Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation†
Potential conflict of interest: Nothing to report.
Abstract
Liver tumor-initiating cells (T-ICs) are capable of self-renewal and tumor initiation and are more chemoresistant to chemotherapeutic drugs. The current therapeutic strategies for targeting stem cell self-renewal pathways therefore represent rational approaches for cancer prevention and treatment. In the present study, we found that Lup-20(29)-en-3β-ol (lupeol), a triterpene found in fruits and vegetables, inhibited the self-renewal ability of liver T-ICs present in both hepatocellular carcinoma (HCC) cell lines and clinical HCC samples, as reflected by hepatosphere formation. Furthermore, lupeol inhibited in vivo tumorigenicity in nude mice and down-regulated CD133 expression, which was previously shown to be a T-IC marker for HCC. In addition, lupeol sensitized HCC cells to chemotherapeutic agents through the phosphatase and tensin homolog (PTEN)–Akt–ABCG2 pathway. PTEN plays a crucial role in the self-renewal and chemoresistance of liver T-ICs; down-regulation of PTEN by a lentiviral-based approach reversed the effect of lupeol on liver T-ICs. Using an in vivo chemoresistant HCC tumor model, lupeol dramatically decreased the tumor volumes of MHCC-LM3 HCC cell line-derived xenografts, and the effect was equivalent to that of combined cisplatin and doxorubicin treatment. Lupeol exerted a synergistic effect without any adverse effects on body weight when combined with chemotherapeutic drugs. Conclusion: Our results suggest that lupeol may be an effective dietary phytochemical that targets liver T-ICs. (HEPATOLOGY 2011.)
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world.1 The curative treatment for HCC is liver transplantation or surgical resection.2, 3 However, 80% of HCC cases are presented at advanced stages and are no longer operable. Even after surgical resection, the long-term prognosis of HCC remains unsatisfactory due to high recurrence rates. For HCC patients in advanced stages, chemotherapy by way of either transarterial chemoembolization or systemically is the second-line treatment. Unfortunately, the overall response rate is unsatisfactory due to the highly chemoresistant nature of the tumor and toxicity of chemotherapeutic agents.4-6
The traditional model of HCC development considers that HCC arises from a series of sequential mutations resulting from genetic instability and/or environmental factors effecting normal cells.7, 8 More recently, a cancer stem cell (CSC) model has been proposed.9-11 According to this new model, HCC development is driven by a small population of cells called tumor-initiating cells (T-ICs). There is evidence supporting the presence of T-ICs in different solid tumors, including brain,12 colon,13 breast,14 prostate,15 skin,16 pancreatic,17 and head and neck cancers.18 Recently, liver T-ICs have been identified by several cell surface antigens such as CD133,19 CD90,20 and epithelial cell adhesion molecule,21 and these T-ICs are capable of self-renewal and are chemoresistant to chemotherapeutic drugs. If the CSC hypothesis is valid, strategies aiming at targeting stem cell self-renewal pathways represent rational approaches for cancer prevention and treatment.
In the past few years, natural dietary substances like those obtained from fruits and vegetables have gained considerable attention for the prevention and/or treatment of many cancers. The potential role of dietary substances in HCC prevention development and therapy is further supported by recent epidemiological studies showing a lower incidence of HCC in Japan and Europe as a result of a high intake of fruits and vegetables.22, 23 Our recent study has demonstrated that Lup-20(29)-en-3β-ol (lupeol), a triterpene found in fruits and vegetables, selectively induced apoptosis of head and neck cancer cells and chemosensitized cisplatin when applied simultaneously.24 In addition, lupeol can suppress head and neck cancer metastasis by reversing the epithelial-mesenchymal transition process.24 Because induction of epithelial-mesenchymal transition and chemoresistance are two major characteristics of T-ICs, based on the molecular actions of lupeol against head and neck cancers, we hypothesized that lupeol specifically targets liver T-ICs.
In this study, we found that lupeol was able to modulate the self-renewal ability of liver T-ICs present in both HCC cell lines and clinical samples by hepatosphere formation assay. Lupeol suppressed tumorigenicity in nude mice by decreasing CD133 expression and chemosensitized HCC cells to chemotherapeutic treatments through the phosphatase and tensin homolog (PTEN)–Akt–ABCG2 pathway. Lentiviral-based PTEN knockdown abolished the suppressive role of lupeol on the self-renewal and chemoresistance of liver T-ICs. Using an in vivo chemoresistant HCC tumor model, lupeol was found to exert a synergistic effect when combined with chemotherapeutic drugs. Our results provide solid evidence that lupeol can inhibit self-renewal and tumor initiation and reverse the chemoresistant nature of liver T-ICs, which are mediated through the PTEN–Akt–ABCG2 signaling pathway.
Abbreviations
DMSO, dimethyl sulfoxide; HCC, hepatocellular carcinoma; lupeol, Lup-20(29)-en-3β-ol; MTT, 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; PTEN, phosphatase and tensin homolog; T-IC, tumor-initiating cell.
Materials and Methods
Cell Lines and Cell Culture.
The human HCC cell lines MHCC-LM3 (from Liver Cancer Institute, Fudan University, Shanghai, China), Huh-7 (Japanese Cancer Research Bank, Tokyo, Japan), and PLC-8024 (Institute of Virology, Chinese Academy of Medical Sciences, Beijing, China) were maintained in Dulbecco's modified Eagle's medium with high glucose (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), 100 mg/mL penicillin G, and 50 μg/mL streptomycin (Gibco BRL) at 37°C in a humidified atmosphere containing 5% CO2. MIHA was kindly provided by J. R. Chowdhury, Albert Einstein College of Medicine, New York.25
Human HCC Tissue Collection and Processing.
Liver tumor tissue specimens were collected from five patients who underwent hepatectomy for HCC between 2008 and 2009 in the Department of Surgery, Queen Mary Hospital, Hong Kong, with Institutional Review Board approval. Tumor tissue from fresh tumors was minced into 1-mm3 cubes and incubated with Liberase TM Research Grade (Roche Diagnostics, Indianapolis, IN) for 5-10 minutes at 37°C. A single-cell suspension was obtained by filtering the supernatant through a 100-μm cell strainer (BD Biosciences, San Jose, CA). Removal of CD45+ cells from within the tumor was performed with a CD45 depletion kit (Miltenyi Biotech, Bergisch Gladbach, Germany).
Treatment of Cells.
A stock solution of lupeol (30 mmol/L) (NW = 426.72) was resuspended in warm alcohol and diluted in dimethyl sulfoxide (DMSO) at a 1:1 ratio. For dose-dependent studies, cells (50% confluent) were treated with lupeol (1-200 μmol/L) for 72 hours in complete Dulbecco's modified Eagle's medium/high-glucose cell medium. For all treatment protocols, the final concentrations of DMSO and alcohol were 0.25% and 0.075%, respectively.
Isolation of CD133+ and CD133− Populations by Flow Cytometry and Magnetic Cell Sorting.
For HCC cell lines, CD133+ tumor cells were sorted on a BD FACSVantage SE (BD Biosciences, San Jose, CA). For clinical HCC samples, CD133+ cells were isolated by way of magnetic cell sorting. Clinical HCC tumor cells were labeled with CD133/1 microbeads and sorted using the Miltenyi Biotec CD133 Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer's instructions. Magnetic separation was performed twice to obtain purity greater than 95% for both CD133+ and CD133− populations. Aliquots of CD133+ and CD133− sorted cells were evaluated for purity with a FAT-ICalibur machine and CellQuest software (BD Biosciences, San Jose, CA) using the phycoerythrin-conjugated anti-human CD133/2 antibody (Miltenyi Biotec). For cell sorting using flow cytometry, cells were stained with the phycoerythrin-conjugated anti-human CD133/1 antibody (Miltenyi Biotec). Isotype-matched mouse immunoglobulins served as controls. Samples were analyzed and sorted on a BD FACSVantage SE (BD Biosciences).
In Vivo Tumorigenicity Experiments.
The effect of lupeol on tumorigenicity was evaluated by both in vitro pretreatment and in vivo administration in mice. First, Huh-7 and PLC-8024 cells were labeled with luciferase as described.24 For in vitro experiment, a total of 1 × 106 cells, with or without pretreatment with 10 μM lupeol for 72 hours, was injected subcutaneously into nude mice. For in vivo lupeol administration, lupeol at a dose of 1 mg/animal was administered intraperitoneally twice per week for 30 days into nude mice right after 1 × 106 cells was inoculated into the nude mice subcutaneously. Imaging was performed using a Xenogen IVIS 100 cooled CCD camera (Xenogen, California) on day 40. The mice were given intraperitoneal injections with 200 μL of 15 mg/mL D-luciferin 15 minutes before imaging. For imaging, the mice were placed in a light-tight chamber, the acquisition time ranged from 3 seconds to 1 minute, and pseudoimages of the emitted light in photons/second/cm2/steradian superimposed over the gray-scale photographs of the animal were taken.
Chemoresistant Tumor Model.
Subcutaneous xenografts were established using the MHCC-LM3 HCC cell line. The animals used to test the treatment were 4- to 6-week-old male athymic nude mice (BALB/c-nu/nu). Treatment was started once the size of the xenograft reached approximately 4 × 4 mm. The mice were randomly assigned into four groups, each consisting of five mice. They were treated with intraperitoneal injection twice per week for 30 days of either (1) lupeol (2 mg/animal) in 0.2 mL of corn oil, (2) 2 mg/kg cisplatin and 2 mg/kg doxorubicin, (3) lupeol (2 mg/animal) in 0.2 mL of corn oil plus 1 mg/kg cisplatin and 1 mg/kg doxorubicin, or (4) 0.2 mL of corn oil alone as the control group. The administration protocol of lupeol was consistent with that of Saleem et al.26
Statistical Analyses.
All statistical analyses were performed using SPSS version 17 for Windows (SPSS Inc., Chicago, IL). Results are presented as the mean ± SD and were analyzed using a Student t test. P < 0.05 was considered statistically significant.
Results
Lupeol Inhibited Hepatosphere Formation and Decreased Self-Renewal Ability of HCC Cells.
Our previous study demonstrated that lupeol treatment induced dramatic cell death in head and neck cancer cell lines in a dose-dependent manner.24 In this study, using 3-(4, 5-cimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay, we found that lupeol inhibited the growth of Huh-7, PLC-8024, and MHCC-LM3 in a dose-dependent manner 72 hours after the administration of lupeol (Table 1). To exclude the possibility that the observation was due to toxicity effect, the effect of lupeol on the nontumorigenic normal liver cell line MIHA was also investigated. MIHA cells were found to be remarkably less sensitive to lupeol administration, with a high IC50 of 280 μM, as compared with those of the HCC cell lines (90, 80, and 120 μM for Huh-7, PLC-8024, and MHCC-LM3, respectively) (Table 2).
| Cell Lines | 0 μM | 5 μM | 10 μM | 15 μM | 30 μM | 60 μM | 90 μM | 120 μM | 150 μM | 200 μM |
|---|---|---|---|---|---|---|---|---|---|---|
| Huh-7 | 0% | 0% | 0% | 0% | 16.5 ± 2.5% | 35.6 ± 5.4% | 50 ± 2.4% | 64.1 ± 5.5% | 92 ± 7.8% | 100% |
| PLC-8024 | 0% | 0% | 0% | 3 ± 0.1% | 19.8 ± 3.3% | 40 ± 5.4% | 55 ± 3.3% | 70.5 ± 5.5% | 99 ± 0.3% | 100% |
| MHCC-LM3 | 0% | 0% | 0% | 0% | 9.5 ± 0.9% | 27.8 ± 1.4% | 39.9 ± 1.4% | 50 ± 6.7% | 78.2 ± 4.5% | 100% |
| Cell Lines | IC10 (μM) | IC30 (μM) | IC50 (μM) |
|---|---|---|---|
| MIHA | 75 ± 3.5 | 190 ± 7.3 | 280 ± 12.2 |
| Huh-7 | 25 ± 4.6 | 50 ± 5.5 | 90 ± 1.1 |
| PLC-8024 | 20 ± 2.2 | 54 ± 1.3 | 80 ± 4.3 |
| MHCC-LM3 | 32 ± 1.9 | 69 ± 7.5 | 120 ± 11.5 |
- MIHA cells were found to be remarkably less sensitive to lupeol administration.
Next, we examined the effects of lupeol on liver T-ICs. One of the properties of T-ICs is the ability to survive under anchorage-independent conditions.27 We therefore determined the effect of lupeol on liver T-ICs in hepatosphere formation, using lupeol at concentrations ranging from 1 to 20 μM on Huh-7 and PLC-8024 cells. Compared with the DMSO control, we found that 10 μM of lupeol completely inhibited hepatosphere formation of cells derived from Huh-7 and PLC-8024 but had no cell growth inhibition on these two cell lines in Table 1. Importantly, lupeol completely inhibited sphere formation in the HCC clinical samples from five patients at 10 μM concentration (Fig. 1A).
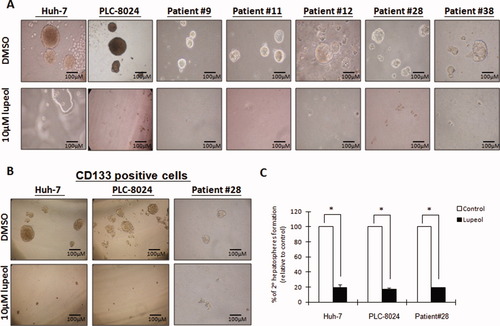
Lupeol at a low dose completely inhibited hepatosphere formation and suppressed the ability to form secondary spheres. (A) Lupeol at 10 μM completely inhibited hepatosphere formation in Huh-7 and PLC-8024 cell lines and in five HCC clinical samples (patients 9, 11, 12, 28, and 38). (B) To further enrich the T-IC population, HCC cells from Huh-7, PLC-8024, and a clinical sample (patient 28) were sorted using the CD133 antibody by way of flow cytometry. The same dose of lupeol also completely inhibited the formation of hepatospheres in these CD133+ HCC cells. (C) Lupeol also inhibited the ability of cells to form secondary hepatospheres by more than 80% compared with DMSO control. *P < 0.001 (Student t test). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
It has previously been demonstrated that CD133+, but not CD133−, cells are capable of generating tumors in severe combined immunodeficiency mice.19 To examine the effect of lupeol on hepatosphere formation in this stem/progenitor cell population, CD133+ HCC cells were further enriched by either flow cytometry (for Huh-7 and PLC-8024) or magnetic cell sorting (for HCC clinical sample), subjected to lupeol treatment, and evaluated for hepatosphere formation. We found that application of 10 μM lupeol completely inhibited hepatosphere formation of the CD133+ cells (Fig. 1B).
Because the ability of sphere formation in serial passages is an indirect marker for stem cell renewal,27 we then determined the effect of lupeol on the primary hepatospheres in serial passaging in the Huh-7 and PLC-8024 cells and the HCC clinical sample shown in Fig. 1B. The addition of 10 μM lupeol to primary hepatospheres remarkably inhibited the ability of the cells to form secondary hepatospheres by more than 80% compared with controls (Fig. 1C).
Lupeol Suppressed Tumorigenicity in Nude Mice and Decreased Expression of Liver T-IC Marker.
One of the distinct properties of T-ICs is to initiate tumor formation.13, 14 Next, we examined the effect of lupeol on the tumor initiation abilities of Huh-7 and PLC-8024 cells upon pretreatment with 10 μM lupeol for 72 hours. The tumorigenic ability was compared between cells with or without lupeol pretreatment (Fig. 2A). The incidence of tumors formed was evaluated 40 days after tumor cell inoculation using a CCD camera. Both PLC-8024 and Huh-7 cells without lupeol pretreatment demonstrated tumor formation 40 days after tumor inoculation (Fig. 2A). Conversely, all lupeol-pretreated HCC cells showed no tumor formation, suggesting the suppressive effect of lupeol on HCC tumorigenicity. No tumor formation was observed even on day 80 (data not shown). To demonstrate the in vivo effect of lupeol on tumorigenesis, continuous lupeol administration at a dose of 1 mg/animal was administered intraperitoneally into nude mice right after 1 × 106 Huh-7 or PLC-8024 cells were inoculated into the nude mice subcutaneously. After 40 days, tumor formation was evaluated using a CCD camera. All Huh-7 or PLC-8024 cells without lupeol treatment showed tumor formation. In vivo lupeol administration inhibited the tumor formation ability of Huh-7 or PLC-8024 cells to 20% and 0% respectively (Fig. 2B). A previous study has demonstrated that CD133+ HCC cells have a greater ability to initiate tumor formation in vivo.19 To examine the effects of lupeol on liver T-ICs, CD133+ cells from Huh-7 and PLC-8024 were first sorted by flow cytometry. CD133 expression was evaluated after the administration of 10 μM lupeol for 72 hours. Western blot analysis revealed that CD133 expression decreased in a time-dependent manner in both Huh-7 and PLC-8024 cells (Fig. 2C). Moreover, using flow cytometry analysis, CD133 expression was found to decrease by 86% and 82% in Huh-7 and PLC-8024 cells, respectively, following lupeol administration (Fig. 2D). Accompanied with decrease of CD133 expression upon lupeol treatment, stemness genes including Sox2, Oct4, Nanog, Nestin, and β-catenin were down-regulated (Fig. 2E).
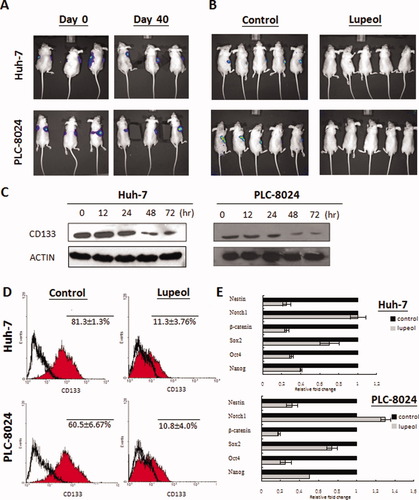
A low dose of lupeol completely inhibited tumorigenicity and decreased expression of CD133 and stemness markers in HCC cells. (A) Lupeol completely suppressed the tumorigenicity of Huh-7 and PLC-8024 cells after pretreatment with 10 μM of lupeol for 72 hours. Three representative mice in each group were shown. On day 0, untreated Huh-7 and PLC-8024 cells were subcutaneously injected into the right side of nude mice, and lupeol-pretreated cells were injected into the left side. Tumor formation was observed in animals injected with untreated cells (right; 6/6) but not in animals injected with untreated cells (left; 0/6) on day 40. (B) In vivo, lupeol suppressed tumorigenicity of Huh-7 and PLC-8024 after corresponding HCC cells were inoculated on the right side of the nude mice. Tumor formation was observed in untreated Huh-7 (5/5) and PLC-8024 (5/5) cells. Administration of lupeol in vivo decreased the tumor-forming abilities of Huh-7 and PLC-8024 to 20% (1/5) and 0% (0/5), respectively. (C) CD133 expression decreased in a time-dependent manner in Huh-7 and PLC-8024 cells upon administration of 10 μM lupeol. (D) Flow cytometry analysis revealed that CD133 expression decreased in both Huh-7 cells (81.3 ± 1.3% to 11.3 ± 3.76%; P < 0.001 [Student t test]) and PLC-8024 cells (60.5 ± 6.67% to 10.8 ± 4.0%; P < 0.001 [Student t test]). (E) Lupeol down-regulated several genes related to stemness in Huh-7 and PLC-8024 cells.
Lupeol Inhibited HCC Cell Growth Synergistically with Chemotherapeutic Agents Through Modulation of PTEN-Mediated ABCG2 Expression.
CD133+ cells demonstrated resistance to chemotherapeutic agents compared with CD133− cells,28 and expansion of the CD133+ population was observed in PTEN knockout mice.29 Based on the results shown in Fig. 2B, we hypothesized that lupeol chemosensitized HCC cells to chemotherapeutic agents through modulation of the PTEN pathway. Using MTT assay, the IC10 and IC30 of Huh-7 and PLC-8024 cells in response to cisplatin and doxorubicin were determined. The IC10 and IC30 values, defined as 10% and 30% growth inhibition of Huh-7 and PLC-8024 cells in response to cisplatin or doxorubicin, were 0.5 and 0.9 μg/mL (Huh-7, cisplatin), 0.3 and 0.6 μg/mL (PLC-8024, cisplatin), 0.7 and 1.45 μg/mL (Huh-7, doxorubicin), and 0.25 and 0.66 μg/mL (PLC-8024, doxorubicin) (Fig. 3A). When lupeol (10 μM) was combined with cisplatin at the IC10 and IC30 doses, growth was inhibited by 32.3% and 58.2%, respectively, in Huh-7 cells and 30.1% and 55.2%, respectively, in PLC-8024 cells (Fig. 3A). Similarly, when lupeol (10 μM) was combined with doxorubicin at the IC10 and IC30 doses, growth was inhibited by 40.2% and 69.2%, respectively, in Huh-7 cells and 37.2% and 61.3%, respectively, in PLC-8024 cells (Fig. 3A). To determine whether this suppressive growth effect of lupeol was mediated through the PTEN pathway, we evaluated PTEN and Akt protein expression when Huh-7 and PLC-8024 were treated with 10 μM lupeol. Western blot analysis revealed that increased PTEN protein levels were accompanied by decreased expression levels of phosphorylated AktSer473 (Fig. 3B). Akt has been shown to regulate ABCG2 expression in stem-like cells in glioma,30 which is important in drug efflux response to chemotherapeutic agents. Consistent with these findings, we observed a decrease in ABCG2 protein expression and a decrease in AktSer473 phosphorylation (Fig. 3B).
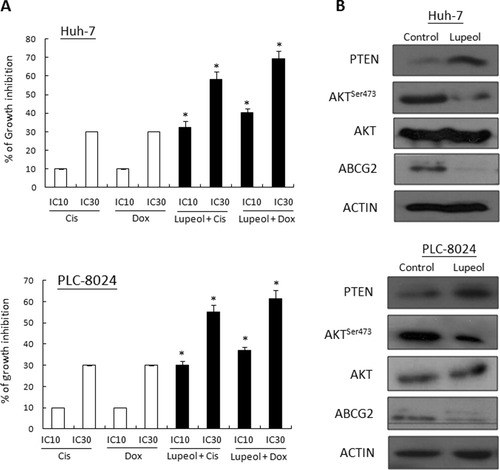
Lupeol exerted a synergistic effect with chemotherapeutic drugs through modulation of the PTEN–Akt–ABCG2 pathway. (A) The effect of cisplatin, doxorubicin, and their combination with lupeol on the growth of Huh-7 and PLC-8024 cells is shown. The cells were exposed to the IC10 and IC30 doses of cisplatin (Cis) or doxorubicin (Dox) for 72 hours, with or without 10 μM lupeol. Columns and bars represent the mean and SD values from three individual experiments performed in duplicate. Differences in cell growth after exposure to cisplatin or doxorubicin, separately or in combination with lupeol, were determined using the Student t test. *P < 0.001. (B) Upon administration of 10 μM lupeol for 72 hours, PTEN was up-regulated in both Huh-7 and PLC-8024 cells. Conversely, AktTSer473 and ABCG2 were down-regulated.
Lentiviral-Mediated PTEN Knockdown Enhanced Self-Renewal Ability and Abolished Lupeol-Induced Chemosensitization.
We evaluated the role of PTEN in chemoresistance and formation of hepatospheres by knocking down PTEN expression in HCC cells using short hairpin RNA knockdown approach. Upon PTEN knockdown in Huh-7 and PLC-8024 cells, AktSer473 expression was up-regulated, whereas CD133 and ABCG2 protein expression was consistently decreased in the two PTEN knockdown clones (#2130 and #31001) each of Huh-7 and PLC-8024 (Fig. 4A). Knockdown of PTEN also decreased hepatosphere formation and the ability to form secondary hepatospheres (Fig. 4B).
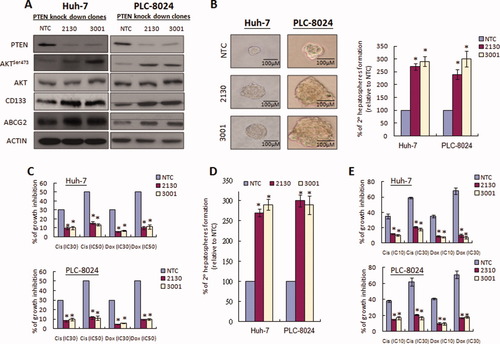
Knockdown of PTEN influenced self-renewal and chemoresistance and reversed the effect of lupeol on liver T-ICs. (A) Knockdown of PTEN in Huh-7 and PLC-8024 cells resulted in up-regulation of AktSer473, CD133, and ABCG2 protein expression in two PTEN knockdown clones (#2130 and #3001) compared with the nontarget control. (B) Knockdown of PTEN also affected the sizes of hepatospheres formed in Huh-7 and PLC-8024 cells. In addition, knockdown of PTEN increased the ability of cells to form secondary hepatospheres by more than two-fold. *P < 0.001 (Student t test). (C) PTEN knockdown clones from Huh-7 and PLC-8024 cells demonstrated enhanced chemoresistance in response to either doxorubicin or cisplatin when compared with the nontarget control (NTC) clones. *P < 0.001 (Student t test). (D) The effect of lupeol on the ability to form secondary hepatospheres and chemoresistance was abolished following PTEN knockdown. *P < 0.001 (Student t test). (E) The chemosensitization effect of lupeol was abolished in the PTEN knockdown clones compared with the non-target control. *P < 0.001 (Student t test).
PTEN down-regulation has been linked to chemoresistance through modulation of the phosphoinositide 3-kinase –Akt pathway in some cancers.31 Next, we determined the change in chemosensitivity upon PTEN knockdown in HCC cells. PTEN knockdown clones from Huh-7 and PLC-8024 cells showed enhanced chemoresistance in response to either cisplatin or doxorubicin compared with the nontarget control clones (Fig. 4C). To examine whether the effect of self-renewal and chemoresistance by lupeol is PTEN-dependent, we compared the self-renewal ability and chemosensitivity between PTEN knockdown HCC cells and nontarget controls upon lupeol treatment. The inhibitory effect of lupeol on the ability of primary spheres to form secondary spheres was significantly diminished in PTEN knockdown clones compared with the nontarget controls (Fig. 4D) (P < 0.001). To examine whether reversal of chemoresistance by lupeol is PTEN-dependent, we compared the chemosensitivity between PTEN knockdown HCC cells and nontarget controls upon lupeol treatment. The chemosensitization effect of lupeol was abolished, as reflected by the marked decrease in inhibition of cell growth in PTEN knockdown clones of Huh-7 and PLC-8024 cells (Fig. 4E).
Combined Lupeol and Chemotherapeutic Drug Treatment Significantly Suppressed Tumor Growth in a Chemoresistant HCC Nude Mouse Model.
We examined the in vivo therapeutic effect of lupeol in the chemoresistant HCC nude mouse model using chemoresistant MHCC-LM3 cells.32 MHCC-LM3 cells were found to be highly chemoresistant, showing approximately 15-fold and approximately four-fold more resistance to doxorubicin and cisplatin, respectively, compared with Huh-7 and PLC-8024 cells by way of MTT assay due to high ABCG2 expression (data not shown). Using this animal model, we examined the effect of lupeol alone as well as in combination with cisplatin and doxorubicin using (1) continuous lupeol administration at a dose of 2 mg/animal (group A), (2) cisplatin (2 mg/kg) and doxorubicin alone (2 mg/kg) (group B), (3) lupeol (2 mg/animal) plus cisplatin and doxorubicin (1 mg/kg and 1 mg/kg) (group C), and (4) corn oil only (group D) as a control. During the experiment, there was no significant decrease in the body weights of the animals in group A (19.9 ± 1.8 g) and group C (20.1 ± 2.2 g) compared with the control group (group D) (16 ± 3.5 g) and in group B (15.3 ± 2.5 g). In fact, the body weights in the former two groups were slightly higher than those of the latter two. These results indicate that lupeol alone or in combination with a low dose of cisplatin and doxorubicin showed no signs of toxicity (infection, diarrhea, or loss of body weight). Histology of the normal organs, such as the tongue, heart, liver, spleen, lung, and kidney, showed no necrosis or significant cell death in hematoxylin and eosin sections (data not shown). The corresponding tumors and their volumes in these four animal groups are shown in Fig. 5A,B. Lupeol significantly reduced the tumor volumes in a manner as potent as the chemotherapeutic treatment by cisplatin and doxorubicin. In addition, lupeol exerted a synergistic effect with low-dose chemotherapeutic drugs, resulting in a dramatic shrinkage of the tumor. Using terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling, we found a remarkable difference in the number of apoptotic nuclei in the tumor tissues treated with lupeol compared with the untreated samples (Fig. 5C). Combined lupeol and cisplatin/doxorubicin treatment significantly induced tumor cell apoptosis compared with the chemotherapeutic drugs alone (Fig. 5C). To further evaluate the molecular changes in the different groups, quantitative polymerase chain reaction analysis using primers against PTEN, CD133, and ABCG2 showed consistent up-regulation PTEN expression in the lupeol-treated groups (groups A and C) compared with the control group (group D) (Fig. 5D). Following treatment with chemotherapeutic drugs, enrichment of the T-IC population was found by increased CD133 and ABCG2 expression in group B. Conversely, the lupeol-treated groups showed the lowest expression of CD133 and ABCG2 expression, suggesting that lupeol can target liver T-ICs.
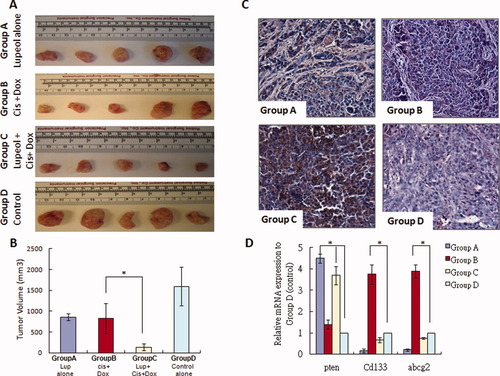
The effect of chemotherapeutic drugs and lupeol in a chemoresistant HCC xenograft model. (A) The in vivo therapeutic effect of lupeol was examined in a nude mice model using chemoresistant MHCC-LM3 cells. The nude mice were randomized into four groups, each consisting of five animals. Each group was treated twice per week for 30 days with either lupeol (2 mg/animal) in 0.2 mL of corn oil (group A), 2 mg/kg cisplatin (Cis) and 2 mg/kg doxorubicin (Dox) (group B), lupeol (2 mg/animal) plus 1 mg/kg cisplatin and 1 mg/kg doxorubicin (group C), or corn oil alone (group D) as the control group. All reagents were administered intraperitoneally. Corresponding tumors were excised from the animals after treatment. (B) Tumor volume from the four groups of animals in W × L2/2. *P < 0.01 (Student t test). (C) By way of terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling, combined lupeol and chemotherapeutic treatment induced tumor cell apoptosis compared with treatment with chemotherapeutic drugs alone. (D) Quantitative polymerase chain reaction analysis revealed that PTEN expression was consistently up-regulated in the lupeol-treated groups (groups A and C) compared with the control group (group D). *P < 0.01 (Student t test). Enrichment of the T-IC population was shown by increased expression levels of CD133 and ABCG2 in group B compared with the control group (group D). *P < 0.01 (Student t test). The lowest expression levels of CD133 and ABCG2 were found in group A.
Discussion
Targeting T-ICs through inhibition of the self-renewal process is an emerging strategy for the treatment of cancer. Because these interventions focus on self-renewal rather than toxicity induction, they are potentially less toxic than conventional chemotherapeutic drugs. In the present study, a low concentration of lupeol (10 μM) was found to inhibit in vitro formation of hepatospheres derived from HCC cell lines and clinical samples. This dose of lupeol had no effect on cell proliferation or viability. In addition, lupeol decreased hepatosphere formation upon serial passaging, suggesting the inhibitory role of lupeol on the self-renewal of stem cells. To our knowledge, this is the first study demonstrating the inhibitory effect of dietary substances on the self-renewal of enriched stem/progenitor cells from clinical tumor samples. At a high concentration, lupeol effectively and selectively inhibited cellular proliferation of HCC cells but exerted a minimal effect on nontumorigenic normal liver cell lines.
Apart from the self-renewal ability, T-ICs are capable of tumor initiation.13, 14 Pretreatment of PLC-8024 and Huh-7 cells with low-dose lupeol suppressed tumor formation on day 40 after tumor inoculation. In addition, no tumor formation was observed even by day 80 (data not shown), suggesting that lupeol suppressed tumor formation rather than simply delaying tumor growth. In addition, lupeol suppressed tumorigenicity of HCC cells upon its continuous intraperitoneal administration. CD133 was recently reported to be a marker of liver T-ICs, which are capable to initiating tumor formation in vivo.19 In this study, we found that CD133 protein levels were consistently decreased in a time-dependent manner using western blot analysis. Further quantification by way of flow cytometry analysis showed that the percentage of CD133 was decreased significantly after the cells were pretreated with lupeol. These data suggest that lupeol suppresses tumorigenicity by decreasing CD133 expression in HCC cells.
T-ICs are thought to be quiescent and thus more resistant to conventional chemotherapy.33 CD133+ HCC cells are more chemoresistant to chemotherapeutic drugs by preferential activation of the Akt pathway.28 Because lupeol suppresses CD133 expression, we hypothesized that lupeol chemosensitized HCC cells to chemotherapeutic drugs. In this study, we have documented a chemosensitization effect of lupeol on HCC cells to treatment with either doxorubicin or cisplatin. Our results have confirmed our previous findings that lupeol chemosensitized head and neck cancer cells to cisplatin treatment.24 In addition, these results indicate that the chemosensitization effect of lupeol is not drug-specific. In addition, we observed that lupeol significantly modulated the PTEN–Akt pathway. The PTEN-Akt pathway has been reported to regulate ABCG2 activity in stem-like cells in gliomas.30 In this study, ABCG2 expression was consistently reduced upon lupeol treatment, and this was accompanied by a decrease in AktSer473 phosphorylation. Thus, the results suggest that lupeol may sensitize HCC cells by down-regulating ABCG2 expression through the PTEN–Akt pathway.
The central role of PTEN in self-renewal and chemoresistance in HCC was studied by knocking down PTEN expression using a lentiviral-based short hairpin RNA approach. Western blot analysis confirmed the regulation of the PTEN–Akt pathway on CD133 and ABCG2 expression in HCC cells. Our result is consistent with recent findings that showed the role of PTEN in the enrichment of stem cells in breast and brain tumors.30, 34 The increased number of hepatospheres formed, and the percentage of cells needed to form secondary spheres also demonstrated the role of PTEN in the self-renewal process. PTEN down-regulation has been linked to chemoresistance through modulation of the phosphoinositide 3-kinase–Akt pathway.31 Along with the increase in ABCG2 expression, we observed a decrease in chemosensitivity upon PTEN knockdown in HCC cells. Most importantly, using the PTEN knockdown approach, the suppressive role of lupeol on self-renewal and chemoresistance was shown to act through the PTEN–Akt–ABCG2 pathway. The mechanism by which lupeol up-regulates PTEN is unknown. Our data revealed that lupeol up-regulated PTEN mRNA levels (data not shown), indicating transcriptional regulation of lupeol on PTEN. Analysis of PTEN's promoter suggests that there are some regulatory factors that modulate PTEN's transcription. Sp1 and c-Jun have also recently been suggested as PTEN transcription factors.35, 36 It is possible that lupeol transcriptionally activates PTEN through these transcription factors.
In this animal model, the highly chemoresistant cell line MHCC-LM3 was chosen for subcutaneous tumor inoculation because this HCC cell line is more chemoresistant compared with the Huh-7 and PLC-8024 cell lines (unpublished data). In addition, MHCC-LM3 has a high ABCG2 expression.37 We found that lupeol shrank the tumor volume by induction of apoptosis. Moreover, lupeol did not show signs of toxicity; importantly, the other organs of the mice showed no histological damage or necrosis. Treatment with lupeol alone had an effect similar to that of cisplatin plus doxorubicin in suppressing tumor growth. However, combined treatment with cisplatin and doxorubicin had severe side effects in terms of decreasing body weight. Our data have shown that lupeol was as potent as cisplatin in terms of decreasing tumor volume. Lupeol combined with a low dose of cisplatin and doxorubicin could effectively suppress tumor growth. More importantly, lupeol given with a low dose of cisplatin and doxorubicin was approximately 11-fold more potent than cisplatin and doxorubicin alone and had no side effects in this animal model. To confirm the in vitro mechanism of lupeol, corresponding RNAs from each group were extracted and quantified by way of quantitative polymerase chain reaction. Enrichment of the stem cell population was shown by the increased levels of CD133 and ABCG2 upon treatment with chemotherapeutic drugs alone. These results further support enrichment of the T-IC population found in lung cancer following chemotherapy.38 Consistent with our in vitro data, lupeol-treated tumors had decreased expression of CD133 and ABCG2 compared with control tumors. If the T-IC hypothesis is correct, this result could explain the chemosensitization effect of lupeol.
To our knowledge, this study is the first in vitro and in vivo demonstration of the anti–T-IC efficacy of lupeol, which acts by modulating the PTEN–Akt–ABCG2 pathway against HCC. Lupeol exerted a significant synergistic and cytotoxic effect without adverse effects when combined with low doses of cisplatin and doxorubicin. Overall, these findings have provided evidence that lupeol may be a dietary phytochemical that has the potential to target liver T-ICs.




