Down-regulation of tumor suppressor a kinase anchor protein 12 in human hepatocarcinogenesis by epigenetic mechanisms†
Potential conflict of interest: Nothing to report.
Abstract
The A kinase anchor protein 12 (AKAP12) is a central mediator of protein kinase A and protein kinase C signaling. Although AKAP12 has been described to act as a tumor suppressor and its expression is frequently down-regulated in several human malignancies, the underlying molecular mechanisms responsible for the AKAP12 reduction are poorly understood. We therefore analyzed the expression of AKAP12 and its genetic and epigenetic regulatory mechanisms in human hepatocarcinogenesis. Based on tissue microarray analyses (n = 388) and western immunoblotting, we observed a significant reduction of AKAP12 in cirrhotic liver (CL), premalignant lesions (DN), and hepatocellular carcinomas (HCCs) compared to histologically normal liver specimens (NL). Analyses of array comparative genomic hybridization data (aCGH) from human HCCs revealed chromosomal losses of AKAP12 in 36% of cases but suggested additional mechanisms underlying the observed reduction of AKAP12 expression in hepatocarcinogenesis. Quantitative methylation analysis by MassARRAY of NL, CL, DN, and HCC tissues, as well as of various tumorigenic and nontumorigenic liver cell lines revealed specific hypermethylation of the AKAP12α promoter but not of the AKAP12β promoter in HCC specimens and in HCC cell lines. Consequently, restoration experiments performed with 5-aza-2′deoxycytidine drastically increased AKAP12α mRNA levels in a HCC cell line (AKN1) paralleled by AKAP12α promoter demethylation. As hypermethylation is not observed in CL and DN, we investigated microRNA-mediated posttranscriptional regulation as an additional mechanism to explain reduced AKAP12 expression. We found that miR-183 and miR-186 are up-regulated in CL and DN and are able to target AKAP12. Conclusion: In addition to genetic alterations, epigenetic mechanisms are responsible for the reduction of the tumor suppressor gene AKAP12 in human hepatocarcinogenesis. (HEPATOLOGY 2010;.)
A kinase anchor proteins (AKAPs) are a diverse group of functionally related scaffolding proteins that target protein kinase A (PKA) and other enzymes, thereby coordinating a range of signaling events.1 Human AKAP12 (synonymous: Gravin/AKAP250) is a large protein up-regulated in contact-inhibited cells and down-regulated by Src, Ras, and PKC.2 Interestingly, AKAP12 is able to modulate both protein kinase A and C, indicating that this protein is involved in the regulation of several signaling pathways. Other effects of AKAP12 are direct sequestration of cyclin D1, inhibition of ERK2 activation, and actin cytoskeleton interaction.3 Cyclin D1 overexpression is a frequent event in hepatocarcinogenesis and has been shown to occur early in the development of hepatocellular carcinoma (HCC) in the mouse.4, 5 SSeCKS, the rodent ortholog of AKAP12, has been demonstrated to act as tumor suppressor in vitro and in vivo.6, 7 This function was also described in human solid neoplasms, such as prostate and gastric cancer,7, 8 but data concerning the role of AKAP12 in human hepatocarcinogenesis are scarce.
The AKAP12 gene locus in human and rodents encodes three major transcripts under the control of three independent promoters, designated α, β, and γ (Fig. 1). The two major protein isoforms of AKAP12, α and β, are expressed ubiquitously in most cell and tissue types, whereas the expression of isoform γ is restricted to testes.9 The proteins translated from each transcript are encoded by a single large exon and share >95% amino acid sequence homology; however, they differ in their N-terminal domains. These isoforms are frequently posttranslationally modified; for example, only the α isoform is myristoylated at the N-terminus, a modification that facilitates AKAP12α association with plasma membranes and vesicles of the endoplasmic reticulum.9
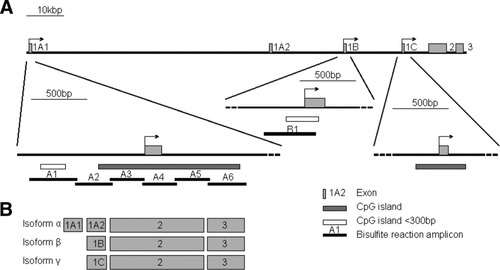
Scheme of the AKAP12 gene. (A) Location of CpG islands and PCR amplicons (A1-A6 and B1). Arrows indicate the predicted transcription start sites of each of the three isoforms. (B) Exon organization for each isoform.
In one of our recent studies analyzing 63 HCCs by aCGH, the AKAP12 gene locus on chromosome 6q24-25.2 showed chromosomal losses in 36% (23/63) of all analyzed cases, whereas gains were observed only in 5% (3/63).10 Yet this is an incomplete explanation of the observed AKAP12 down-regulation in HCC. Here, we show protein expression data of the tumor suppressor AKAP12 in a large series of human liver specimens, containing typical pathohistological features of hepatocarcinogenesis. In order to elucidate mechanisms of AKAP12 down-regulation, we have analyzed genetic, epigenetic, and posttranscriptional mechanisms. In summary, we here propose three different mechanisms of AKAP12 down-regulation in hepatocarcinogenesis: microRNA (miRNA) interference in preneoplastic lesions, genetic alterations, and AKAP12α promoter hypermethylation in HCCs.
Abbreviations
aCGH, array-based comparative genomic hybridization; AKAP12, a kinase anchor protein 12; 5-aza-dC, 5-aza-2′deoxycytidine; CL, cirrhotic liver; DN, dysplastic nodule; FFPE, formalin-fixed paraffin-embedded; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; miRNA, microRNA; NL, normal liver; PCR, polymerase chain reaction; PHH, primary human hepatocytes; PT, noncirrhotic peritumorous tissue; SSeCKS, Src-suppressed C kinase substrate; TMA, tissue microarray; UTR, untranslated region.
Materials and Methods
Patients and Tissue Specimens.
A total of 388 human liver tissue samples were evaluated by tissue microarrays (TMAs). TMA#1 (n = 225) contained two representative areas (diameter: 0.6 mm) of 14 histologically normal liver (NL), 38 dysplastic nodule (DN), 135 hepatocellular carcinoma (HCC; grading: 29 × G1, 76 × G2, 30 × G3), 14 cirrhotic (CL), and 24 noncirrhotic peritumorous (PT) liver tissue samples. The independently designed, processed, and evaluated TMA#2 (n = 163) contained two representative areas (diameter: 0.6 mm) of 20 NL, 77 HCC (grading: 13 × G1, 55 × G2, 9 × G3), and 66 PT tissue samples (see Supporting Table 7 and Supporting Fig. 1). All specimens were fixed in 4% formalin (pH 7.4) and embedded in paraffin. Tissue specimens were obtained from the tissue bank of the National Center of Tumor Diseases (Heidelberg, Germany). All specimens were surgically resected at the University of Heidelberg and histologically classified according to established criteria by three pathologists (TL, MAK, and PS). The study was approved by the institutional ethics committee (206/05). TMAs were processed as previously described.11
Immunohistochemistry.
Immunohistochemical analysis was performed according to standard protocols using the avidin biotin complex-method and diaminobenzidine as chromogen. AKAP12 immunohistochemistry of TMA#1 was performed using a goat polyclonal anti-AKAP12 antibody (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). AKAP12 immunohistochemistry of TMA#2 was performed using a mouse monoclonal anti-AKAP12 antibody (dilution 1:100; Abcam, Cambridge, MA). All sections were counterstained with hemalum. Specificity of the reaction was controlled by omitting the primary antibody. Immunohistochemistry of factors used in the correlation analysis was performed as described.11
Immunoblotting.
Western immunoblotting was performed using the following primary antibodies: goat polyclonal anti-AKAP12 (dilution 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and a mouse monoclonal anti-AKAP12 antibody (dilution 1:1000; Abcam, Cambridge, MA). For further information, see Supporting Information.
Tissue Microarray Analysis.
For semiquantitative immunohistochemical assessment of AKAP12 expression, the product of the scores of staining intensity and percentage of immunoreactive cells was calculated based on the following scoring system: the intensity ranged from 0 = negative, 1 = low, 2 = medium, to 3 = high; the quantity comprised 0 = no expression, 1 = positivity in less than 10%, 2 = positivity in 10% to 50%, 3 = positivity in 51% to 80%, and 4 = positivity in more than 80% of hepatocytes or tumor cells. The final immunohistochemical score (IHS; ranging from 0 to 12) was obtained by multiplication of the intensity score and the quantity score according to IRS scoring. For comparison of staining results, we further defined a scoring index comprising three different expression scores for AKAP12 based on the calculated product of cytoplasmic intensity and quantity of immunoreactive cells: 0-4 = absent/low expression; 5-8 = moderate expression; and 9-12 = high expression. Nonparenchymal cells were not counted. Evaluation was performed independently by two pathologists (B.G. and A.W.).
Cell Lines.
The human liver tumor cell lines HepG2 and Hep3B were both obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). HuH7 and PLC/PRF/5 cell line were obtained from JHSF (Osaka, Japan).12, 13 The human liver tumor cell line AKN1 was kindly provided by R. Bartenschlager (Division of Molecular Virology, University of Heidelberg, Germany).14 HepG2 cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI), Hep3B cells were cultured in minimal essential medium (MEM), and HuH7, PLC/PRF/5, and AKN1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (all media were obtained from PAA Laboratories, Cölbe, Germany) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (Sigma, St. Louis, MO) in an atmosphere containing 5% CO2.
DNA Isolation and Bisulfite Conversion.
High-molecular weight DNA was isolated from fresh-frozen NL, CL, DN, and HCC tissue samples and cultured cells using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). DNA from formalin-fixed paraffin-embedded (FFPE) tissue was isolated using the QIAamp DNA FFPE Tissue kit (Qiagen) with an additional 16 hours proteinase K digestion. Genomic DNA was converted by bisulfite treatment using the EZ DNA methylation kit (Zymo Research, Orange, CA).
DNA Methylation Analysis.
Fresh-frozen tissue samples included 16 HCC, six CL, six microdissected CL (CL microdissected), 17 NL, and five human liver tumor cell lines. DN were obtained from 11 FFPE tissue specimens. Regions for quantitative DNA methylation analysis covered the CpG islands around the respective transcription start sites of the two AKAP12 isoforms α and β (Fig. 1) and were targeted by amplicons of up to 460 bp in size (see Supporting Table 4). In total, our analysis covered 44% of the genomic CpG sites of the AKAP12α promoter and 41% of genomic CpG sites of AKAP12β promoter. The amplicons A1 and A2, and B1 were the most representative genomic regions of all analyzed amplicons in both isoforms for depicting methylation differences between normal, preneoplastic, and neoplastic tissue (data not shown). Smaller amplicons which were located in those most informative regions were used to characterize methylation levels of FFPE DN samples.
Bisulfite-converted DNA was polymerase chain reaction (PCR) amplified, in vitro transcribed, base-specifically cleaved by RNase A, and subjected to matrix-assisted laser desorption, ionization-time-of-flight mass spectrometry (MassARRAY technique; Sequenom, San Diego, CA) as described.15 DNA methylation standards (0%, 20%, 40%, 60%, 80%, and 100% methylated genomic DNA) were used for data correction.
Microdissection of CL Samples.
To assure that the methylation analysis of CL samples was not contaminated by nonparenchymal cells, microdissection was performed. CL tissue samples were cut into 18-μm-thick sections using a cryostat (Leica CM1850; Leica Microsystems, Wetzlar, Germany) and laser microdissection was performed as described.16
5-aza-dC Treatment.
Human HCC cell lines (AKN1, HepG2, and HuH7) were incubated for 72 hours with 10 μmol/L 5-aza-dC (Sigma-Aldrich, St. Louis, MO) with a medium change every 24 hours. Genomic DNA was isolated from treated cells as described and total RNA was purified using Trizol reagent (Invitrogen, Karlsruhe, Germany).
Reverse Transcription and Quantitative Real-Time PCR.
Quantitative mRNA expression analysis of AKAP12 isoforms and reference genes was performed on a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) using the Absolute QPCR SYBR Green Mix (Thermo Scientific, Waltham, MA) as described.17 Briefly, total RNA from the cell lines (1 μg) was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) and oligodT primers or random hexamers for tissue samples. (For primers and real-time PCR conditions, see Supporting Table 5.) Reaction products were characterized by melting point analysis and relative quantification with efficiency correction (LightCycler Software 4, Roche Diagnostics). Mean normalized ratios were determined for each sample, using HPRT1, TBP, and ACTB as reference genes.
miRNA Purification and Quantification in FFPE and Fresh-Frozen Tissues.
For purification of miRNA from FFPE tissue sections we used the miRNeasy FFPE Kit according to the manufacturers' instructions (Qiagen). miRNAs were isolated from fresh-frozen tissues using Trizol (Invitrogen). For FFPE tissues, cDNA was synthesized using miScript Reverse Transcription kit (Qiagen), and real-time quantitative PCR (qPCR) was performed using miScript SYBR Green PCR kit and specific human miScript assays for hsa-miR-183 and hsa-miR-186 (Qiagen) in an ABI-Prism 7300 Real-time PCR system (Applied Biosystems, Darmstadt, Germany). RNU6B was used as endogenous reference RNA.
Functional Interaction Studies of miRNAs and AKAP12.
Human miR-183 and miR-186 were cloned into the pCMX-PL1 expression plasmid by PCR amplification of ±100 bp of the pre-miRNA sequence as annotated in the UCSC, hg18. The conserved 3′end of the AKAP12 3′-untranslated region (3′UTR) was cloned by insertion of hybridized oligonucleotides (120 bp) into the 3′end of the Firefly luciferase gene in the pMirReport plasmid (Ambion, Austin, TX). AKAP12 UTR/miRNA interactions were performed by expression in HEK293T cells using a TK-Renilla plasmid (Promega, Madison, WI) as a transfection control. Regulation of endogenous AKAP12 mRNA was determined by overexpression of miRNAs followed by RNA isolation using Trizol; cDNA synthesis, and qPCR as described.
Statistical Analyses and Software.
Data are presented as mean values ± SD. The Spearman rank coefficient was used as a statistical measure of association. For TMA correlation analysis, r > 0.3 and P < 0.001 were considered as biologically relevant. To account for multiple testing (TMA-data), the α-level was adjusted according to Bonferroni. The statistical comparison between two groups was accomplished with the nonparametric Mann-Whitney U test (SPSS version 11).
Results
Down-Regulation of AKAP12 in CLs, DNs, and in HCCs.
A previous study of our group demonstrated losses of genomic material coding for the AKAP12 gene locus in 36% of human HCCs.10 As data about AKAP12 expression in the process of human hepatocarcinogenesis are scarce, we aimed to define alterations of AKAP12 protein levels in hepatocarcinogenesis. We detected AKAP12 expression by immunohistochemistry using TMAs containing a total number of 388 human liver tissue samples, including NL, CL, DN, and HCC. Immunohistochemistry showed a strong cytoplasmic staining pattern in hepatocytes, with a partly granular, partly homogeneous appearance (Fig. 2A). Statistical analyses of TMA#1 revealed a significantly higher relative immunoreactivity of AKAP12 in human NL compared to HCCs (P < 0.0001, Fig. 2B). According to our scoring index, 72% of NL samples showed a high or moderate AKAP12 expression, whereas in the HCC group only 27% (G1), 36% (G2), and 23% (G3) of samples showed a high or moderate expression. Overall, in 68% of the HCC samples AKAP12 expression dropped below the 25th percentile compared to the NL group. AKAP12 expression in noncirrhotic PT was comparable to NL, whereas in CL and DN a significant down-regulation of AKAP12 was observed (P < 0.01, P < 0.05; Fig. 2B). Focusing on the group of DN and HCC (all differentiation grades), we detected a statistically significant down-regulation of AKAP12 correlating with the reduced differentiation grade from DN toward G3-HCC (P < 0.01; Fig. 2B). TMA#2 (n = 163; containing NL, PT, and HCC specimens) confirmed the results of TMA#1 (see Supporting Table 7 and Supporting Fig. 1).
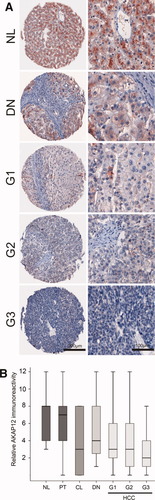
Down-Regulation of AKAP12 correlates with dedifferentiation in human hepatocarcinogenesis. (A) In NL, a strong cytoplasmic expression of AKAP12 is observed in hepatocytes, whereas in DN and in HCC a distinct reduction of AKAP12 was detectable. Original magnification: ×50 (left), ×200 (right). (B) Box plot illustration of TMA analyses demonstrate AKAP12 expression in NL and a significant stepwise reduction in CL, DN (CL: P < 0.01; DN: P < 0.05), and further in the group of HCCs (P < 0.0001).
TMA results were confirmed by analyzing protein extracts of human NL tissues and HCC samples of various differentiation grades by western immunoblot. In NL specimens, AKAP12 was strongly or at least moderately expressed, whereas in HCC samples, AKAP12 expression was reduced or not detectable. Semiquantitative densitometry of western immunoblots revealed a 10-fold to 100-fold higher AKAP12 expression in NL compared to HCC (Fig. 3A). In addition, we examined AKAP12 expression in various hepatic cell lines and in primary human hepatocytes (PHH). These immunoblots showed a reduced or absent AKAP12 expression in the HCC cell lines, whereas in PHH, AKAP12 expression was clearly detectable. Semiquantitative densitometry of western immunoblots revealed a 30-fold to 600-fold higher AKAP12 expression in PHH compared to HCC cell lines (Fig. 3B).
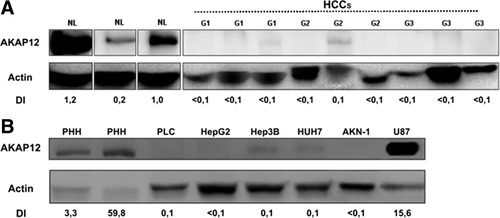
Western blot analysis shows AKAP12 down-regulation in HCC tissue lysates and in HCC cell lines. (A) HCC tissue samples of various differentiation grades (G1-G3) show a 10-fold to 100-fold reduction of AKAP12 expression when compared to NL tissue samples. (B) HCC cell lines (PLC/PRF/5, HepG2, Hep3B, HuH7, AKN1) show a 30-fold to 600-fold reduction of AKAP12 signal when compared to PHH. A glioblastoma cell line (U87) served as a positive control. Densitometry was performed and is indicated as density index (DI, normalized ratio).
Correlation Analysis of AKAP12 Expression With Clinical Parameters, Proliferation Rate, and Other Factors Involved in Hepatocarcinogenesis.
Correlation analysis for AKAP12 expression and the proliferation marker Ki-67 showed a statistically significant inverse correlation (r = −0.318; P < 0.001). AKAP12 levels did not show any correlation at the protein level with other factors involved in hepatocarcinogenesis. More interestingly, no correlation of AKAP12 with cyclin D1 was detected. No significant statistical correlation was detected after testing etiological factors, such as chronic viral hepatitis (HBV and HCV), clinical parameters, tumor staging (TNM), or gender with AKAP12 levels (see Supporting Table 2 and 3).
Isoform-Specific AKAP12 mRNA Expression in Tissue Samples.
Because the used antibodies recognize the C-terminal domain of both AKAP12 isoforms, we separately examined AKAP12α and β expression at the mRNA level in NL, CL, and HCC tissues (Fig. 4A,B). We found that AKAP12α was the predominant isoform expressed in all tissues. AKAP12α mRNA expression showed a statistically significant decrease during hepatocarcinogenesis, correlating with TMA protein data.
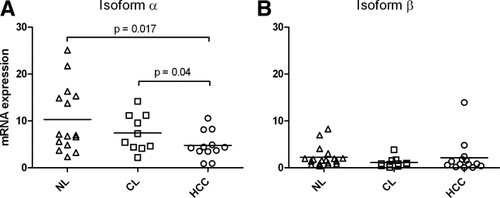
AKAP12 mRNA expression in tissue samples. (A) AKAP12α mRNA levels in fresh-frozen tissue samples of NL, CL, and HCC. Horizontal bars indicate mean values. HCC tissue samples show statistically significant lower AKAP12α mRNA levels compared to CL and NL (P < 0.05; Mann-Whitney U test). (B) AKAP12β mRNA levels in fresh-frozen tissue samples of NL, CL, and HCC. Horizontal bars indicate mean values. NL, CL, and HCC tissue samples do not show statistically significant differences in AKAP12β mRNA expression. In comparison with (A), AKAP12α is the predominantly expressed isoform in all subgroups.
Regulation of AKAP12 Expression by DNA Methylation.
In a cohort of 63 HCCs recently analyzed by aCGH, the AKAP12 gene locus on chromosome 6q24-25.2 showed chromosomal losses in 36% (23/63) and gains in 5% (3/63) of cases.10 In addition, no alterations of the AKAP12 gene locus on chromosome 6q24-25.2 were detectable in all analyzed cell lines (see Supporting Table 1). However, these data do not sufficiently explain the distinct decrease of AKAP12 protein expression observed in this study. As a possible suppressive mechanism, we investigated the DNA methylation of promoter-related CpG islands of both AKAP12 isoforms in NL, CL, DN, and HCC samples by quantitative MassARRAY analysis (Fig. 1). In tumor samples, hypermethylation was detected in the AKAP12α promoter (Fig. 5A,B), but not in the AKAP12β promoter (Fig. 5C,D). Methylation analysis of the AKAP12β promoter showed a methylation value higher than 10% only for one HCC sample. For the AKAP12α promoter, a mean methylation of 12% for NL and CL, 15% for DN, and of 30% for HCC was observed. As high amounts of fibroblasts, infiltrating immune cells, and other nonparenchymal cells may inflict on the genuine methylation status of hepatocytes in CL, microdissection of hepatocytes was performed. However, the methylation values of CL specimens after microdissection (18%) did not significantly differ from undissected samples of the same tissues.
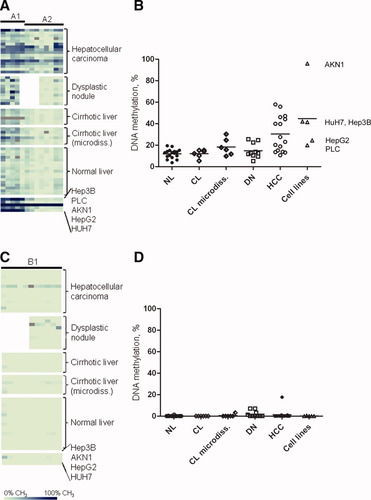
MassARRAY analysis of AKAP12 promoter methylation. (A) Quantitative DNA methylation analysis of the AKAP12α promoter region. Each column represents a group of one to five CpGs analyzed; each row represents a sample. Percentages of methylation span from 0% to 100%. Gray squares indicate unavailable data. (B) The mean percentage of methylation for each sample represented in (A). Horizontal bars represent mean methylation values for each group. HCC tissue samples show statistically significant hypermethylation when compared to NL (P < 0.001), CL (P < 0.01), and DN tissue samples (P < 0.01; Mann-Whitney U test). (C) Quantitative DNA methylation analysis of the AKAP12β promoter region using MassARRAY. (D) The average percent methylation for each sample represented in (C).
Elevated DNA methylation levels of the AKAP12α promoter were also detected in HCC cell lines. This analysis revealed DNA methylation of 96% (AKN1), 42% (HuH7), 41% (Hep3B), 24% (HepG2), and 20% (PLC/PRF/5) (Fig. 5A,B). Methylation analysis of the AKAP12β promoter in cell lines showed methylation values lower than 1% (Fig. 5C,D). Hypermethylation of the AKAP12α promoter was confirmed by an independent method (combined bisulfite restriction analysis; see Supporting Fig. 2).
To verify the functional relationship between promoter hypermethylation and loss of AKAP12 gene expression, methylation and mRNA expression levels of both isoforms were compared before and after treatment with 5-aza-dC in cell lines AKN1, HepG2, and HuH7. Isoform-specific mRNA expression of AKAP12 differed between untreated hepatic cell lines and PHH but confirmed protein data (Fig. 3B; Fig. 6A). The 5-aza-dC treatment resulted in a decrease in AKAP12α promoter methylation in the highly methylated AKN1 cell line (Fig. 6B), accompanied by a strong increase in AKAP12α mRNA expression (Fig. 6C), demonstrating a relationship between AKAP12α expression and methylation of its promoter. In HepG2 and HuH7 cells, which showed lower methylation levels than AKN1, demethylation as well as re-expression of 5-aza-dC was moderate. Similarly, only a marginal increase in expression was detected for isoform β with its unmethylated promoter (Fig. 6B,C). Data were confirmed in two independent experiments (see Supporting Table 6).
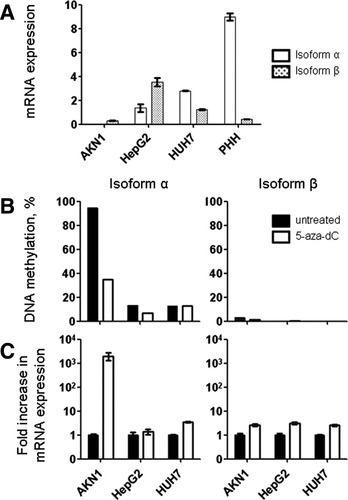
5-aza-dC treatment causes DNA demethylation and reactivation of the hypermethylated AKAP12α gene in AKN1 cells. (A) Isoform-specific AKAP12 mRNA expression levels in various untreated HCC cell lines and PHH. (B) 5-aza-dC treatment strongly reduces promoter methylation levels of AKAP12α in AKN1 cells compared to HepG2 and HuH7 cells, which display lower baseline methylation levels. AKAP12β is unmethylated in all cell lines tested (black bars, untreated cells; white bars, cells treated with 10 μmol/L 5-aza-dC for 72 hours). (C) 5-aza-dC treatment causes the re-expression of AKAP12α in AKN1 cells. Only minor increases in expression of AKAP12α in HuH7 cells and AKAP12β in all cell lines are observed. Histograms represent the average value of three technical replicates of the quantitative real-time PCR, with the corresponding SD.
Regulation of AKAP12 Expression by miRNAs.
Although we have demonstrated that silencing of AKAP12 is associated with DNA hypermethylation in HCC, promoter methylation does not explain the loss of expression in earlier stages of hepatocarcinogenesis. Thus, we postulated that a posttranscriptional mechanism may cause silencing in CL and DN. A candidate list of up-regulated miRNAs was established by a literature search of miRNA expression profiles in CL and HCC,18 which was cross-referenced with miRNAs predicted to target the 3′UTR of AKAP12 using miRWalk, a composite software suite of several miRNA target prediction programs (http://www.ma.uni-heidelberg.de/apps/zmf/mirwalk). This search strategy identified miR-183 and miR-186 as potential regulators of AKAP12 in hepatocarcinogenesis. We then performed an expression analysis of miR-183 and miR-186 in FFPE and fresh-frozen tissue samples. Expression analysis in FFPE samples showed a significant up-regulation of miR-183 (P < 0.01, all groups versus NL) and a trend toward miR-186 up-regulation in DN and CL tissues (Fig. 7A). Further analysis of miR-186 expression from fresh-frozen tissues showed an up-regulation of miR-186 in CL (see Supporting Fig. 3; P < 0.05). We next determined if miR-183 and miR-186 can directly regulate AKAP12 levels. The 3′UTR of AKAP12α/β harbors three and four putative target sites for miR-183 and miR-186, respectively, as predicted by TargetScan (Fig. 7B).19 The highly conserved 3′ end of the AKAP12 3′UTR contains two putative binding sites for each miRNA and was cloned into the 3′ end of Firefly luciferase. Nucleotide sequences were mutated to ablate either both miR-183 or both miR-186 sites. MiR-183 and miR-186 were cloned into expression plasmids and transfected in HEK293T cells (see Supporting Fig. 4). Expression of increasing amounts of both miRNAs caused a dose-dependent increase in the mature miRNAs above endogenous levels (range 9 to 27 and 1.8 to 20-fold for miR-183 and miR-186, respectively). Coexpression of miR-183 with the wild-type (WT) and 186-mutated UTR resulted in a measurable (1.3-fold) decrease in luciferase expression as compared to the 183-mutated UTR (Fig. 7C). Coexpression of miR-186 with the WT UTR resulted in a distinct decrease (two-fold) in luciferase expression compared to the 186-mutated UTR. Expression of miR-186 in HEK293T cells resulted in a 2-fold reduction of endogenous AKAP12 mRNA levels (Fig. 7D). Expression of miR-183 did not reduce endogenous AKAP12 levels. Both α and β isoforms were reduced to similar levels following miRNA-dependent mRNA knockdown (data not shown).
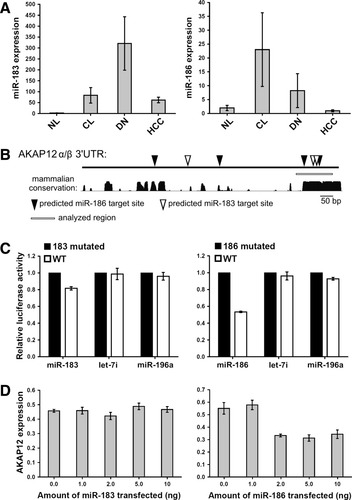
AKAP12 regulation by miR-183 and miR-186 in hepatocarcinogenesis. (A) Expression levels of miR-183 and miR-186 relative to RNU6B in NL (n = 5), CL (n = 13), DN (n = 5), and HCC (n = 12) in FFPE tissues. Error bars represent standard errors of the mean. MiR-183 is significantly higher expressed in CL, DN, and HCC versus NL (P < 0.01; P < 0.001; Mann-Whitney U test). (B) Schematic representation of the AKAP12 3′UTR depicting the position of putative miR-183 and miR-186 binding sites, the region analyzed for functional miRNA/UTR interactions, and the level of mammalian sequence conservation (UCSC, hg18). (C) Functional interaction assays were performed by expressing the highly conserved (3′end) portion of the AKAP12 3′UTR linked to the luciferase gene in HEK293T cells. MiR-183, miR-186, and control (Let-7i and miR-196a) miRNAs were coexpressed. Luciferase expression is displayed relative to the 3′UTR where the corresponding predicted binding sites were mutated. Error bars represent the SD of three independent experiments. (D) Endogenous AKAP12 mRNA expression levels following expression of increasing amounts of miR-183 and miR-186 expression plasmids in HEK293T cells. Data represent the average of α and β isoforms. Error bars represent the SD of three technical replicates.
Discussion
Down-regulation and tumor suppressor activity of the scaffold protein AKAP12 has been shown in several malignancies,7, 8, 20 but, so far the role of AKAP12 in hepatocarcinogenesis is almost completely unknown. Here, we present protein expression data of AKAP12 in a large number of human liver tissue specimens (n = 388), revealing a significant down-regulation of AKAP12 in HCC compared to NL. Remarkably, CL and DN already showed reduced AKAP12 expression, suggesting that down-regulation of AKAP12 is an early event in hepatocarcinogenesis. Indeed, recent findings in prostate cancer have led to the theory that AKAP12 may act as a key player in early stages of carcinogenesis.6 TMA data demonstrate that AKAP12 expression is progressively down-regulated during HCC progression. Accordingly, we propose a two-step model of AKAP12 down-regulation in hepatocarcinogenesis, with a first significant down-regulation from NL to CL and DN. The second step takes place during HCC progression. Because expression characteristics and functional data obtained in prostate and gastric cancer suggest a tumor suppressive function of AKAP12,6, 8 its down-regulation in the majority of CL and DN may contribute to the increased risk of malignant transformation.
Because little is known about interaction of AKAP12 with other factors, we correlated AKAP12 expression at the protein level with the expression of other factors involved in hepatocarcinogenesis. Cyclin D1 overexpression is a common finding in hepatocarcinogenesis, which has been shown to occur very early in hepatocarcinogenesis in mouse models.4, 5 In NIH3T3 cells, it has been shown that SSeCKS expression induces G1 arrest marked by a decrease in cyclin D1 expression.21 Interestingly, our study did not reveal a statistically significant inverse correlation of AKAP12 with cyclin D1, although our TMA analysis showed increasing cyclin D1 levels during hepatocarcinogenesis. As expected, AKAP12 showed an inverse correlation with the proliferation marker Ki-67.
As we previously demonstrated, AKAP12 down-regulation may partly be caused by chromosomal loss of the AKAP12 gene locus (see Supporting Table 1).10 However, this finding did not sufficiently explain down-regulation of AKAP12 in most HCCs. Because aberrant methylation status has been identified to be of mechanistic and prognostic significance in human HCC,22 we tested epigenetic alterations in the AKAP12 promoter region. Our study demonstrates hypermethylation of AKAP12α promoter in human HCC specimens and in various HCC cell lines. Thus, gene silencing by promoter hypermethylation may be the cause for the significant decrease of AKAP12 protein levels in HCC cells. This concept of AKAP12 down-regulation is in line with studies in lung and gastric cancer which described the AKAP12 gene as a target for epigenetic silencing.8, 23 Although existing antibodies fail to distinguish between AKAP12 isoforms, data on AKAP12α and β transcripts suggest that hypermethylation of the AKAP12α promoter is predominantly responsible for epigenetic silencing of AKAP12. This is supported by the fact that the highly methylated HCC cell line AKN1 decreased AKAP12α promoter methylation after 5-aza-dC treatment resulting in increased expression of AKAP12α mRNA. The distinct hypermethylation of only the AKAP12α promoter seems to be specific for human HCC, because data obtained in other malignancies, e.g., gastric cancer, showed a hypermethylation of both, AKAP12α and β promoter region.8 A coordinate control between the AKAP12 promoters might be involved in hepatocarcinogenesis; however, our data in human HCC do not support this hypothesis.
Interestingly, methylation analyses did not show hypermethylation of the AKAP12 promoter in CL and DN, although AKAP12 was down-regulated at the protein level. Because methylation analysis of CL tissue specimens was originally performed without the use of microdissection, we assumed that a “dilution effect” by nonparenchymal cells (mainly fibrocytes and fibroblasts) might conceal hypermethylation of hepatocytes in these samples. However, even improved analysis employing microdissected CL samples of the same tissue specimens failed to confirm promoter hypermethylation as the cause of AKAP12 down-regulation in CL and DN. In search of posttranscriptional mechanisms for AKAP12 down-regulation we detected an alternative regulatory mechanism in CL and DN by miR-183 and miR-186. Both of these miRNAs are up-regulated in the precancerous stages where promoter hypermethylation is absent; and, via a direct interaction with the AKAP12 transcript, both miRNAs can regulate mRNA levels to various degrees, with miR-186 demonstrating a strong ability to regulate endogenous transcript levels. Regarding the observed genetic and epigenetic alterations in HCC, this represents an interesting interplay between different epigenetic regulatory mechanisms in the course of human hepatocarcinogenesis. A connection between epigenome and miRNome and alteration in the balance of this complicated network as a possible mechanism leading to cancer has been described recently.24
Additional mechanisms may also account for AKAP12 down-regulation. In CL, it could be shown that a histone deacetylase inhibitor influences SSeCKS expression.25 Apart from aberrant patterns of histone modification, involvement of chromatin modifications in the expression of the AKAP12α isoform was recently shown by its re-expression after treatment of mouse fibroblasts with a histone deacetylase inhibitor.26 Different models support the hypothesis that CpG island methylation may follow histone modification to stably lock silenced genes.27 It is therefore conceivable that the observed de novo DNA methylation of the AKAP12α promoter in HCCs may be also triggered by histone modifications which are already present in CL.
In summary, the data presented here demonstrate that the tumor suppressor AKAP12 is down-regulated during hepatocarcinogenesis in a stepwise manner: early in cirrhosis and in premalignant lesions, and late in HCC dedifferentiation. We could identify different epigenetic mechanisms responsible for this stepwise down-regulation. In CL and DN, down-regulation of AKAP12 is at least partly caused by interaction of two specific miRNAs, whereas in HCC genetic loss and to a significant extent hypermethylation of the AKAP12α promoter are responsible for AKAP12 reduction.
Acknowledgements
We thank Peter Waas, Anna-Lisa Lackner, and Otto Zelezny (Division of Epigenomics and Cancer Risk Factor, German Cancer Research Center), and Eva Eiteneuer and John Moyers (Institute of Pathology, University of Heidelberg) for their excellent technical assistance.




