Noncirrhotic presinusoidal portal hypertension is common in cystic fibrosis–associated liver disease†
Potential conflict of interest: Nothing to report.
We read with great interest the article by Lewindon et al.,1 who demonstrated the importance of liver biopsies in the detection of cystic fibrosis–associated liver disease (CFLD). They demonstrated that in CF complications of portal hypertension can occur before the onset of cirrhosis as determined by liver biopsy. They could partially attribute this to sampling error, although a great effort was made to reduce sampling error by performing dual pass biopsies. However, we are not convinced that sampling error is the main issue. We believe, from Lewindon et al.1 and our own findings, that CFLD often presents as noncirrhotic portal hypertension (NCPH) in which the development of portal hypertension precedes that of cirrhosis.
In a retrospective analysis of CF patients of two centers (University Hospitals Leuven, Leuven, Belgium, and Cliniques St Luc, Université Catholique de Louvain, Brussels, Belgium), we gathered biopsies of 12 patients with portal hypertension (10 with esophageal varices, 11 with splenomegaly and thrombocytopenia, and one with previous surgical shunting procedure). Of these 12, there were only five with possible cirrhosis on biopsy. The other seven had varying degrees of fibrosis (F3 in four patients, F2 in one patient, F1 in one patient, and F0 in one patient) and therefore represent NCPH. Four of these seven were scored on liver explants and therefore sampling error certainly did not play a role.
In two of these patients, we were able to perform hemodynamic measurements that revealed a hepatic venous pressure gradient of 5 and 9 mm Hg, respectively, despite the presence of esophageal varices. This is clear proof of an important presinusoidal component in the portal hypertension consistent with NCPH.2 Using this technique, there is also no sampling error.
Portal hypertension out of proportion with the fibrosis suggests NCPH and therefore an important vascular component. Analysis of our biopsies revealed portal branch venopathy in all the patients with NCPH (most notably, absence of portal veins in more than 40% of portal tracts3) (Fig. 1). These findings were clearly more prevalent in our patients with NCPH than in a reanalyzed control group4 of 20 patients with CFLD without portal hypertension (P = 0.008) adding to the evidence of a presinusoidal vascular component.
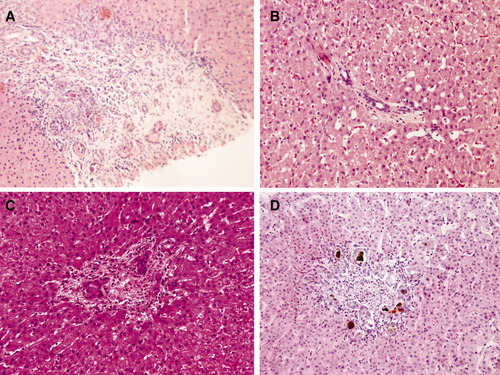
Portal branch venopathy: absence of portal veins. Hematoxylin-eosin stain of four portal tracts from different liver biopsies of patients with CF (original magnification: ×200). None of these portal tracts have demonstrable portal vein branches. Bile inspissations are present in (A; asterix) and (D; brown pigment). Note that (B) has no inflammatory infiltrate.
The development of this portal branch venopathy remains obscure. It could be due to spillover of the inflammatory infiltrate of the bile ducts (as suggested in other biliary diseases with presinusoidal portal hypertension5), due to microthrombosis (platelets are hyperactive in CF6), or due to primary endothelialitis (CF is associated with a rise in markers of vasculitis7).
Although the findings of Lewindon et al. and our findings demonstrate the importance of liver biopsies in CF, extreme care must be taken not to underestimate the degree of portal hypertension based on these biopsies. In view of the good hepatic synthetic function, management of patients with CF who have NCPH should probably seek the alleviation of this portal hypertension by shunting procedures (that is, transjugular intrahepatic portosystemic shunt) rather than referring these patients for liver transplantation. Also in that respect, performing liver biopsies and hemodynamic measurements seems indicated.
References
Peter Witters M.D., Ph.D.* **, Louis Libbrecht M.D., Ph.D.** , Tania Roskams M.D., Ph.D. , Kris De Boeck* , Lieven Dupont§§, Marijke Proesmans M.D., Ph.D.* , François Vermeulen M.D.* , Birgitta Strandvik¶¶, Anders Lindblad M.D.11, Xavier Stéphenne12, Etienne Sokal M.D., Ph.D.12, Serge Gosseye M.D.13, Sam Heye , Geert Maleux , Raymond Aerts Ph.D.§, Diethard Monbaliu M.D.§, Jacques Pirenne M.D., Ph.D.§, Ilse Hoffman*, Frederik Nevens M.D.¶ **, David Cassiman M.D., Ph.D.¶ **, * Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium, Department of Pathology, University Hospitals Leuven, Leuven, Belgium, Department of Interventional Radiology, University Hospitals Leuven, Leuven, Belgium, § Abdominal Transplant Surgery, University Hospitals Leuven, Leuven, Belgium, ¶ Department of Hepatology, University Hospitals Leuven, Leuven, Belgium, ** Liver Research Facility, Katholieke Universiteit Leuven, Leuven, Belgium, Department of Pathology, Ghent University Hospital, Ghent, Belgium, Department of Pediatrics, Cystic Fibrosis Center, University Hospitals Leuven, Leuven, Belgium, §§ Department of Pulmonology, Cystic Fibrosis Center, University Hospitals Leuven, Leuven, Belgium, ¶¶ Department of Biosciences and Nutrition, NOVUM, Karolinska Institutet, Stockholm, Sweden, 11 Cystic Fibrosis Center, Department of Pediatrics, Sahlgrenska University Hospital, Goteborg, Sweden, 12 Department of Pediatrics, Cliniques St Luc, Université Catholique de Louvain, Brussels, Belgium, 13 Department of Pathology, Cliniques St Luc, Université Catholique de Louvain, Brussels, Belgium.




