Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis†
Potential conflict of interest: Nothing to report.
fax: (34)-918854526.
Abstract
Systemic activation of the inflammatory immune system contributes to the progression of cirrhosis with ascites. Immune cells become activated after interacting at the mesenteric lymph nodes (MLNs) with bacteria translocated from the gut, and thereafter reach the bloodstream through recirculation. It is unknown whether systemic activation of the immune system is present in pre-ascitic cirrhosis, in which gut bacterial translocation has not been described. The purpose of this study was to determine whether systemic activation of the immune system initiates in rats with compensated carbon tetrachloride (CCl4)-induced cirrhosis, and if so to establish the activation site of immune cells. We studied the activation status of immune cells in peripheral blood, MLNs, and hepatic lymph nodes (HLNs). Systemic inflammation was present in rats with cirrhosis, as shown by expansion (P < 0.01) of circulating total and inflammatory monocytes and recently activated CD134+ T helper (Th) cells. The same populations of cells were increased (P < 0.01) in MLNs and HLNs. Bacterial translocation was absent in rats with cirrhosis or control rats, but bacterial DNA fragments were present in the MLNs of 54% of rats with cirrhosis. The liver was the source of activated immune cells present in the blood, as shown by the direct correlation between activated Th cells in the blood and HLNs, but not in MLNs, and the normalization by gut decontamination with antibiotics of activated cells in MLNs, but not in the blood or HLNs. Conclusion: In experimental cirrhosis, systemic activation of the immune system occurs before ascites development and is driven by recirculation of cells activated in HLNs. In addition, in compensated cirrhosis, bacterial DNA fragments reach the MLNs, where they elicit a local inflammatory response. (HEPATOLOGY 2010;52:2086-2095)
The immune system is a complex network of cells and molecules that plays a relevant role in the defense against infections through its ability to recognize and develop a response against non–self-antigens.1 The effector defensive response is associated with the induction of an orchestrated cascade of events that involve activation of immune system cells and production of cytokines at the systemic and/or local level. Although the inflammatory response is essential for maintaining tissue homeostasis, protecting against infection, and mediating immune responses, it can also contribute to tissue injury.2
The immune system is abnormally activated at the systemic level in patients and experimental models with cirrhosis and ascites.3-5 The alteration is characterized by expansion of activated lymphocytes and monocytes in peripheral blood and an increased production of proinflammatory cytokines.3-5 It has been claimed that in cirrhosis with ascites, this systemic inflammatory response is mainly induced and maintained by the interaction of cells of the immune system with bacteria that have translocated from the intestinal lumen at the mesenteric lymph nodes (MLNs). Thereafter, recirculation of activated immune cells extends the inflammation response to the peripheral blood.5-7 Activated immune cells can migrate to the tissues and modify the function of somatic cells, such as vascular endothelial and brain cells, and contribute to the nonhepatic clinical expression of cirrhosis.3, 8-10
Despite the pivotal role of systemic activation of the immune system in cirrhosis, it is unknown whether this abnormality already exists in the compensated pre-ascitic stage of the disease. It is possible to hypothesize that the liver, the main organ of inflammation in cirrhosis, has a crucial role as a source of abnormally activated monocytes and lymphocytes. Such a particular role of the liver appears to be particularly relevant in rats with cirrhosis at the preascitic stage, in which gut bacterial translocation is not increased.11 The aim of this study was to investigate whether there is in fact systemic activation of the inflammatory immune system in rats with preascitic compensated carbon tetrachloride (CCl4)-induced cirrhosis, and if so to establish the pivotal site where immune system cells become activated.
Abbreviations
APC, allophycocyanin; CCl4, carbon tetrachloride; FITC, fluorescein isothiocyanate; HLN, hepatic lymph node; IL-6, interleukin-6; MLN, mesenteric lymph node; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; Tc, T cytotoxic; Th, T helper; TNFα, tumor necrosis factor α.
Materials and Methods
Animals
Male Wistar rats (Harlan, Horst, The Netherlands) were used for all experiments. Animals were fed a standard laboratory diet with water and food provided ad libitum. All experiments were approved by the Spanish animal welfare authorities and performed in accordance with the animal care guidelines of our institution. All studies were conducted according to the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985) and in compliance with local regulations.
Induction of Cirrhosis
Cirrhosis was induced by CCl4 feeding by gavage on a weekly basis, along with phenobarbital added to the drinking water. The initial 20-μL dose of CCl4 was subsequently increased, depending on the animal's weekly change in body weight. Animals were sacrificed at 12 weeks, when cirrhosis without ascites is almost constantly present.12 Experiments were performed 7 days after the last CCl4 dose. Cirrhosis was confirmed by way of trichrome staining of livers.
Instrumentation
Experiments were performed in 8-hour fasted animals under sterile conditions. Anesthesia was induced with isofluorane (Forane; Abbott Laboratories, Madrid, Spain). The abdomen was opened, and 5-10 mL of peripheral blood was obtained by way of aortic puncture. After blood collection, the lymph nodes of the ileocecal area (MLNs) and hepatic hilum (hepatic lymph nodes [HLNs]) were aseptically removed. Thereafter, the liver was perfused through the portal vein with a prewarmed digestion buffer, cut into small pieces, and enzymatically digested as described.13, 14
Study Design
The phenotype and activation status of lymphocyte and monocyte subpopulations in the different immune system compartments (MLNs, HLNs, liver, and peripheral blood) were examined in rats with preascitic cirrhosis (n = 28) and in healthy, phenobarbital-treated age- and sex-matched rats (n = 20). A subgroup of rats with preascitic cirrhosis (n = 14) received a 2-week course of broad-spectrum oral nonabsorbable antibiotics (norfloxacin 10 mg/kg/day and vancomycin 16 mg/day; Sigma-Aldrich, St. Louis, MO) or placebo to investigate the impact of enteric bacterial products on immune cells. Finally, we examined the phenotype and activation status of immune cell subpopulations in rats receiving the first three doses of CCl4 (n = 5) or only phenobarbital in drinking water (n = 5).
Methods
Peripheral blood mononuclear cells were separated by way of Histopaque-1083 (Sigma-Aldrich) density gradient centrifugation. Single mononuclear cell suspensions from MLNs and HLNs were obtained by pressing the nodes through a 150-μm pore mesh (Sefar Maissa SA, Madrid, Spain) and from the liver by a modification of the method of Crispe.13, 14 Briefly, perfused livers were digested with media containing collagenases (type I, Invitrogen, Grand Island, NY; type IV, Sigma-Aldrich) and DNase I (Roche, Mannheim, Germany). The resultant cell suspension was passed through a stainless mesh and centrifuged to obtain a cell pellet depleted of hepatocytes.
Surface Immunofluorescence and Quantitative Flow Cytometry.
Proportions of monocyte, B cell, and T cell subpopulations were determined in cell suspensions obtained from peripheral blood, MLNs, HLNs, and liver by way of four-color immunofluorescence and flow cytometry in a FACScalibur cytometer using Cell Quest software (Becton-Dickinson, San Jose, CA). Analyses were performed using FlowJo software (Tree Star, San Carlos, CA). Cell suspensions were incubated with combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)-, allophycocyanin (APC)-, and AlexaFluor647-labeled monoclonal antibodies (Table 1). The rat monoclonal antibodies (BD Pharmingen, San Diego, CA, and Serotec, Kidlington, Oxford, UK) used were: APC-CD3 (1F4), PE-Cy5.5-CD4 (OX-38), PerCP-CD8a (OX8), FITC-CD134 (OX 40), PE-CD62L (HRL1), AlexaFluor647-CD11b (OX-42), PE-NKR-P1A (10/78), FITC-CD43 (W3/13), FITC-CD45RA (OX 33), PerCP-RT1B (OX 6), PE-OX62 (MRC OX-62), and PE-CD80 (3H5). Immune cell numbers in lymph nodes, liver, and blood were counted in a Neubauer chamber. Absolute cell counts of mononuclear cell subpopulations (cells/node × 10−3, cells/liver × 10−3 and cells/μL of blood) were calculated by multiplying the absolute number by the proportion of each subpopulation established by flow cytometry.
| Immune Cell Subsets | Function Associated With the Indicated Surface Receptors |
|---|---|
| T cells | |
| CD3 | Signal transduction module in the T cell receptor for antigen (TCR/CD3 complex); expressed in all T cell subsets |
| CD4 | TCR/CD3 coreceptor that recognizes MHC class II molecules, which present antigens to Th cells; Th cell marker |
| CD8 | TCR/CD3 coreceptor that recognizes class I MHC molecules, which present antigens to Tc cells; identifies Tc cell marker |
| CD62L | Member of the selectin adhesion molecule family that is required for lymphocyte homing to peripheral lymph nodes and is rapidly shed from lymphocytes upon activation; the level of CD62L expression distinguishes naive from effector T cells |
| CD134 | Also termed OX-40 receptor; a costimulatory molecule that identifies a subpopulation of recently activated Th cells |
| B cells | |
| CD45RA | Isoform of the leukocyte common antigen that identifies B cells |
| CD80 | One of the accessory molecules that play an important role in T cell–B cell costimulatory interactions |
| Monocytes | |
| CD11bbright | Integrin expressed on the surface of monocyte lineages |
| NK-RP1Amed | NKR-P1A has been detected at low levels on peripheral blood monocytes, and its expression is up-regulated specifically in a subpopulation of large monocytes with phagocytic capacity |
| CD43 | Rat monocytes can also be divided into CD43low and CD43high subsets with distinct migratory properties in vivo; expression is down-regulated in inflammatory monocytes |
| Dendritic cells | |
| OX-62 | Rat αE2 integrin expressed by myeloid dendritic cells and a subset of T cells |
| RT1B | Class II MHC: responsible for antigen presentation to CD4+ Th cells; expressed on the surface of dendritic cells |
- Abbreviation: MHC, major histocompatibility complex.
Bacteriological Study.
Samples of MLN were inoculated in thioglycollate (Scharlab, Barcelona, Spain) and incubated at 37°C for 48 hours. Specific microorganisms were identified by a manual biochemical test or automated system (Microscan; Baxter, Irvine, CA). Bacterial translocation from the intestinal lumen was defined as the presence of viable organisms (i.e., a positive bacteriological culture result from the MLNs).8, 15, 16
DNA Isolation, Amplification, and Sequencing.
MLNs were homogenized in PBS by way of sonication (UP100H Ultrasonic Processor, Hielscher, Teltow, Germany). Genomic DNA from homogenized MLNs was isolated using the QIAmp Tissue Kit (Qiagen, Hilden, Germany). Bacterial DNA was identified by running a broad-range polymerase chain reaction followed by nucleotide sequencing of a conserved region of the 16SrRNA gene.17
Inflammatory Markers in MLN and Serum, and Aminotransferases in Serum.
Serum samples and homogenized MLNs were stored at −80°C until analysis. Enzyme-linked immunosorbent assay kits (Biosource International, CA, and R&D Systems, Minneapolis, MN) were used to determine tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) according to the manufacturers' instructions. The sensitivity detection limits were 5 pg/mL and 8 pg/mL, respectively. All experiments were performed in duplicate. Serum concentrations of aspartate aminotransferase and alanine aminotransferase were determined using an automatic analyzer (Beckman Coulter, Villepinte, France).
Statistical Analysis
Results are presented as the mean ± SD. Quantitative variables were analyzed using a Fisher's exact test and an unpaired Student t test. Correlations between selected variables were assessed by way of linear regression analysis. The level of statistical significance was set at P < 0.05.
Results
Seventeen out of 45 animals (38%) died during cirrhosis induction. On the day of the experiment, cirrhosis was present in all the animals on CCl4, as shown by histological assessment of the livers (data not shown), and the peritoneal cavity was free of ascites. The rats with cirrhosis showed higher serum aminotransferase levels (P < 0.01) than controls (aspartate aminotransferase, 162 ± 71 versus 64 ± 21 IU/L; alanine aminotransferase, 51 ± 14 versus 21 ± 2 IU/L).
Expansion of Activated T Helper Cells and Monocytes and Rise in Proinflammatory Cytokine Concentrations in the Peripheral Blood of Rats With Preascitic Cirrhosis.
We first examined the presence of systemic immune system disturbance in rats with cirrhosis. Compared with controls, there was marked expansion of activated T helper (Th) cells, B cells, and monocytes in the peripheral blood of rats with cirrhosis (Table 2). Expansion of activated Th cells was indicated by a 5.2-fold increase (P < 0.001) in the subset of Th cells expressing the costimulatory receptor CD134, a marker of recent activation, and by a 1.6-fold increase (P < 0.01) in the subset of effector Th cells, identified as those that have lost the selectin adhesion molecule CD62L.18 The CD11bbright monocyte population was also markedly expanded and showed signs of activation, including a 2.9-fold increase (P < 0.001) in the number of inflammatory monocytes,19 which are those with phagocytic activity and the ability to migrate to inflamed tissues.20, 21 Other antigen-presenting cells (such as dendritic cells) were also expanded (2.2-fold) in the peripheral blood of rats with cirrhosis. In agreement with the increased number of activated Th cells and monocytes, levels of proinflammatory cytokines were significantly higher in the circulation of rats with cirrhosis (TNFα, 19.4 ± 0.6 versus 8.9 ± 0.2 pg/mL [P < 0.05]; IL-6, 58.2 ± 1.2 versus 50.6 ± 2.8 pg/mL, P < 0.05).
| Immune Cell Subsets | Peripheral Blood (cells/μL) | MLNs (cells/node × 10−3) | HLNs (cells/node × 10−3) | |||
|---|---|---|---|---|---|---|
| Rats With Preascitic Cirrhosis (n = 28) | Control Rats (n = 20) | Rats With Preascitic Cirrhosis (n = 28) | Control Rats (n = 20) | Rats With Preascitic Cirrhosis (n = 28) | Control Rats (n = 20) | |
| T cells (CD3+) | 1,243 ± 554 | 1,087 ± 522 | 7,222 ± 3,199‡ | 5,208 ± 2,890 | 2,583 ± 1,340* | 1,495 ± 799 |
| Total Th (CD4+ CD8−) | 896 ± 397 | 761 ± 359 | 4,724 ± 2,875 | 3,768 ± 1,968 | 2,098 ± 1,088† | 1,077 ± 666 |
| Recently activated Th (CD134+) | 89 ± 49* | 17 ± 11 | 458 ± 252* | 254 ± 160 | 319 ± 114* | 68 ± 37 |
| Effectory Th (CD62L−) | 106 ± 60‡ | 66 ± 41 | 1387 ± 663* | 809 ± 253 | 987 ± 473* | 228 ± 102 |
| Total Tc (CD4− CD8+) | 307 ± 170 | 293 ± 164 | 1344 ± 805 | 1328 ± 801 | 407 ± 249 | 390 ± 127 |
| Effectory Tc (CD62L−) | 38 ± 25 | 32 ± 20 | 190 ± 73 | 143 ± 68 | 53 ± 35 | 34 ± 22 |
| B cells (CD45RA+) | 871 ± 385† | 580 ± 345 | 5,589 ± 2,362* | 3,214 ± 1,657 | 1,449 ± 672† | 699 ± 308 |
| Activated B cells (CD80+) | 24 ± 19‡ | 10 ± 7 | 132 ± 70† | 50 ± 60 | 73 ± 53† | 19 ± 18 |
| Monocytes (CD3−CD45RA− NK-RP1Ahigh−CD11bbright+) | 491 ± 202* | 126 ± 70 | 54 ± 42‡ | 17 ± 11 | 38 ± 28† | 6 ± 4 |
| Inflammatory monocytes (CD43−NK-RP1Amed+) | 88 ± 55* | 30 ± 17 | 38 ± 15‡ | 8 ± 5 | 18 ± 14† | 3 ± 2 |
| Dendritic cells (CD3−CD45RA− OX62+RT1Bbright+) | 11 ± 9‡ | 5 ± 3 | 88 ± 69‡ | 38 ± 27 | 63 ± 43† | 12 ± 11 |
- * P < 0.001 versus control rats.
- † P < 0.01 versus control rats.
- ‡ P < 0.05 versus control rats.
Th Cells and Monocytes Become Activated in HLNs of Rats With Preascitic Cirrhosis.
The number of intrahepatic Th cells increased by 1.4-fold (4,667 ± 2,481 versus 3,281 ± 1,107 cells/liver × 10−3 [P < 0.05]) and that of Th cells expressing the CD134 receptor by 4.8-fold in rats with cirrhosis compared with controls (785 ± 411 versus 163 ± 101 cells/liver × 10−3 [P < 0.01]). The latter finding was concurrent with expansion of the total number of activated effector (CD62L−) Th cells (4,910 ± 3,340 versus 1,450 ± 401 cells/liver × 10−3 [P < 0.05]). As expected,22 the livers of rats with cirrhosis showed a significantly lower number of natural killer cells compared with controls (3,097 ± 2,002 versus 5,433 ± 2,679 cells/liver × 10−3 [P < 0.01]), but there were no signs of activation of other immune cell populations, such as B cells expressing the CD80+ receptor, or monocyte/macrophages.
It has been established that there is an intense immune system cell trafficking between the liver and its draining lymph nodes (the HLNs), which are located along the hepatic artery as far as the portal vein.23, 24 The HLNs of rats with cirrhosis showed marked enlargement (27 ± 12 versus 13 ± 6 mg [P < 0.05]), likely because of the significant expansion of T cells (1.7-fold), B cells (2.1-fold), and monocytes (6.3-fold). Activation of HLN Th cells was shown by significantly increased (P < 0.001) numbers of recently activated CD134+ Th cells, as well as of those that had lost the selectin adhesion molecule CD62L. The number of inflammatory monocytes was higher by six-fold in the HLNs of rats with cirrhosis (Table 2).
Bacterial DNA Fragments in MLNs of Rats With Preascitic Cirrhosis Start a Local Immune Response in the Absence of Microbiological Evidences of Bacterial Translocation.
We have noted that an orchestrated immune response cascade initiated by enteric bacteria in MLNs contributes to the systemic inflammation of experimental cirrhosis with ascites.5 However, gut bacterial translocation, although apparent in rats with cirrhosis,11, 16 is distinctively absent in rats without ascites.11 As in the HLNs, the MLNs of rats with cirrhosis showed significant (P < 0.05) simultaneous expansion of T cells (1.4-fold), B cells (1.7-fold), and monocytes (3.2-fold), accounting for an increased lymph node weight (24 ± 12 versus 15 ± 7 mg [P < 0.05]). Indeed, the subpopulations of recently activated CD134+ and CD62L− Th cells were expanded (P < 0.001), as were the numbers of B cells expressing the CD80+ receptor and monocytes expressing the activation marker NKR-P1A (Table 2). Of note, we also observed the marked expansion of dendritic cells (by 2.3-fold [P < 0.05]) in the MLNs of rats with cirrhosis.
Thereafter, we explored the contribution of enteric bacteria to the activation of MLNs and circulating immune system cells. Although no episodes of bacterial translocation were detected in rats with cirrhosis or control rats (culture-negative MLNs), bacterial DNA was demonstrated in the MLNs of 15 of the 28 rats with cirrhosis (53.6%) (Table 3) and in no control animals (P < 0.01). As illustrated in Fig. 1, there is a close association between the immune system alteration observed in the MLNs of rats with cirrhosis and the presence of bacterial DNA fragments. Indeed, the numbers of activated Th cells, B cells, and monocytes in the MLNs of rats with cirrhosis without bacterial CpG motifs were similar to those observed in control rats. Accordingly, levels of the proinflammatory cytokines TNFα and IL-6 were only elevated in the MLNs of rats with cirrhosis and bacterial DNA (Fig. 2).
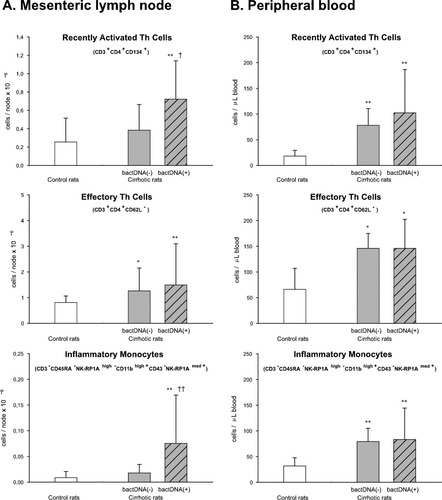
Immunophenotypic profile of Th cells and monocytes in the (A) MLNs and (B) peripheral blood of control rats and rats with preascitic cirrhosis with (+) or without (−) bacterial DNA fragments (bactDNA) in the MLNs. *P < 0.05 versus control rats. **P < 0.01 versus control rats. †P < 0.05 versus bactDNA (−) rats with cirrhosis. ††P < 0.01 versus bactDNA (−) rats with cirrhosis.
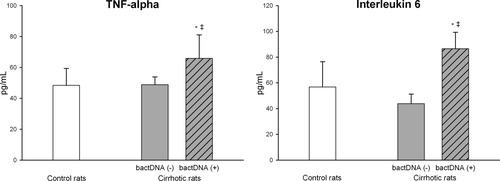
Levels of TNFα and IL-6 in the MLNs of control rats and rats with preascitic cirrhosis with (+) or without (−) bacterial DNA fragments (bactDNA) in the MLNs. *P < 0.05 versus control rats. **P < 0.01 versus control rats. †P < 0.05 versus bactDNA (−) rats with cirrhosis. ††P < 0.01 versus bactDNA (−) rats with cirrhosis.
| Study Goup | MLNs |
|---|---|
| Rats with cirrhosis | |
| n = 11 | Escherichia coli |
| n = 3 | Pseudomonas spp. |
| n = 1 | Klebsiella |
| n = 13 | Negative |
| Control rats | |
| n = 20 | Negative |
HLNs Are the Main Source of Activated Immune Cells Contributing to Systemic Inflammation in Rats With Preascitic Cirrhosis.
We went on to examine the relative contributions of liver/HLN and/or enteric bacterial driven-mesenteric inflammation to the activated immune system cells observed in the circulation of rats with cirrhosis. To this end, we analyzed the activation status of immune cells in peripheral blood according to the presence of bacterial DNA in MLNs and in response to bowel decontamination with nonabsorbable antibiotics, as well as correlations among activated immune cells in the compartments studied. As shown in Fig. 1, the numbers of total and activated Th cells and monocytes in the peripheral blood of rats with cirrhosis without bacterial DNA in MLNs were significantly greater than in control animals, but similar to those observed in rats with cirrhosis with bacterial DNA.
Bowel decontamination normalized the number and activation state of immune cells in the MLN, but did not affect immune cell subpopulations in peripheral blood or HLN (Table 4). We did not detect fragments of bacterial DNA in the MLNs of any of the antibiotic-treated rats with cirrhosis. Indeed, the broad-spectrum nonabsorbable antibiotics abrogated the expansion of recently activated CD134+ and CD62L− Th cells, inflammatory monocytes, and dendritic cells in the MLNs of rats with cirrhosis, whose values were no longer significantly different from those found in control animals. In contrast, antibiotics lacked any significant effects on the distribution and activation status of immune cells in the HLNs and peripheral blood of rats with cirrhosis (Table 4).
| Immune Cell Subsets | Peripheral Blood (cells/μL) | MLNs (cells/node × 10−3) | HLNs (cells/node × 10−3) | |||
|---|---|---|---|---|---|---|
| Placebo-Treated Rats With Cirrhosis (n = 7) | Antibiotic-Treated Rats With Cirrhosis (n = 7) | Placebo-Treated Rats With Cirrhosis (n = 7) | Antibiotic-Treated Rats With Cirrhosis (n = 7) | Placebo-Treated Rats With Cirrhosis (n = 7) | Antibiotic-Treated Rats With Cirrhosis (n = 7) | |
| T cells (CD3+) | 1,411 ± 424 | 1,235 ± 447 | 6,417 ± 3,712 | 3,111 ± 1,477† | 2,631 ± 1,483 | 2,783 ± 1,411 |
| Total Th (CD4+ CD8−) | 982 ± 262 | 902 ± 300 | 4,765 ± 2,880 | 2,390 ± 1,125† | 2,109 ± 1,261 | 2,274 ± 1,798 |
| Recently activated Th (CD134+) | 99 ± 57 | 94 ± 60 | 501 ± 357 | 155 ± 90* | 349 ± 164 | 376 ± 193 |
| Effectory Th (CD62L−) | 137 ± 40 | 207 ± 132 | 1791 ± 796 | 598 ± 230‡ | 905 ± 513 | 847 ± 412 |
| Total Tc (CD4− CD8+) | 326 ± 171 | 306 ± 152 | 1426 ± 594 | 669 ± 344‡ | 451 ± 204 | 426 ± 498 |
| Effectory Tc (CD62L−) | 32 ± 18 | 36 ± 45 | 255 ± 151 | 72 ± 31 | 57 ± 46 | 24 ± 14 |
| B cells (CD45RA+) | 925 ± 380 | 903 ± 438 | 5,820 ± 2,495 | 2,979 ± 1,216† | 1,638 ± 650 | 1,321 ± 854 |
| Activated B cells (CD80+) | 30 ± 19 | 26 ± 14 | 175 ± 110 | 55 ± 30* | 67 ± 31 | 55 ± 48 |
| Monocytes (CD3−CD45RA− NK-RP1Ahigh−CD11bbright+) | 570 ± 319 | 320 ± 182 | 70 ± 45 | 15 ± 8† | 44 ± 21 | 38 ± 20 |
| Inflammatory monocytes (CD43−NK-RP1Amed+) | 72 ± 41 | 66 ± 35 | 41 ± 27 | 6 ± 3† | 16 ± 9 | 16 ± 10 |
| Dendritic cells (CD3−CD45RA− OX62+RT1Bbright+) | 15 ± 7 | 14 ± 6 | 119 ± 72 | 29 ± 17† | 64 ± 38 | 90 ± 46 |
- * P < 0.001 versus placebo-treated rats with cirrhosis.
- † P < 0.01 versus placebo-treated rats with cirrhosis.
- ‡ P < 0.05 versus placebo-treated rats with cirrhosis.
Notably, we observed direct correlation between the percentage of recently activated Th cells (r = 0.59, P < 0.01) and inflammatory monocytes (r = 0.64, P < 0.01) found in the blood and HLNs of individual rats with cirrhosis (Fig. 3), further supporting the role of HLNs as a source of activated immune cells that recirculate toward peripheral blood.
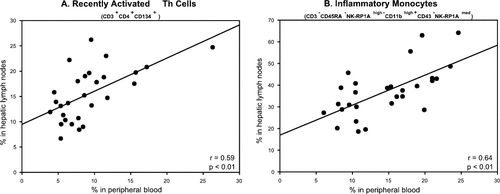
Direct correlation between the percentage of (A) recently activated (CD134+) Th (CD3+CD11bbright−CD4+CD8−) cells and (B) inflammatory (CD43−NK-RP1Amed+) monocytes (CD3−CD45RA−NK-RP1Ahigh−CD11bbright+) present in the blood and HLNs of rats with preascitic cirrhosis.
Absence of Inflammation in Peripheral Blood or in MLNs of Rats on a Short Course of CCl4.
We also investigated the effect of a short-course of CCl4 on the immune cells of the MLNs, HLNs, and peripheral blood of rats before chronic liver damage becomes established (Supporting Information Table 1). Our results indicate similar numbers of CD134+ and CD62L−-Th cells and of inflammatory monocytes in peripheral blood and MLNs in rats on a short course of CCl4 and in controls. However, animals receiving three doses of CCl4 showed a discrete expansion (P < 0.05) of CD134+ and CD62L− Th cells and of inflammatory monocytes (2.0-, 2.4-, and 2.6-fold increases, respectively) in HLNs.
Discussion
In this study, we tested the hypotheses that (1) systemic activation of the inflammatory immune system occurs in the compensated, preascitic stage of experimental cirrhosis and (2) this immune activation is mainly induced in the draining lymph nodes of the liver and/or intestine. Our findings indicate that in rats with cirrhosis, the proinflammatory immune system is activated at the systemic level before ascites appearance. Such a proinflammatory state is concurrent with expansion of activated T cells and monocytes in the HLNs, which at this stage of cirrhosis constitutes the main source of the expanded activated immune cells present in the peripheral blood. In addition, this study reveals that translocation of enteric bacterial products, as assessed by the presence of bacterial DNA in the MLNs of animals with cirrhosis, occurs in rats without ascites and starts off an inflammatory response restricted to the local environment.
The finding of an intense expansion in the peripheral blood of recently activated CD134+- and effector CD62L−-Th cells and inflammatory monocytes, along with increased serum levels of proinflammatory cytokines, is consistent with activation of the inflammatory immune system at the systemic level in pre-ascitic experimental cirrhosis. These data are consistent with a limited number of reports that show expansion of activated monocytes and/or augmented concentrations of proinflammatory cytokines in the peripheral blood of patients with compensated cirrhosis4, 25, 26; however, to the best of our knowledge, this had not yet been shown in experimental compensated cirrhosis. As in other tissues, immune system responses to hepatic antigens and cellular lesion products can take place in regional draining lymph nodes (HLNs).23 Activated immune cells recirculate after leaving the HLNs and thereafter can home in different organs, including the inflamed liver. Indeed, direct correlation was observed between circulating activated Th cells and inflammatory monocytes and these cells present in the HLNs, but not those in the liver.
Our previous study conducted in rats with cirrhosis and ascites identified the MLNs as the source of a systemic immune response triggered by enteric bacteria that thereafter reach the peripheral blood by recirculation of activated immune system cells.5 This is in contrast with the situation in rats with compensated cirrhosis, in which recirculation of immune cells preferentially triggered in hepatic but not mesenteric draining lymph nodes seems to be the main source of the activated immune system cells present in the blood. This contention is supported by (1) abrogation by nonabsorbable antibiotics of the ongoing proinflammatory immune response in MLNs, but not in HLNs or peripheral blood, and (2) direct correlation between HLNs and blood proportions of recently activated Th cells and inflammatory monocytes. Thus, activated immune cells that leave the HLNs and recirculate in peripheral blood preferentially account for the systemic immune activation observed in rats with preascitic cirrhosis.
Our detection of passage of bacterial DNA fragments to the MLNs in rats with preascitic cirrhosis is of particular interest. To date, viable (i.e., positive culture) or nonviable (i.e., DNA fragments) bacteria in the MLNs had only been reported in rats with cirrhosis and ascites.6, 11, 16, 27 Similarly, passage of enteric bacterial products to the bloodstream, as shown by increased serum lipopolysaccharide-binding protein or bacterial DNA in serum, has only been demonstrated in patients with cirrhosis and ascites.3, 10, 17, 28 In a setting of cirrhosis with ascites, bacterial translocation results from enteric bacterial overload, deranged intestinal permeability, and probably also impaired intestinal immunity, which is unable to eliminate the translocated microorganisms.6, 16, 29 The detection of bacterial genome fragments but not of viable bacteria in the MLNs of our rats with preascitic cirrhosis indicates that the mechanisms leading to passage of enteric bacteria to the MLNs are also operative at the pre-ascitic stage of experimental cirrhosis. However, and in contrast to rats with ascites, a functional intestinal immune system is able to eradicate the accessing bacteria. Interestingly, in our study, bacterial CpG motifs, which are immunologically active components of bacterial DNA,30 were able to elicit an inflammatory response in the MLNs with expansion of activated mononuclear cells and production of proinflammatory cytokines. Remarkably, the immune system at the MLNs was able to maintain the inflammatory response to bacterial DNA fragments at the local level. This was revealed by a lack of correlation between the expansion of activated immune cells at the systemic level and the presence of bacterial DNA at the MLNs or bowel decontamination with antibiotics.
We sought to detect systemic inflammation in rats with CCl4 cirrhosis, given that it is the most widely used and clearly characterized toxin-based experimental model of cirrhosis. This model has been shown to effectively mimic many of the features of human cirrhosis associated with toxic damage.31-33 Thus, intoxication with CCl4 elicits a liver response that sequentially involves acute liver damage (at 2-3 weeks of intoxication), fibrosis (at 4-6 weeks), and cirrhosis (at 12 weeks).31-33 The inflammation observed in our experimental model at the systemic level was attributed to cirrhosis and not to the liver inflammatory response to CCl4 for the following reasons: (1) No systemic immune system abnormalities were produced after a short course of CCl4. We determined the effects of CCl4 by examining the phenotype and activation status of cell subpopulations in different compartments of the immune system before cirrhosis developed. It has been reported that a single dose or a few doses of CCl4 lead to acute liver damage characterized by steatosis, necrosis, and apoptosis of hepatocytes.31, 34, 35 However, at least 4 weeks of CCl4 administration are needed for liver fibrosis to develop.34, 36 After the short course of CCl4, we observed a slight inflammation response at the HLNs, but not the MLNs or peripheral blood. This finding is in agreement with the results from other laboratories, which indicate neither gut wall damage nor bacterial translocation to MLNs in rats receiving a short course of orally administered CCl4.37 Thus, the immunological disturbance observed in our rats with cirrhosis at the preascitic stage cannot be ascribed to a direct effect of CCl4 on immune system cells, nor to a secondary response to the non–cirrhosis-related liver damage induced by CCl4. (2) Similarly, systemic inflammation in other experimental models of cirrhosis, such as biliary cirrhosis, provides additional support linking the inflammatory response in peripheral blood detected here to cirrhosis rather than to CCl4 toxicity. Indeed, activation of circulating monocytes and of Th cells has been shown in mice and rats with preascitic cirrhosis induced by bile duct ligation.9, 14 (3) The presence of significant transaminitis in our rats with cirrhosis, indicating severe inflammation and hepatocellular necrosis, would have weakened our model and the proposed link between systemic inflammation and cirrhosis.
The notion of a systemic inflammatory immune response associated with cirrhosis is also supported by the observed increases in serum TNFα and IL-6 levels. However, in view of the notorious variability among the available assays, these slight yet significant increases in the concentrations of both cytokines should be interpreted with caution. Nevertheless, it should also be noted that, in sharp contrast to the acute systemic inflammatory reaction of the immune system produced in response to intense stimulation (e.g., intravenous lipopolysaccharide injection, Jarisch-Herxheimer reaction), increases in serum levels of proinflammatory cytokines in chronic local or systemic inflammation are characteristically moderate. In addition, the volume of distribution of TNFα is high, such that a mild increase in serum TNFα could mean a dramatic increase in the number of extracellular TNFα molecules.38 Finally, TNFα is an active molecule, and slight increases in its serum levels could induce substantial biological effects on immune and nonimmune cells.38
In conclusion, the results of this study suggest a critical role of the liver and its draining lymph nodes in inducing the systemic immune disturbance of compensated cirrhosis. They also extend the view of cirrhosis as a disease in which immunomediated mechanisms, which change from the compensated (pre-ascitic) to the decompensated (ascitic) stage, play a key pathogenetic role. Expansion of activated immune cells in the peripheral circulation and a rise in proinflammatory cytokines occurs in experimental compensated cirrhosis. However, unlike in cirrhosis with ascites, the predominant activation site of recirculating immune cells seems to be the draining lymph nodes of the liver and not the MLNs. The molecular and cellular mechanisms underlying this newly discovered immunological effect of the liver with cirrhosis remain to be elucidated.
Acknowledgements
The authors thank Ana Burton for her assistance with the English translation.




