Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway†‡
Potential conflict of interest: Nothing to report.
Supported by Research Grants Council Competitive Earmarked Research Grant CUHK (GRF 473008, GRF 478108, and CUHK/CRF/09).
Abstract
The paired box 5 (PAX5) is a member of PAX transcription factors family involved in the regulation of embryonic development. However, the role of PAX5 in carcinogenesis is largely unclear. We identified that PAX5 is involved in human cancer by methylation-sensitive representational difference analysis. We examined the biological functions and related molecular mechanisms of PAX5 in hepatocellular carcinoma (HCC). Promoter methylation of PAX5 was evaluated by methylation-specific polymerase chain reaction (PCR) and bisulfite genomic sequencing (BGS). The functions of ectopic PAX5 expression were determined by viability assay, colony formation, and cell cycle analyses, along with in vivo tumorigenicity assays. The PAX5 target signal pathway was identified by promoter luciferase assay, chromosome immunoprecipitation (ChIP), and pathway PCR array. PAX5 is expressed in normal human liver tissue, but silenced or down-regulated in 83% (10/12) of HCC cell lines. The mean expression level of PAX5 was significantly lower in primary HCCs as compared to their adjacent normal tissues (P < 0.0001). The promoter methylation contributes to the inactivation of PAX5. Restoring PAX5 expression in silenced HCC cell lines suppressed cell proliferation, induced apoptosis in vitro, and inhibited tumor growth in nude mice (P < 0.0001). The pathway luciferase reporter assay indicated that PAX5 activated p53 and p21 signaling. ChIP analysis demonstrated that PAX5 directly bound to the p53 promoter. The antitumorigenic function of PAX5 was at least up-regulated by p53 and its downstream targets including tumor necrosis factor, Fas ligand, leucine-rich repeats, and death domain-containing, poly(rC) binding protein 4, p21, and growth arrest and DNA-damage-inducible alpha. Conclusion: PAX5 is frequently inactivated by promoter methylation in HCC. PAX5 appears to be a functional tumor suppressor involved in liver carcinogenesis through direct regulation of the p53 signaling pathway. (HEPATOLOGY 2011)
The incidence of hepatocellular carcinoma (HCC) has been rapidly growing, with a prediction of further doubling in the next 20 years.1 Although the molecular mechanisms of the pathogenesis of HCC remain unclear, inactivation of tumor-related genes through promoter hypermethylation has been demonstrated to play an important role in the development of this disease.2 Considerable efforts have now focused on identifying novel gene targeting by promoter methylation so as to unravel the molecular mechanisms for the inactivation of tumor suppressive pathways that contribute to hepatocarcinogenesis and to design better treatments to reduce its mortality.
The paired box (PAX) genes are a family of transcription factors composed of nine members with crucial roles in tissue development, cellular differentiation, migration, and proliferation.3 PAX genes are classified into subgroups according to the structural similarity: some contain a full homeodomain (PAX3, PAX4, PAX6, and PAX7), whereas others contain a partial homeodomain (PAX2, PAX5, and PAX8) or none at all (PAX1 and PAX9). PAX5 was originally identified as a B-cell-specific transcription factor, and it potentially promotes B-cell commitment by repressing lineage-inappropriate gene expression and reinforcing B-cell-specific gene expression.4-6 Depletion of PAX5 resulted in developmental defects of B cells.7 Until now, the role of PAX5 in tumor development remained unclear. The inappropriate expression of PAX5 has been found in several malignancies.3, 8-10 However, overexpression of PAX5 in vivo does not generally lead to cancer,11 whereas loss of PAX5 contributes to cell proliferation and invasion in mammary cancer cells lines MCF-7 and MDA-231,12 suggesting that aberrant inactivation of PAX5 may contribute to tumorigenesis. We recently identified that PAX5 is differentially methylated in human cancer by methylation-sensitive representational difference analysis. In this study we discovered the frequent loss of PAX5 expression due to promoter methylation in HCC. Further functional studies revealed that ectopic expression of PAX5 resulted in significant suppression of HCC growth by inducing apoptosis, which is mediated by directly upregulation of p53 and its downstream molecules. Our results support PAX5 functions as a novel tumor suppressor in hepatocarcinogenesis.
Abbreviations
Aza, 5-aza-2'-deoxycytidine; BCL2, B-cell lymphoma 2; BGS, bisulfite genomic sequencing; ChIP, chromatin immunoprecipitation; Fas-L, Fas ligand; GADD45, increasing growth arrest and DNA-damage-inducible; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HCC, hepatocellular carcinoma; LITAF, lipopolysaccharide-induced tumor necrosis factor-α factor; LRDD, leucine-rich repeats and death domain containing; MSP, methylation specific PCR; Noxa, Phorbol-12-myristate-13-acetate-induced protein 1; p21 (CDKN1A), cyclin-dependent kinase inhibitor 1A; p53, tumor protein 53; PARP, poly (ADP-ribose) polymerase; PAX, paired box; PCBP4, poly(rC) binding protein4; PIDD, p53-induced protein with a death domain; PUMA, p53 up-regulated modulator of apoptosis; RPRM, reprimo, TP53 dependent G2 arrest mediator candidate; TNF, tumor necrosis factor; TSA, trichostatin A; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling.
Materials and Methods
Cell Lines and Tissue Samples.
Twelve HCC cell lines (Hep3B, hUH4, hUH6, hUH7, Mahlavu, SNU398, SNU423, SNU449, SNU475, PLC-5, SNU387, and HepG2) were used in this study. Cell lines were maintained in RPMI or Dulbecco's modified Eagle's medium (DMEM) medium (Gibco BRL, Rockville, MD) with 10% fetal bovine serum. Human normal adult tissue RNA samples were purchased commercially (Stratagene, La Jolla, CA, or Millipore Chemicon, Billerica, MA).
The paired tissue samples from primary liver cancer and adjacent nontumor sites were obtained from 35 HCC patients during operation prior to any therapeutic intervention. All of the samples were subsequently verified by histology. Informed consent was given by all patients. The study protocol was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong.
RNA Extraction, Semiquantitative Reverse-Transcription Polymerase Chain Reaction (RT-PCR), and Real-Time PCR Analyses.
Total RNA was extracted from cell pellets or tissues using Quizol reagent (Qiagen, Valencia, CA). Semiquantitative RT-PCR was performed using the Go-Taq DNA polymerase (Promega, Madison, WI) with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. Real-time PCR was performed using SYBR Green master mixture on HT7900 system (Applied Biosystems). Primer sequences are listed in Table 1.
| Primer Name | Sequence (5'-3') |
|---|---|
| RT-PCR | |
| PAX5-F | GTCCATTCCATCAAGTCCTG |
| PAX5-R | TTGCTGACACAACCATGGCT |
| P21-F | CTGCGTTCACAGGTGTTTCT |
| P21-R | GTGACAGGTCCACATGGTCTT |
| P53-F | AAGACTCCAGTGGTAACCTACTG |
| P53-R | ATCTAAGCTGGTATGTCCTACTC |
| P73-F | CACGTTTGAGCACCTCTGGA |
| P73-R | CGTGCTCCGCTTTCTTGTAA |
| GADD45-F | GCCTGTGAGTGAGTGCAGAA |
| GADD45-R | CAGGCACAACACCACGTTAT |
| β-actin-F | GTCTTCCCCTCCATCGTG |
| β-actin-R | AGGGTGAGGATGCCTCTCTT |
| BGS | |
| PAX5BGS-F | TTTTTTTAAAAGTATTTGTTTGGT |
| PAX5BGS-R | CACCTTCTATTAAAACATAC |
| MSP | |
| PAX5MSPm-F | AAATAAAAATTCGGTTTGCGTTC |
| PAX5MSPm-R | AAACATACGCTTAAAAATCGCG |
| PAX5MSPu-F | TAAAAATAAAAATTTGGTTTGTGTTT |
| PAX5MSPu-R | TTAAAACATACACTTAAAAATCACA |
5-Aza-2'-deoxycytidine (5-Aza) Treatment.
Cell lines (Hep3B, HepG2, SNU387, SNU398, PLC-5) with silenced PAX5 expression were treated with 2 μM of the DNA demethylating agent 5-Aza (Sigma, St. Louis, MO), with or without 300 nmol/L histone deacetylase inhibitor Trichostatin A (TSA) for 5 days. DNA and total RNA were extracted using Quizol reagent (Qiagen).
Methylation-Specific PCR (MSP) and Bisulfite Genomic Sequencing (BGS).
Genomic DNA was extracted from the cell pellets and tissues using QIAamp DNA Mini kit (Qiagen, Hilden, Germany). DNA was chemically modified with sodium metabisulphite.13 The bisulfite-modified DNA was amplified by using primer pairs that specifically amplify either methylated or unmethylated sequences of the PAX5 genes (Table 1).
BGS was performed to characterize the methylation density in the promoter of PAX5 using the BigDye Terminator Cycle Sequencing kit version 1.0 (Applied Biosystems). Ten CpG sites spanning the −292 and −132bp regions were evaluated. Sequences were analyzed by using SeqScape software (Applied Biosystems) and Bioedit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
PAX5 Expression Plasmid Construction.
Complementary DNA (cDNA) corresponding to the full-length PAX5 was obtained by RT-PCR amplification of normal human stomach cDNA with primers specific to PAX5. The PCR aliquots were subcloned into mammalian expression vector pcDNA3.1-TOPO TA (Invitrogen, Carlsbad, CA).
Colony Formation Assay.
Colony formation assay was performed using monolayer culture. Cells (4 × 105/well) were plated in a 6-well plate and transfected with expression plasmids pcDNA3.1-PAX5 or the empty vector pcDNA3.1 (4 μg each) using lipofectamine 2000 (Invitrogen). After 48 hours of transfection, cells were collected and seeded (1 × 104/well) in a fresh 6-well plate, and selected with G418 for 10 days. Colonies (≥50 cells/colony) were counted after staining with crystal violet solution. All the experiments were performed in triplicate wells three times.
Cell Viability Assay.
Cell viability was measured by the MTS assay (Promega). Briefly, the cells (2 × 103/well) were stably transfected with pcDNA3.1-PAX5 or the empty vector in a 96-well plate for 1, 3, 5, or 7 days. Twenty μL of reaction solution containing 333 μg/mL MTS and 25 μM phenazine ethosulfate was added to cells in 100 μL culture medium, incubated at 37°C for 1.5 hours, and measured at a wavelength of 490 nm.
Flow Cytometry.
Cell cycle distribution was determined by flow cytometry. Briefly, after 12 hours of synchronization by serum starvation, the stably transfected HCC cells with pcDNA3.1-PAX5-expressing vector or pcDNA3.1 empty vector were restimulated with 10% fetal bovine serum (FBS) for 24 hours. Cells were fixed in 70% ethanol and stained with 50 μg/mL propidium iodide (BD Pharmingen, San Jose, CA). The cells were then sorted by FACSCalibur (BD Biosciences, Franklin Lakes, NJ) and cell-cycle profiles were analyzed by WinMDI v. 2.9 software (Scripps Research Institute, La Jolla, CA). Cells undergoing apoptosis were detected as sub-G1 population because of loss of fragmented DNA.
In Vivo Tumorigenicity.
Hep3B cells (1 × 107 cells in 0.1 mL phosphate-buffered saline [PBS]) transfected with PAX5 or pcDNA3.1 were injected subcutaneously into the dorsal left flank of 4-week-old male Balb/c nude mice (5/group). Tumor diameter was measured every 2-3 days for 4 weeks. Tumor volume (mm3) was estimated by measuring the longest and shortest diameter of the tumor and calculated as described.14 An orthotopic HCC mouse model was also established to determine the intrahepatic tumorigenicity. Subcutaneous tumors were harvested 1 week after subcutaneous injection and cut into 1.0 mm3 pieces. One piece was then implanted into the left liver lobe of each mouse (6/group). The mice were sacrificed after 2 weeks and the tumor size and tumor weight were measured. All experimental procedures were approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
Immunostaining and In Situ DNA Nick End Labeling.
Terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay was performed with the Dead End Colorimetric TUNEL System (Promega) following the manufacturer's protocol. Nuclei with clear brown staining were regarded as TUNEL-positive apoptotic cells. The apoptosis index was calculated as the percentage of TUNEL-positive cell after counting at least 1,000 cells.
Dual-Luciferase Reporter Assay.
To investigate the signaling pathways modulated by PAX5, a series of signaling pathway luciferase reporters were examined in PAX5-transfected HCC cells, including nuclear factor (NF)-κB-luc (5 × NFκB binding sites), p53-luc (14 × p53 binding sites), p21-luc (2.1 kb p21 promoter), AP1-luc (7 × AP1 binding sites), SRE-luc (5xSRE binding elements), and TOPFlash (4 × TCF binding sites). The cell lines (HepG2 and Hep3B) stably transfected with pcDNA3.1-PAX5 or pcDNA3.1 (1 × 105 cells/well) in 24-well plates and were cotransfected with luciferase report plasmid (0.1 μg/well) and pRL-cytomegalovirus (CMV) vector (2.5 ng/well) using lipofectamine 2000 (Invitrogen). Cells were harvested 48 hours posttransfection and luciferase activities were analyzed by the dual-luciferase reporter assay system (Promega).
Chromatin Immunoprecipitation (ChIP) Assay.
ChIP analysis was performed to study transcription factor PAX5 binding to target DNA by using Red ChIP Kit (Diagenode, Belgium) as described.15 The immunoprecipitated and input DNA in HepG2/vector and HepG2/PAX5 cells was used as template for quantitative PCR (qPCR) analysis using the primers listed in Table 1.
p53 Signaling Pathway PCR Array.
Gene expression profiles of HepG2 cells stably transfected with pcDNA3.1-PAX5 or pcDNA3.1 vector were analyzed by Human p53 Signaling Pathway RT2 Profiler PCR Array (SABiosciences, Frederick, MD). This array contains 84 functionally well-characterized genes related to p53-mediated signal transduction (http://www.sabiosciences.com). Genes expression with fold changes of more than or less than 1.5 were considered biologically significant.
Western Blot Analysis.
Total protein was extracted and protein concentration was measured by the Bradford DC protein assay (Bio-Rad, Hercules, CA). Forty micrograms of protein from each sample were separated on 10% Bis/Tris-polyacrylamide gel through electrophoresis and blotted onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ). Blots were immunostained with primary antibodies at 4°C overnight and secondary antibody at room temperature for 1 hour. Proteins were visualized using ECL Plus Western Blotting Detection Reagents (RPN2132, GE Healthcare).
Statistical Analysis.
The results are expressed as mean ± standard deviation (SD). The PAX5 expression level in primary HCC tissues and their adjacent normal tissues were compared by the paired sample t test. A Mann-Whitney U test was performed to compare the variables of the two sample groups. The difference in tumor growth rate between the two groups of nude mice was determined by repeated-measures analysis of variance. P < 0.05 was considered statistically significant.
Results
PAX5 Was Frequently Down-Regulated or Silenced in HCC Cell Lines and Primary Tumors.
We first examined the messenger RNA (mRNA) expression of PAX5 in 12 HCC cell lines, 35 primary HCCs, and their corresponding adjacent nontumor tissues. PAX5 transcript was reduced or silenced in 83% (10/12) of HCC cell lines, but was readily expressed in the normal liver tissue (Fig. 1A). PAX5 expression was significantly down-regulated in primary HCCs as compared with adjacent nontumor tissues (P < 0.0001) (Fig. 1B), suggesting an aberrant gene silencing of PAX5 in HCC.
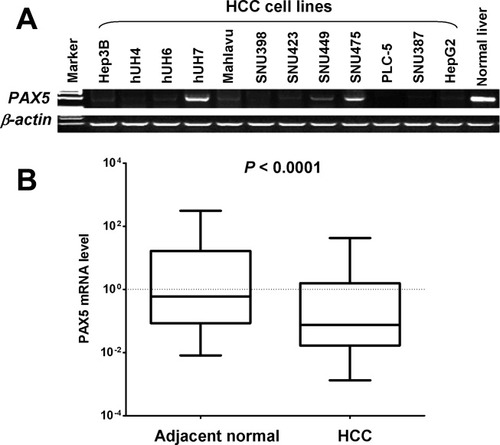
PAX5 frequently silenced or down-regulated in HCC cell lines and primary HCCs. (A) The expression profile of PAX5 mRNA in HCC cell lines and normal liver tissue by semiquantitative RT-PCR with β-actin as an internal control. (B) PAX5 mRNA expression was significantly down-regulated in primary HCCs as compared with adjacent noncancer tissues as determined by quantitative real-time PCR (n = 35). PAX5 expression level was normalized with the β-actin mRNA level.
PAX5 Hypermethylation Correlated with Transcriptional Silencing.
It has been well documented that hypermethylation in the CpG-rich promoter region is a critical event leading to the epigenetic inactivation of tumor suppressor genes in cancers. We next explored the methylation status of the PAX5 promoter by using MSP (Fig. 2A). Full or partial methylation was detected in HCC cell lines (Hep3B, huH4, huH6, Mahlavu, SNU398, and PLC5), which showed silenced or down-regulated PAX5 expression, whereas methylation was not detected in the cell lines with PAX5 expression (hUH7, SNU475, and SNU449) (Fig. 2A).
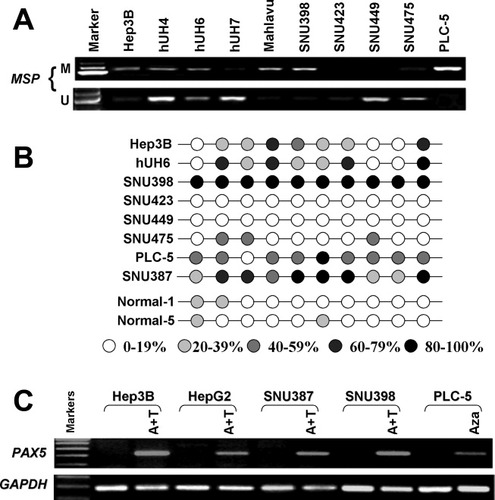
Promoter methylation status of PAX5. (A) Promoter methylation of PAX5 in HCC cell lines was determined by methylation-specific PCR (MSP). M, methylated DNA; U, unmethylated DNA. (B) BGS analysis confirmed the methylation status of PAX5 in HCC cell lines and normal controls. (C) PAX5 mRNA expression was restored after treatment with demethylation reagent 5-Aza (A), with or without trichostatin A (T) in five HCC cell lines.
The methylation density within the PAX5 promoter region was then characterized and validated by BGS (Fig. 2B). The BGS results were consistent with those of MSP in which dense methylation was found in methylated cell lines by MSP, but not in normal liver tissues (Fig. 2B).
To confirm whether the promoter methylation is involved in the silencing of PAX5, five cell lines with silenced PAX5 expression including Hep3B, HepG2, SNU387, SNU398, and PLC-5 were treated with 5-Aza combined with or without trichostatin A. This treatment resulted in the restoration of PAX5 expression in all cell lines examined (Fig. 2C), further implicating that the transcriptional silence of PAX5 was mediated by promoter methylation.
Ectopic Expression of PAX5-Suppressed HCC Cell Growth.
The frequent inactivation of PAX5 in HCC cell lines but not in normal liver tissue suggested that PAX5 may play a role in tumor growth. We thus examined the growth-suppressive effect through ectopic expression of PAX5 in HepG2 and Hep3B, which showed no PAX5 expression. Reexpression of PAX5 in the stable transfected HepG2 and Hep3B cells was confirmed by RT-PCR (Fig. 3A1) and western blot (Fig. 3A2). Ectopic expression of PAX5 in these HCC cell lines caused a significant decrease in cell viability (Fig. 3B). The inhibitory effect on HCC cell growth was further confirmed by colony formation assay. The colonies formed in PAX5-transfected cells were significantly fewer in number and smaller in size than in empty vector-transfected cells (down to 44%-54% of vector control, P < 0.01) (Fig. 3C).

PAX5 inhibited HCC cell growth. (A) Ectopic expression of PAX5 mRNA (A1) and protein (A2) in HepG2 and Hep3B cell lines was evidenced by RT-PCR and western blot, respectively. (B) PAX5 significantly inhibited cell viability in HCC cell lines. (C) The effect of PAX5 on cancer cell growth was further confirmed by colony formation assay. Left panel shows the representative images of the colony formation in HCC cells transfection with pcDNA3.1/PAX5 or empty vector (pcDNA3.1). Quantitative analysis of colony numbers is shown in the right panel. Data are mean ± SD, *P < 0.01. (D) Protein expression of cleaved caspase-7, -8, -9 and nuclear enzyme poly (ADP-ribose) polymerase (PARP) was evaluated by western blot. GAPDH was used as loading control. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Induction of Apoptosis by PAX5.
We examined the contribution of apoptosis to the observed growth inhibition in HCC cells derived by PAX5. The number of HepG2 cells with sub-G1 DNA content after PAX5 transfection was substantially increased compared with the control vector transfection (24.75% ± 2.09% versus 33.11% ± 2.06%; P < 0.05). Apoptosis was further assessed by immunoblot detection of the active form of caspase-7, caspase-8, caspase-9, and poly (ADP-ribose) polymerase (PARP). As shown in Fig. 3D, overexpression of PAX5 enhanced the levels of active caspase-7, -8, -9, and PARP.
PAX5 Inhibits Tumor Growth in Nude Mice.
The subcutaneous tumor growth curve of Hep3B stably transfected with PAX5 or empty vector in vivo is shown in Fig. 4A. The tumor volume was significantly lower in PAX5-transfected nude mice as compared to the vector control mice (P < 0.0001). At the end of experiments tumors were isolated and weighed. The mean tumor weight was significantly less in PAX5-transfected nude mice as compared with the vector control mice (P < 0.05) (Fig. 4B), indicating that PAX5 acts as a tumor suppressor in hepatocarcinogenesis. The apoptotic index in the xenograft tumor of nude mice was evaluated using a TUNEL assay. HCCs from the PAX5 group displayed significantly more apoptotic cells as compared with control group (1.4% ± 0.6% versus 2.4% ± 0.7%; P < 0.01, Fig. 4C). Moreover, the mouse orthotopic xenograft model of liver cancer with transplanted human HCC derived from Hep3B were transfected with PAX5 or empty vector. Both the tumor volume and tumor weight in mice transfected with PAX5 were significantly decreased compared with that of control mice (Fig. 4D).
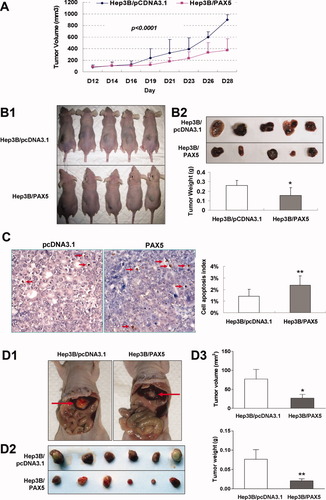
PAX5 retarded tumor growth in vivo. (A) Subcutaneous tumor growth curve of PAX5-expressing Hep3B cells in nude mice was compared with vector (pcDNA3.1) transfected cells. The PAX5 group showed a retarded tumor growth compared to the vector group (P < 0.0001). The data are means ± SD (n = 5/group) of three separate experiments. (B1) A representative picture of tumor growth in nude mice subcutaneously inoculated with PAX5 or vector. (B2) Histogram represents mean of the tumor weight from the PAX5 and vector groups. (C) Representative TUNEL staining of xenografted tumor derived from Hep3B cells transfected with PAX or control vector (pcDNA3.1). An increase in the number of TUNEL-positive cells (brown-stained nuclei, red arrows) is evident in PAX-transfected tumors (×200). (D) Effects of PAX5 on orthotopic tumor growth in nude mouse. (D1) Representative image of orthotopic tumor appearance in the liver derived from Hep3B cells transfected with PAX5 or control vector. (D2) Pictures of the isolated tumors from the liver of each mouse (n = 6/group). (D3) Histogram shows average of the tumor volume and weight from the livers of PAX5 group and control group. Quantitative results are shown on the right. Data are mean ± SD. *P < 0.05, **P < 0.01.
Tumor Suppressive Property by PAX5 Is Mediated by p53 Signaling Pathways.
To gain insights into the downstream signaling pathways modulated by PAX5 in tumor inhibition, we performed promoter-luciferase activity assays using several pathway luciferase reporters including p53-Luc, p21-Luc, NF-κB-Luc, AP-1-Luc, SRE-Luc, and TOPFlash. Ectopic expression of PAX5 increased p53 and p21 luciferase reporter activities in HepG2 cells, whereas no significant activity changes in NF-κB, AP-1, SRE, and TOPFlash pathway reporters were observed (Fig. 5A). Moreover, reexpression of PAX5 in Hep3B, known as a p53 depleted cell line, failed to change p53 and p21 luciferase reporter activities (data not shown), which confirmed that PAX5 is an important positive modulator of the p53/p21 pathway in HepG2.
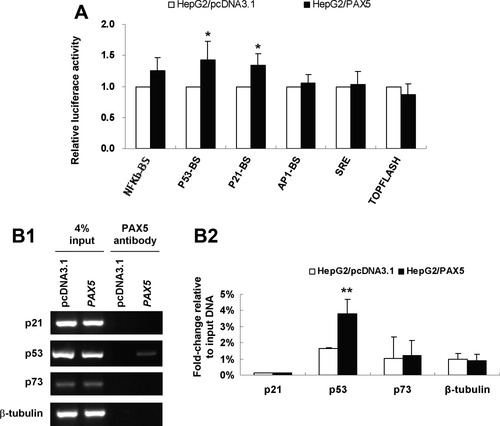
PAX5 induced p53 reporter activity through binding to the promoter of p53. (A) To screen for PAX5 target signaling pathways, a serial of promoter-luciferase assays (p53-luc, p21-luc, NF-κB-luc, AP1-luc, SRE-luc, and TOPFlash) were performed in PAX5 stably transfected HepG2 cells compared with vector control cells. Luciferase activities were determined by dual luciferase assay system at 48 hours posttransfection. (B) Functional interaction between PAX5 and the promoter of its targets. (B1) HepG2 cells were transfected with PAX5-pcDNA3.1 or pcDNA3.1, then cross-linked with formaldehyde and lysed. The soluble chromatin was immunoprecipitated with the anti-PAX5 antibody. Primers were designed to detect the promoter region of p21, p53, p73, and β-tubulin, respectively. The immunoprecipitated DNA was subjected to semiquantitative ChIP-PCR assay (B1) and quantitative ChIP-qPCR validation (B2). Data are mean ± SD. *P < 0.01, **P < 0.001.
We further evaluated whether the observed PAX5-mediated p53 and P21 activities were associated with direct promoter binding ChIP assay using specific PAX5 antibody, performed in HepG2 cells followed by PCR targeting the promoter regions (Fig. 5B). ChIP-qPCR assay indicated that PAX5 binds to the promoter of p53 (P < 0.01), but not p21 in HepG2 cells (Fig. 5B).
To further determine the downstream mediators of the p53 signaling pathway derived by PAX5, gene expression profiles in PAX5 stably transfected HepG2 was analyzed by p53 signaling pathway PCR array. When compared with empty vector-transfected cells, PAX5 modulated p53 downstream target genes involved in apoptosis, proliferation, cell cycle, and DNA repair (Table 2). PAX5 increased the expression of proapoptotic genes including p53 family members, p73 and p63, tumor necrosis factor (TNF), Fas ligand (Fas-L), leucine-rich repeats, and death domain containing (LRDD). PAX5 also exerted antiproliferative effect by increasing the expression of cyclin-dependent kinase inhibitor 1A (p21, CDKN1A), poly(rC) binding protein4 (PCBP4), Reprimo, and P53-dependent G2 arrest mediator candidate (RPRM). Moreover, PAX5 exerted a DNA repair effect by inducing the expression of DNA-damage-inducible gene (GADD45). Semiquantitative RT-PCR and/or western blot confirmed that ectopic expression of PAX5 was associated with up-regulation of p53, p21, p73, Fas-L, GADD45, phorbol-12-myristate-13-acetate-induced protein 1 (Noxa), and p53 up-regulated modulator of apoptosis (PUMA) in HepG2 cells (Fig. 6A).
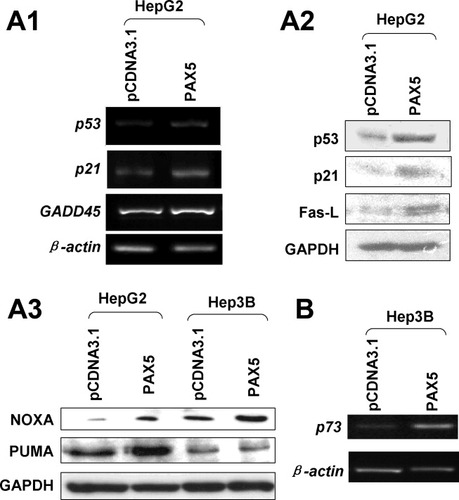
(A) RT-PCR (A1) and western blot (A2) analyses were performed to confirm the elevated gene expression in PAX5-transfected HepG2 cells. (A3) The protein expression of Noxa and PUMA was detected in HepG2 and Hep3B cells by western blot. (B) p73 expression was enhanced in Hep3B cells transfected with PAX5 by RT-PCR.
| GenBank Accession | Gene Name | Symbol | Fold Change | Gene Function |
|---|---|---|---|---|
| NM_005427 | Tumor protein P73 | P73 | 7.7 | Apoptosis |
| NM_003722 | Tumor protein p73-like, TP73L | P63 | 1.8 | Apoptosis |
| NM_000594 | Tumor necrosis factor (TNF superfamily, member 2) | TNF | 2.3 | Apoptosis |
| NM_000639 | Fas ligand (TNF superfamily, member 6) | Fas-L | 1.8 | Apoptosis |
| NM_018494 | Leucine-rich repeats and death domain containing | LRDD | 1.7 | Apoptosis |
| NM_000389 | Cyclin-dependent kinase inhibitor 1A (p21Waf1, Cip1) | CDKN1A | 1.7 | Proliferation |
| NM_010418 | Poly(rC) binding protein4 | PCBP4 | 5.9 | Cell growth |
| NM_019845 | Reprimo, TP53 dependent G2 arrest mediator candidate | RPRM | 3.4 | Cell cycle arrest |
| NM_001924 | Growth arrest and DNA-damage-inducible, alpha | GADD45A | 1.5 | DNA repair |
We evaluated the expression of p73 in Hep3B. Our result showed that reexpression of PAX5 in Hep3B induced p73 expression (Fig. 6B). However, no direct interaction between PAX5 and p73 promoter was observed in the ChIP-qPCR assay either in HepG2 (Fig. 5B) or Hep3B (data not shown). The proapoptotic protein Noxa was also increased in Hep3B transfected with PAX5 (Fig. 6A3).
Discussion
In this study we found that PAX5 expression was frequently absent or down-regulated in HCC cell lines in vitro, and was also significantly decreased in primary HCC tissues compared with their adjacent nontumor tissues in vivo (P < 0.0001), suggesting PAX5 would be a candidate tumor suppressor in the pathogenesis of HCC. The reduced expression is associated with promoter methylation, as confirmed by promoter methylation analyses and pharmacological demethylation treatment, implicating DNA methylation as the principle regulatory mechanism of PAX5 inactivation in HCC. There are unmethylated alleles in the SNU423 cell line with no expression detected by RT-PCR, indicating that other transcription-regulating mechanisms such as histone modification or transcriptional repressors may also contribute to the gene silencing.16, 17
Silencing of PAX5 might abolish tumor suppression so as to contribute to tumorigenesis. We tested the putative tumor suppressor function of PAX5 in human HCC by both in vitro and in vivo assays. Reexpression of PAX5 in two silenced HCC cell lines (HepG2 and Hep3B) showed significant growth-suppressing effect by inhibition of cell viability and colony formation. The diminution of tumor growth in PAX5-reexpressed cells was further confirmed in nude mice both in a subcutaneous xenograft model and in an orthotopic liver implantation model (Fig. 4). Increased apoptosis was revealed in PAX5-reexpressed HepG2 cells by FACScan analysis and in PAX5-reexpressed Hep3B by TUNEL staining. The induction of apoptosis by PAX5 was mediated through a caspase-dependent pathway including activation of caspase-8, an initiator caspase, followed by direct cleavage of downstream effector caspase, caspase-9, which further processes other effector caspase members such as caspase-7 to initiate a caspase cascade. These effectors further stimulated the proteolytic cleavage of PARP which facilitates cellular disassembly and apoptosis. Collectively, we indicate for the first time that PAX5 functions as a tumor suppressor in hepatocarcinogenesis. Other reports have shown that PAX5 was commonly mutated in human acute B-cell leukemia18 and loss of PAX5 in late B cells could initiate lymphoma in mice.19 Our findings have highlighted the importance of PAX5 as a potential tumor suppressor in solid cancer.
Mapping the target genes regulated by PAX5, a nuclear transcription factor, in a malignant situation will be important for understanding the molecular basis of PAX5 function. We demonstrated that increased p53 activity was induced by PAX5 in HepG2 using luciferase reporter assay (Fig. 5A). We further indicated that PAX5 directly binds to p53 promoter in HepG2 cells by ChIP-qPCR (Fig. 5B). Consistent with our result, a very recent study showed that reexpression of PAX5 increased the level of p53 mRNA in mammary carcinoma cell line MCF7.13 However, negative dependence between the quantity of PAX5 expression and the expression of p53 in ependymoma and bladder tumors has also been reported.20, 21 The differential responses may occur due to the varied cancer cell types.
To better define the tumor suppressive effect of PAX5 through activation of p53 in liver carcinogenesis, we examined the downstream consequences of p53 by overexpression of PAX5 using a p53 signaling pathway PCR array. P53 target genes modulating the apoptosis, cell growth, and DNA repair pathways were characterized (Table 2; Fig. 7). We observed that PAX5-mediated apoptosis occurs through the p53 pathway by up-regulation of extracellular death ligand TNF, Fas-L, and LRDD. Induction of p53 has been reported to increase lipopolysaccharide-induced tumor necrosis factor-α factor (LITAF), which in turn up-regulates the transcription of TNF.22 TNF is a cytokine involved in tumorigenesis inhibition. Dysregulation of TNF has been implicated in a variety of human cancers.23 Moreover, TNF has been identified to initiate apoptosis through activating several downstream signaling events, including the induction of p53 accumulation.24 Fas-L, a member of the TNF family, interacts with Fas-R to form the death-inducing signaling complex, which initiates the extrinsic apoptosis pathway through activation of caspase-8, an initiator caspase, followed by direct cleavage of downstream effector caspases.25, 26 LRDD is also known as p53-induced protein with a death domain (PIDD). The expression of LRDD is regulated by p53 to induce cell apoptosis in response to DNA damage.27 P73 and p63, similar to their homolog p53, regulate apoptosis during DNA damage.28 P63 regulates the caspase-8 apoptotic pathway.29 In addition, P53/p73 target genes Noxa and PUMA were up-regulated by PAX5, which are proapoptosis proteins from the B-cell lymphoma 2 (BCL2) family.30 Noxa protein can undergo BH3 motif-dependent localization and activate caspase-dependent cell death.31 PUMA is likely to mediate cell apoptosis through the cytochrome c/Apaf-1-dependent pathway.32 Therefore, the up-regulation of p53-mediated proapoptotic genes induced by PAX5 may explain the apoptotic effect exerted by PAX5 (Fig. 7).
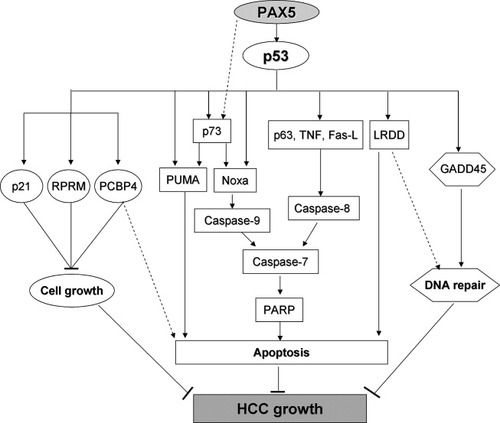
Schematic diagram of PAX5 function as a tumor suppressor through regulating p53 signaling pathway. (1) PAX5 up-regulated the effectors of p53-dependent apoptosis, including extracellular death ligands TNF, Fas-L, adaptor protein LRDD, p53 family members p63 and p73, and Bcl2 family member Noxa and PUMA, which in turn induced caspase dependent cell apoptosis; (2) PAX5 increased p53 transcriptional target genes p21, RPRM, and PCBP4 resulting in cell growth arrest; (3) PAX5 induced p53-dependent DNA repair gene GADD45, which protects against tumorigenesis through maintaining genomic stability.
We found that the antiproliferative effect derived by PAX5 is at least due to the up-regulation of p21, RPRM, and PCBP4, which are transcriptionally regulated by p53. P21 is a critical cyclin E/CDK2 and cyclin D/CDK4 inhibitor, mediating p53-dependent cell cycle G1 phase arrest.33 The induction of RPRM and PCBP4 also contributed to suppress cell proliferation by inducing cell cycle arrest in G2/M.34, 35 The antitumorigenesis property exerted by PAX5 in HCC may also result from the induction of DNA repair genes (GADD45, LRDD). GADD45, transcriptionally activated by p53,36 has been implicated in the maintenance of genomic stability37 and mediates G2/M phase arrest in a wildtype p53-dependent manner.38 Aside from GADD45, LRDD is also reported to be involved in genome stability by DNA repair.39 Collectively, through modulating p53 and its downstream target genes, PAX5 could functions as a tumor suppressor gene by promoting apoptosis, growth arrest, and DNA repair.
We also observed that reexpression of PAX5 in Hep3B induced growth arrest, indicating besides the p53 pathway, another signal transducer may also be involved in the modulation of cellular growth. The p53/p63/p73 family is a family of tumor suppressor genes with overlapping and distinct functions. p73 can activate p53-regulated genes and suppress growth or induce apoptosis.40 In keeping with this, we demonstrated an up-regulation of p73 in Hep3B following the induction of PAX5 (Fig. 6B). It was reported that apoptosis induced in Hep3B cells is always associated with p73 accumulation and mitochondrial dysregulation.41, 42 P73 is known as a tumor suppressive protein with structural and functional resemblance to p53.43 P73 can partially substitute mutant p5344 to promote growth arrest or apoptosis similar to p53.45-47 We also found that the pro-apoptotic protein Noxa was upregulated in Hep3B with PAX5 expression (Fig. 6A3). This is consistent with reports that Noxa expression could be p53-independent48 and that p73 induced Noxa expression in p53-deficient cancer cells.49 Collectively, these findings suggest a possible mechanism by which PAX5 suppresses HCC growth in Hep3B by way of the p73 signaling pathway. The precise downstream molecules of p73 by which it mediates such effects are worthy of future studies.
In conclusion, we identified a novel functional tumor suppressor gene PAX5 inactivated by promoter methylation in liver cancer. PAX5 contributes to suppression of hepatocarcinogenesis by inhibiting cell proliferation and inducing cell apoptosis through direct regulating p53 signaling pathway.




