs,16 and it does not depend on inhibitory molecules such as IL-10 and TGF-β. However, it bears a resemblance to T cell anergy, which is triggered by incomplete stimulation through APCs.25 The development of the HSC veto function involves initial mutual interactions with T cells stimulated by APCs. This eventually results in T cells being completely inhibited from proliferating and entering a differentiation program by mechanisms that need to be addressed in future studies. A previous study identified a function of IFN-γ in inducing B7-H1 expression, which mediates the HSC-induced protection of islet grafts from T cell–mediated rejection.28 We also observed a contribution of IFN-γ to the regulation of CD54 on HSCs, which influences subsequent veto function (data not shown), and this is consistent with a general contribution of IFN-γ to the immune-regulatory capacity of HSCs. It is important to note that the HSC veto function does not affect T cell viability. Because naive T cells constitute a sizable population in the liver29 and DCs migrate within the space of Disse toward the portal tract,30 HSCs apparently occupy a strategic position for interfering with naive T cell activation in the liver by DCs. Interestingly, HSCs do not seem to influence tolerogenic antigen presentation by LSECs in vivo because cross-presentation by LSECs results in naive CD8 T cell recruitment to the liver.31
Persistent hepatic inflammation is accompanied by the development of fibrosis; this is caused by the activation and proliferation of extracellular matrix–producing HSCs, which differentiate into myofibroblasts.11 Because this activation is followed by increased expression of CD54,32 which, as we have shown here, increases the ability of HSCs to function as third-party veto cells, it is likely that CD54 expression on HSCs results in protection from a self-amplifying feedback loop in which inflammation drives further local T cell stimulation and expansion and leads to further deterioration of local inflammation and increased fibrotic processes. Interestingly, hepatocytes do not show any veto effect on T cell activation, although they express low levels of CD54. This not only means a unique role for HSCs in the prevention of T cell stimulation but also indicates that CD54 exerts inhibitory effects only beyond a certain absolute level of expression.
Exogenous IL-2 can overcome the HSC-induced veto effect on T cell stimulation, which is similar to the effect of IL-2 in breaking T cell anergy.11 This result implies that the local release of IL-2 from memory or previously activated T cells can overcome the third-party veto effect of HSCs on T cell stimulation. In support of this notion, we observed that T cell immunity was initiated in the liver when animals were vaccinated shortly before the experiment, and this led to the hepatic accumulation of T cells capable of releasing IL-2 locally.33 Thus, the hepatic infiltration of larger numbers of activated CD4 or CD8 T cells, as observed during chronic inflammation associated with a persistent viral infection,34 may overcome local tolerogenic mechanisms in the liver because LSEC-induced tolerance is also overcome by exogenous IL-2.35
Collectively, the results presented here support the existence of a functional barrier in the liver: sinusoidal cells (i.e., HSCs and LSECs but not hepatocytes) veto the local stimulation of T cells by either directly impeding T cell activation or impairing DC function. This barrier may hinder the local induction of T cell immunity in the inflamed liver in order to prevent autoimmunity and may attenuate excessive self-amplifying T cell–mediated inflammation in fibrosis, but it leaves unaltered important innate immune functions that control the spread of infectious microorganisms.36 Interestingly, this barrier can be overcome by exogenous IL-2, which may be employed as a therapeutic principle to abrogate local tolerogenic mechanisms and thus increase T cell immunity during persistent viral infections or in patients with hepatocellular carcinoma.
Acknowledgements
The authors acknowledge support from the Central Animal Facility and the Flow Cytometry Core Facility. They thank Cristina Amparo Hagmann for her critical reading of the article.




