Conjugation is essential for the anticholestatic effect of NorUrsodeoxycholic acid in taurolithocholic acid–induced cholestasis in rat liver†‡
Potential conflict of interest: Nothing to report.
Data were in part reported at the Annual Meeting of the American Association for the Study of Liver Diseases, Boston, November 2-6, 2007, and were published in part in abstract form in Hepatology 2007;46(4 Suppl):338A-339A
Abstract
NorUDCA (24-norursodeoxycholic acid), the C23-homolog of ursodeoxycholic acid (UDCA), showed remarkable therapeutic effects in cholestatic Mdr2 (Abcb4) (multidrug resistance protein 2/ATP-binding cassette b4) knockout mice with sclerosing/fibrosing cholangitis. In contrast to UDCA, norUDCA is inefficiently conjugated in human and rodent liver, and conjugation has been discussed as a key step for the anticholestatic action of UDCA in cholestasis. We compared the choleretic, anticholestatic, and antiapoptotic properties of unconjugated and taurine-conjugated UDCA (C24) and norUDCA (C23) in isolated perfused rat liver (IPRL) and in natrium/taurocholate cotransporting polypeptide (Ntcp)-transfected human hepatoma (HepG2) cells. Taurolithocholic acid (TLCA) was used to induce a predominantly hepatocellular cholestasis in IPRL. Bile flow was determined gravimetrically; bile acids determined by gas chromatography and liquid chromatography/tandem mass spectrometry; the Mrp2 model substrate, 2,4-dinitrophenyl-S-glutathione (GS-DNP) was determined spectrophotometrically; and apoptosis was determined immunocytochemically. The choleretic effect of C23-bile acids was comparable to their C24-homologs in IPRL. In contrast, TnorUDCA, but not norUDCA antagonized the cholestatic effect of TLCA. Bile flow (percent of controls) was 8% with TLCA-induced cholestasis, and unchanged by coinfusion of norUDCA (14%). However, it was increased by TnorUDCA (83%), UDCA (73%) and TUDCA (136%). Secretion of GS-DNP was markedly reduced by TLCA (5%), unimproved by norUDCA (4%) or UDCA (17%), but was improved modestly by TnorUDCA (26%) or TUDCA (58%). No apoptosis was observed in IPRL exposed to low micromolar TLCA, but equivalent antiapoptotic effects of TUDCA and TnorUDCA were observed in Ntcp-HepG2 cells exposed to TLCA. Conclusion: Conjugation is essential for the anticholestatic effect of norUDCA in a model of hepatocellular cholestasis. Combined therapy with UDCA and norUDCA may be superior to UDCA or norUDCA monotherapy in biliary disorders in which hepatocyte as well as cholangiocyte dysfunction contribute to disease progression. (HEPATOLOGY 2010;52:1758-1768)
Chronic cholestatic liver diseases such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) are characterized by impaired biliary secretion of bile acids and other potentially harmful cholephiles. Intrahepatic accumulation of endogenous hydrophobic bile acids, together with cytokine- and chemokine-mediated inflammatory bile ductular and liver parenchymal injury, may contribute to development of fibrosis and cirrhosis in chronic cholestatic disorders. The ratio of toxic to less toxic bile acids correlates with severity of liver injury.1
The hydrophilic C24 bile acid, ursodeoxycholic acid (UDCA) improves biochemical and histological features in PBC, halts progression to cirrhosis in the majority of patients with PBC, normalizes life expectancy in two-thirds of patients with PBC2-5 and currently represents the only approved therapy for PBC.6 In PSC, its therapeutic long-term benefit remains so far unclear although serum liver tests and surrogate markers of long-term prognosis improved during UDCA treatment in pilot trials.6 Mechanisms of action of UDCA in cholestatic disorders have not yet been fully resolved.7 Stimulation of impaired hepatocellular secretion by mainly posttranscriptional mechanisms, detoxification of bile, antiapoptotic effects in hepatocytes and cholangiocytes, and stimulation of cholangiocyte HCO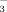 secretion may contribute to the beneficial effect observed during UDCA treatment in cholestatic disorders.7
secretion may contribute to the beneficial effect observed during UDCA treatment in cholestatic disorders.7
NorUDCA is a C23 homolog of UDCA. C23 bile acids are found only in trace amounts in human bile.8 They are poorly conjugated with taurine and glycine in liver, and their pharmacokinetic properties differ from their naturally occurring C24 homologs.8 NorUDCA has recently been shown to exert therapeutic effects superior to those of UDCA in multidrug resistance protein 2 (Mdr2)/ATP-binding cassette b4 (Abcb4) knockout mice, a murine model of nonsuppurative, sclerosing cholangitis.9, 10 The mechanisms of action of norUDCA in Mdr2−/− mice remain obscure.10 NorUDCA is a potent (hyper-)choleretic bile acid as a result of cholehepatic shunting.8, 10 Whether this property may explain, at least in part, the anticholestatic, anti-inflammatory, and antifibrotic effects of norUDCA in Mdr2−/− mice9, 10 still remains unresolved. Most importantly, it is unclear whether norUDCA, like UDCA, may exert anticholestatic effects at the level of the hepatocyte.7, 11
Lithocholic acid conjugates are the most potent cholestatic agents among the major human bile acids.12 Taurolithocholic acid (TLCA)-induced cholestasis is an established experimental model of hepatocellular cholestasis.12-15 TLCA seems to exert its short-term cholestatic effects by stimulation of retrieval of key canalicular transport proteins such as the bile salt export pump (Bsep, Abcb11) and conjugate export pump (Mrp2, Abcc2)13, 16 in hepatocytes via phosphatidylinositol 3-kinase (PI3K)-dependent and putatively protein kinase C ε (nPKCε)-dependent posttranscriptional mechanisms.14, 17
TLCA and other hydrophobic bile acids are potent apoptotic stimuli at low micromolar levels in hepatocytes.18 In contrast, UDCA conjugates are effective antiapoptotic agents in liver.19-21 It is yet unknown whether norUDCA exerts antiapoptotic properties in liver cells.
The aim of this study was to compare the anticholestatic potential of norUDCA with that of its taurine conjugate as well as to compare the effects of both molecules with those of UDCA and its taurine conjugate in TLCA-induced hepatocellular cholestasis using the single-pass isolated perfused rat liver. In addition, the antiapoptotic properties were tested in human HepG2 hepatoma cells transfected with natrium/taurocholate cotransporting polypeptide (Ntcp) to insure adequate conjugated bile acid uptake.
Abbreviations
bisnorUDCA, bisnorursodeoxycholic acid; CDNB, 1-chloro-2,4-dinitrobenzene; GS-DNP, 2,4-dinitrophenyl-S-glutathione; IPRL, isolated perfused rat liver; KRB, Krebs-Ringer bicarbonate buffer; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LDH, lactate dehydrogenase; norUDCA, norursodeoxycholic acid; PBC, primary biliary cirrhosis; PI3K, phosphatidylinositol 3-kinase; TCDCA, taurochenodeoxycholic acid; TLCA, taurolithocholic acid; TnorUDCA, tauronorursodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid.
Materials and Methods
Materials.
TnorUDCA was synthesized according to Tserng et al.22 For other materials, see Supporting Information data.
Isolated Rat Liver Perfusion.
Male Sprague-Dawley rats (205 ± 24 g) were purchased from Charles River (Sulzfeld, Germany). They had unlimited access to rodent chow and water, were subjected to a 12-hour day-night rhythm, and received humane care. The technical procedure of isolated rat liver perfusion has been described in detail previously.11, 13, 14, 23 Rat livers were perfused in a nonrecirculating fashion with oxygenated Krebs-Ringer bicarbonate buffer (KRB, 37°C, 95% O2/5% CO2) for 45 minutes. Then, bile acids (UDCA, TUDCA, TCDCA, norUDCA, TnorUDCA, their C22 homolog bisnorUDCA, and/or TLCA) or the carrier dimethyl sulfoxide (DMSO) only (controls) were added continuously to the perfusion buffer for 70 minutes to reach portal venous concentrations of 10 μmol/L (TLCA) or 25 μmol/L (other bile salts) or 0.1% vol/vol (DMSO). CDNB (30 μmol/L), the precursor of the model Mrp2 substrate, 2,4-dinitrophenyl-S-glutathione (GS-DNP), was administered from minute 65 to 75 via the portal vein. At this concentration, saturation of hepatobiliary GS-DNP secretion has been observed in the IPRL.24 For determination of bile flow and calculation of hepatobiliary organic anion and bile acid secretion, bile was collected in pretared tubes and measured gravimetrically; GS-DNP was determined in 5-minute intervals, whereas bile salts were measured in bile samples pooled between minute 45 and 65, minute 65 and 85, and minute 85 and 105 for gas chromatography, and between minute 45 and 65 for liquid chromatography–tandem mass spectrometry (LC-MS/MS), respectively. In addition, bile acids were determined by gas chromatography in hepatovenous effluate pooled between minute 55 and 115.
Quantification of Bile Acid Levels in Bile, Liver Tissue, and Hepatovenous Effluate by Capillary Gas Chromatography.
Quantification of bile acid levels by capillary gas chromatography was performed as described earlier.25, 26 For details, see Supporting Information Data.
Characterization of Biliary Bile Acids by LC-MS/MS.
Biliary bile acids were further characterized by LC-MS/MS in order to discriminate between conjugated and nonconjugated bile acids as described earlier.27 Biliary bile acid concentrations lower than 0.01 mmol/L were set as zero.
Hepatobiliary Anion Secretion.
Biliary secretion of the Mrp2 substrate, GS-DNP, was determined spectrofluorometrically as described earlier.13, 14 For details, see Supporting Information Data.
Hepatovenous Efflux of Lactate Dehydrogenase.
For quantification of hepatocellular damage, activity of lactate dehydrogenase (LDH) in the hepatovenous effluate was determined by an enzymatic assay as described.28
Immunofluorescence Assessment of Apoptosis in Rat Liver Tissue.
Active caspase-3 and cleaved cytokeratin 18 were determined by immunofluoresecence on cryosections of rat liver tissue as described previously.25, 29 Caspase-3–positive hepatocytes with concomitant cytokeratin intermediate filament breakdown were counted in 40 different high-power fields per sample for quantification of apoptotic cell death.
Immunocytochemical Quantification of Apoptosis in Ntcp-Transfected HepG2 Hepatoblastoma Cells.
Human HepG2 hepatoblastoma cells were stably transfected with a pcDNA3.1/Na+-taurocholate cotransporting polypeptide (Ntcp) construct (Ntcp-HepG2 cells).30 After cultivation, the cells were incubated in minimal essential medium (Eagle) with bile acids or DMSO (0.025%, vol/vol; control) for 4 hours. After fixation, Ntcp-HepG2 cells were incubated with an anti-cleaved caspase-3 antibody for quantification of apoptosis. Subsequently, cells were incubated with a secondary anti-rabbit immunoglobulin G antibody. Nuclear DNA fragmentation was disclosed with Hoechst 33342. The studies were conducted on a Zeiss Axiovert 135TV microscope. Living and dead cells were counted by two examiners independently, and results were expressed as percentage of total cells. For details, see Supporting Information Data.
Quantification of Apoptosis in Ntcp-Transfected HepG2 Hepatoblastoma Cells by Immunoblotting and Fluorescence Techniques.
Ntcp-transfected HepG2 cells were cultured and exposed to bile acids. Quantification of apoptosis was performed by immunoblotting31 and fluorescence techniques. For details, see Supporting Information Data.
Statistics.
Results were expressed as mean ± standard deviation (SD). Differences between the various groups were assessed for statistical significance by analysis of variance (ANOVA) with Tukey's post-hoc test. Statistical significance was assumed when P values were <0.05.
Results
Taurine Conjugation Is Essential for norUDCA's Anticholestatic Effect on Bile Flow.
Bile flow in rat livers was 1.1 ± 0.1 μL/minute/g liver (n = 65) after 45 minutes of perfusion with KRB, reflecting an adequate secretory function of IPRL. Addition of CDNB (30 μmol/L), the precursor of the Mrp2 model substrate GS-DNP, between minute 65 and 75 led to a transient increase of bile flow due to its choleretic property as observed previously.11, 13, 14, 31 Bile flow in controls was stable for the following 70 minutes. Addition of TLCA (10 μmol/L) resulted in a pronounced decrease of bile flow to 8% of controls as described previously.11, 13, 14, 31 In contrast, addition of the hydrophilic C23 and C24 homologs norUDCA, UDCA, TnorUDCA, and TUDCA (25 μmol/L each) led to an increase of bile flow, i.e., choleresis, to 209% (P < 0.01), 254% (P < 0.01), 180% (P < 0.01), and 137% (not significant, n.s.) of controls under noncholestatic conditions (Fig. 1A, Table 1).
Effect of norursodeoxycholic acid (norUDCA), its 24- and 22-homologs, UDCA and bisnorUDCA, and the taurine conjugates, TnorUDCA and TUDCA, on bile flow in the (A) absence and (B) presence of the cholestatic bile acid, taurolithocholic acid (TLCA) in isolated perfused rat livers. Rat livers were continuously perfused with bile acids from minute 45 to minute 115 (TLCA, 10 μmol/L; other bile acids 25 μmol/L, each). 1-Chloro-2,4-dinitrobenzene (CDNB, 30 μmol/L), the precursor of the Mrp2 substrate, 2,4-dinitrophenyl-S-glutathione (GS-DNP), was infused between minute 65 and 75. Bile was collected in 5-minute intervals. Results are expressed as means ± SD, n = 4, each. (a) The hydrophilic bile acids, norUDCA (Δ), TnorUDCA (▴), and UDCA (□) increase bile flow under noncholestatic conditions in comparison to controls (choleretic effect). (b) TLCA (•)-induced cholestasis is antagonized by TnorUDCA (▴), but not norUDCA (Δ), and by TUDCA (▪) more than UDCA (□) (anticholestatic effect).
| Treatment | Bile Flow (μL/50 Minutes/g Liver) | GS-DNP Secretion (nmol/50 Minutes/g Liver) | LDH Efflux After 115 Minutes (mU/Minute/g Liver) |
|---|---|---|---|
| Control | 65.2 ± 2.0 | 1037 ± 79 | 14.2 ± 23.2 |
| TLCA | 5.5 ± 2.1 | 52 ± 14 | 67.4 ± 17.7 |
| UDCA | 165.7 ± 40.9 ** | 838 ± 258 | 27.7 ± 32.3 |
| TUDCA | 89.5 ± 6.3 | 839 ± 99 | 6.0 ± 5.3 # |
| norUDCA | 136.2 ± 22.6 ** | 876 ± 333 | 7.1 ± 5.9 |
| TnorUDCA | 117.4 ± 10.9 ** | 839 ± 119 | 8.3 ± 8.2 # |
| bisnorUDCA | 71.4 ± 12.6 | 820 ± 140 | 20.7 ± 19.8 |
| TCDCA | 63.9 ± 24.9 | 526 ± 225 * | 48.9 ± 28.3 |
| TLCA + UDCA | 47.7 ± 15.0 ## | 174 ± 72 | 44.0 ± 19.3 |
| TLCA + TUDCA | 88.5 ± 8.8 ## | 600 ± 87 ## | 15.0 ± 3.6 |
| TLCA + norUDCA | 8.9 ± 3.0 | 39 ± 19 | 52.6 ± 41.3 |
| TLCA + TnorUDCA | 53.9 ± 26.3 ## | 273 ± 106 # | 64.9 ± 34.7 |
| TLCA + bisnorUDCA | 4.7 ± 2.1 | 30 ± 16 | 29.5 ± 13.5 |
| TLCA + TCDCA | 52.2 ± 13.7 ## | 484 ± 163 ## | 100.9 ± 22.2 ** |
| TLCA + norUDCA + UDCA | 37.8 ± 10.5 # | 95 ± 61 | 49.8 ± 33.9 |
| TLCA + TnorUDCA + TUDCA | 142.6 ± 11.6 ## | 772 ± 105 ## | 13.2 ± 4.9 |
- Results are expressed as means ± SD of 4-5 experiments each. *P <0.05 vs. control; **P < 0.01 vs. control; #P < 0.05 vs. TLCA; ##P < 0.01 vs. TLCA. For statistical analysis, please also refer to Fig. 2 and to the text.
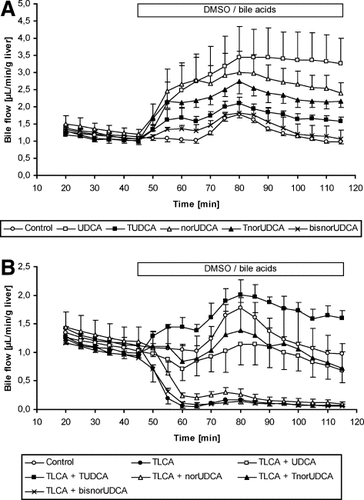
In the presence of TLCA (10 μmol/L), the anticholestatic properties of C23 bile acids markedly differed (Figs. 1B and 2, Table 1). TnorUDCA reversed TLCA-induced cholestasis (bile flow 83% of controls). In sharp contrast, norUDCA failed to counteract the cholestatic effect of TLCA (bile flow 14% of controls). The effect of TnorUDCA, but not norUDCA was comparable to the anticholestatic effect of UDCA (bile flow 73% of controls) and TCDCA (bile flow 80% of controls), but was inferior to TUDCA (bile flow 136% of controls, P < 0.05). TUDCA was more potent than UDCA (P < 0.01) and TCDCA (P < 0.05) in antagonizing TLCA-induced impairment of bile flow. Combined administration of TUDCA and TnorUDCA (25 μmol/L each; bile flow = 219% of controls) was far more efficient in reversing TLCA-induced cholestasis than combined administration of unconjugated UDCA and norUDCA (25 μmol/L each; bile flow 58% of controls; P < 0.01) or administration of only TUDCA (25 μmol/L) or TnorUDCA (25 μmol/L) (both P < 0.01). BisnorUDCA, the C22 homolog of UDCA, lacking one more methylene group in the side chain than norUDCA, had neither choleretic nor anticholestatic properties (Fig. 1, Table 1) in the model of IPRL.
Thus, taurine conjugation is a key prerequisite for the anticholestatic effect of C23 and putatively C24 bile acids in experimental hepatocellular cholestasis.
TUDCA Is Superior to TnorUDCA in Reversing Impaired Organic Anion Secretion in TLCA-Induced Cholestasis.
After administration of the GS-DNP precursor, CDNB (30 μmol/L), for 10 minutes via the portal vein, biliary secretion of GS-DNP was 1037 ± 79 nmol/50 minutes/g liver in controls. Administration of TLCA (10 μmol/L) reduced hepatobiliary GS-DNP secretion to 5% of controls as observed earlier.14 NorUDCA had no effect on TLCA-induced impairment of GS-DNP excretion, and its taurine conjugate, TnorUDCA partially rescued GS-DNP secretion (P < 0.05; Table 1, Fig. 2B). TUDCA and TCDCA, but not UDCA (P < 0.01; Fig. 2B, Table 1), and more effectively than TnorUDCA (P < 0.01 and P < 0.05; Table 1, Fig. 2B) reversed the inhibiting effect of TLCA on hepatobiliary organic anion secretion. Combined administration of unconjugated UDCA and norUDCA (25 μmol/L, each) did not antagonize impairment of organic anion secretion in TLCA-induced cholestasis. In contrast, a combined administration of their taurine-conjugates (TUDCA + TnorUDCA, 25 μmol/L, each) reversed impaired organic anion secretion more effectively than TnorUDCA alone (P < 0.01), but not TUDCA alone.
TnorUDCA, but not norUDCA, and TUDCA more than UDCA antagonize the cholestatic effect of TLCA on (A) bile flow and (B) organic anion secretion in isolated perfused rat liver. Cumulative bile flow and biliary 2,4-dinitrophenyl-S-glutathione (GS-DNP) secretion of experiments shown in Fig. 1B were calculated for 50 minutes. Results are expressed as means ± SD, n = 4-5, each. *P < 0.05, **P < 0.01, ANOVA post hoc test (Tukey).
Thus, TUDCA more than TnorUDCA effectively antagonized TLCA-induced impairment of biliary organic anion secretion, whereas UDCA and norUDCA were not effective.
Levels of NorUDCA in Bile Are Lower Than Those of UDCA in Perfused Rat Livers.
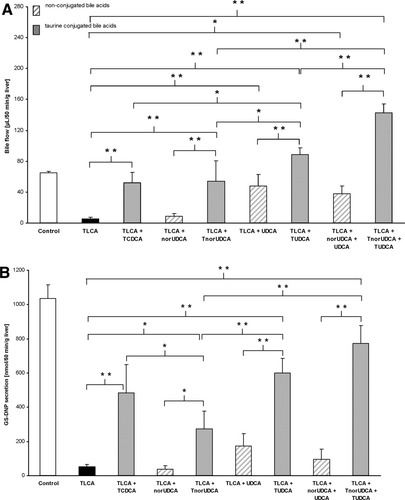
Levels of total (unconjugated + conjugated) norUDCA and UDCA in bile, liver tissue, and hepatovenous effluate were determined using a gas chromatographic technique (Fig. 3). Levels of total UDCA in bile were higher than those of norUDCA under physiological and cholestatic conditions when administered at equimolar concentration (25 μmol/L; Fig. 3A). Levels of UDCA in liver tissue were not different from levels of norUDCA under control conditions, but were higher under cholestatic conditions (Fig. 3B). A total of 9% ± 7% of norUDCA was glucuronidated under control conditions, whereas 40% ± 15% of norUDCA was glucuronidated under cholestatic conditions. Bile acid levels in the hepatovenous effluate as an indirect measure of sinusoidal bile acid uptake were not different (Fig. 3C).
Biliary secretion of norUDCA is lower than that of UDCA in isolated perfused rat livers. Levels of norUDCA and UDCA in (A) bile, in (B) liver tissue, and in (C) hepatovenous effluate after administration of the respective 3α,7β diOH bile acid via the portal vein were determined in experiments shown in Figs. 1 and 2 using a gas chromatographic technique (see methods). Bile was pooled between minute 45 and 65, minute 65 and 85, and minute 85 and 105. The hepatovenous effluate was pooled between minute 55 and 115. Results are expressed as means ± SD, n = 3-4, each. In the last interval (minute 85 to 105) of the group TLCA+norUDCA is n = 1 due to minute amounts of bile available. **P < 0.01; *P < 0.01 versus norUDCA; #P < 0.05 versus TLCA+norUDCA; §P < 0.01 versus TLCA+norUDCA, ANOVA post hoc test (Tukey). Bile acid concentration (y axis) relates to the concentration of UDCA or norUDCA measured.
NorUDCA Is Poorly Conjugated With Taurine in Rat Liver.
After administration of norUDCA (25 μmol/L), only low levels of norUDCA were detected in bile of IPRL by LC-MS/MS (0.73 ± 0.12 mmol/L total, i.e., conjugated and unconjugated, norUDCA, n = 4) of which only 3% were conjugated with taurine (Supporting Information Table 1). Biliary levels of total norUDCA tended to be even lower in TLCA-induced cholestasis (0.20 ± 0.12 mmol/L total norUDCA, n = 2) (Supporting Information Table 1, Fig. 4). Again, taurine conjugates formed only 5% of total norUDCA confirming the low rate of hepatic taurine conjugation of norUDCA as described previously in human8 and mouse10 liver.
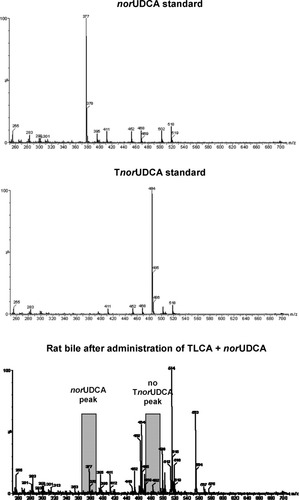
NorUDCA is barely conjugated with taurine in isolated perfused rat liver. Representative mass spectra of a norUDCA standard, a TnorUDCA standard, and a bile sample collected from rat liver perfused with TLCA (10 μmol/L) + norUDCA (25 μmol/L) are shown. No TnorUDCA peak was detected in this sample. Major peaks: m/z = 377, norUDCA; m/z = 464, glycocholic and glycomuricholic acid; m/z = 484, TnorUDCA; m/z = 514, taurocholic acid; m/z = 553, norUDCA glucuronide.
After administration of TnorUDCA (25 μmol/L), considerably higher levels of TnorUDCA were detected in bile of IPRL in the absence of TLCA (22.13 ± 1.22 mmol/L, n = 4, 100% taurine-conjugated) and in the presence of TLCA (16.03 ± 3.86 mmol/L, n = 4, 100% taurine-conjugated) accompanied by the anticholestatic action of TnorUDCA. Thus, taurine-conjugation is essential for the anticholestatic property of norUDCA in IPRL.
After administration of UDCA (25 μmol/L), levels of UDCA detected in bile of IPRL by LC-MS/MS (21.34 ± 4.97 mmol/L total UDCA, n = 4) were more than 29-fold higher than those of norUDCA after administration of equivalent doses (see above and Supporting Information Table 1; P < 0.01). 35% of UDCA were conjugated with taurine. Thus, UDCA is also incompletely conjugated in our experimental setup. Unconjugated UDCA is secreted into bile and, similar to norUDCA, should undergo cholehepatic shunting.
Lack of Hepatocyte Damage in Rat Liver Tissue Exposed to Low Micromolar TLCA in IPRL.
Administration of the potentially toxic bile acid TLCA did not significantly affect hepatovenous LDH release at low micromolar concentrations. Coadministration of TLCA with UDCA, TUDCA, norUDCA, TnorUDCA, and bisnorUDCA had no effect, either. Only coadministration of TLCA and TCDCA markedly increased hepatovenous LDH release (P < 0.01) (Table 1).
Active caspase-3 and cytokeratin 18 breakdown as markers of apoptosis were determined immunohistochemically in IPRL tissue exposed to bile acids at low micromolar concentrations for 70 minutes (Fig. 5). In control livers, the mean number of apoptotic cells was 0.3 ± 0.2 per field (40 fields per liver). Neither TLCA alone (0.7 ± 1.0 apoptotic cells per field) nor coadministration of TLCA with norUDCA (0.2 ± 0.2 apoptotic cells per field) or TnorUDCA (0.1 ± 0.1 apoptotic cells per field) affected apoptotic cell death during the perfusion period.
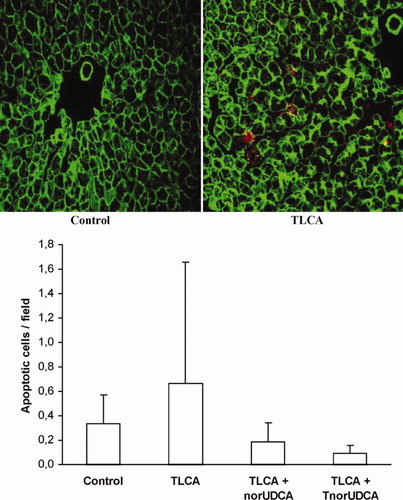
TLCA (10 μmol/L) does not induce relevant apoptosis in isolated perfused rat liver. Rat livers were snap-frozen at the end of experiments shown in Figs. 1B and 2. Apoptotic cells were quantified in cryosections of the livers by staining of activated caspase-3 (yellow) and cytokeratin 18 (red). Results are expressed as means ± SD, n = 4 each.
Thus, the choleretic and anticholestatic effects of C23 and C24 bile acids administered at low micromolar concentrations in this experimental study were not affected by bile acid-induced cell damage as determined by enzymatic and immunofluorescence techniques.
TUDCA and TnorUDCA Reduce TLCA- and GCDCA-Induced Apoptosis in Ntcp-Transfected HepG2 Cells.
Because apoptosis was not induced during short-term administration of TLCA in IPRL, we used Ntcp-transfected HepG2 cells in order to compare potential antiapoptotic properties of TnorUDCA and TUDCA. Apoptotic cells were identified by immunocytochemical visualization of cleaved caspase-3 and by nuclear fragmentation with Hoechst 33342 staining. Under control conditions, 1.5 ± 1.0% of total cells were apoptotic. Addition of TLCA at a low concentration of 5 μmol/L led to a distinct increase of the rate of apoptotic cell death to 65.5 ± 34.1% of cells (P < 0.01 versus controls). Coadministration of the hydrophilic bile acids TUDCA (75 μmol/L) or TnorUDCA (75 μmol/L) both led to a reduction of TLCA-induced apoptosis to 24.5 ± 14.8% (TLCA + TUDCA; P < 0.05 versus TLCA) and 6.3 ± 1.9% apoptotic cells (TLCA + TnorUDCA; P < 0.01 versus TLCA) (Fig. 6).
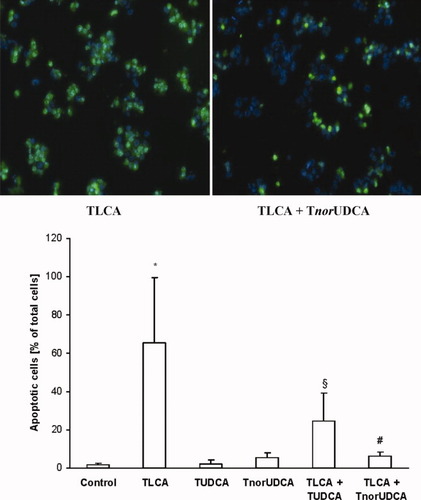
TnorUDCA (75 μmol/L) and TUDCA (75 μmol/L) reduce TLCA (5 μmol/L)-induced apoptosis in Ntcp-transfected HepG2 cells. Ntcp-transfected HepG2 cells were incubated with different bile acids or the carrier only (control). Apoptotic cells were identified by a positive signal for activated caspase-3 (green) and detection of nuclear fragmentation with Hoechst 33342 staining. Results are expressed as means ± SD, n = 4 each. *P < 0.01 versus controls; #P < 0.01 versus TLCA; §P < 0.05 versus TLCA, ANOVA post hoc test (Tukey).
GCDCA also induced apoptosis in Ntcp-transfected HepG2 cells as determined by immunoblotting of cleaved caspase-3 and caspase-9 (Fig. 7). Coadministration of either TnorUDCA or TUDCA reduced the rise of cleaved caspase-3 induced by GCDCA (Fig. 7). The antiapoptotic effect of TUDCA was superior to that of TnorUDCA as indicated by more effective reduction of GCDCA-induced caspase-3/7 activation (P < 0.01) (Fig. 7). In addition, a more than six-fold increase of cytochrome c release after administration of GCDCA when compared to controls (P < 0.01) tended to be reversed by TUDCA more than TnorUDCA (Fig. 7).
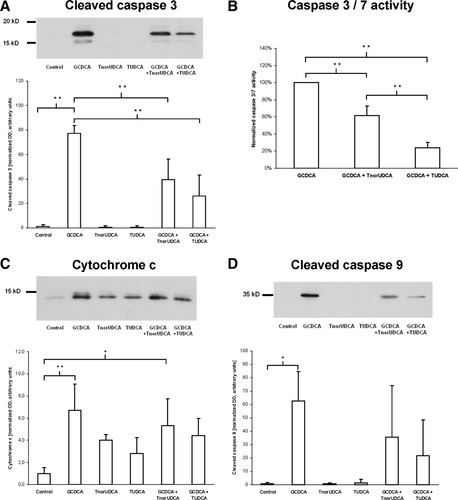
TnorUDCA and TUDCA reduce GCDCA-induced apoptosis in Ntcp-transfected HepG2 cells. Ntcp-transfected HepG2 cells were incubated with different bile acids (GCDCA, TnorUDCA, TUDCA) at a concentration of 100 μmol/L each or with 0.9% NaCl (controls). Apoptosis was quantified by immunoblotting against cytochrome c, cleaved caspase-9 and caspase-3, and by determination of caspase-3/7 activity. For details, please refer to the methods section. Results are expressed as means ± SD, n = 3-4 each. *P < 0.05, **P < 0.01, ANOVA post hoc test (Tukey).
Thus, both TnorUDCA and TUDCA were effective in reducing bile acid-induced apoptosis of human hepatoma cells at moderate micromolar concentrations.
Discussion
The C23-homolog of UDCA, norUDCA, exerts potent anticholestatic, anti-inflammatory, antiproliferative, and antifibrotic effects when administered to Mdr2−/− mice, an experimental model of fibrosing/sclerosing cholangitis.9, 10, 32 The present study aimed at testing norUDCA in TLCA-induced cholestasis in IPRL, an experimental model of acute hepatocellular rather than cholangiocellular cholestasis12-14, 16 to gain further insights into the differential hepatocellular mechanisms of action of UDCA and its derivatives. Our data show that norUDCA exerts choleretic effects in normal IPRL (Fig. 1A, Table 1), but does not exert any anticholestatic effects in the experimental model of TLCA-induced hepatocellular cholestasis in IPRL (Figs. 1B and 2, Table 1). In contrast, its taurine conjugate, TnorUDCA was moderately effective, but still inferior to TUDCA in antagonizing the cholestatic effect of TLCA in IPRL (Figs. 1B and 2, Table 1). Thus, conjugation of norUDCA is essential for an anticholestatic effect in TLCA-induced hepatocellular cholestasis in IPRL.
Taurine-conjugated UDCA was also more effective than unconjugated UDCA in antagonizing the cholestatic effect of TLCA in IPRL (Figs. 1B and 2, Table 1) suggesting that conjugation is essential for the anticholestatic effect not only of norUDCA, but also of UDCA in hepatocellular cholestasis. Why then does unconjugated UDCA, in contrast to norUDCA, exert moderate anticholestatic effects? Our data clearly show that UDCA, but not norUDCA is effectively conjugated in rat liver, resulting in sufficient taurine conjugation during a single passage of more than a third of UDCA molecules secreted into bile. Effective conjugation of UDCA, but not norUDCA has previously been described in different species including human, rabbit, and mouse8, 10, 33 and may explain the marked difference in the effects of UDCA and norUDCA on TLCA-induced cholestasis in the present single-pass perfusion study in rat liver. However, it has to be kept in mind, that in mice fed norUDCA chronically, taurine conjugates of norUDCA accumulated to more than 20% of biliary bile acids.10 Nonetheless, in the Mdr2−/− mouse, TnorUDCA is much less effective than norUDCA.10 The accumulation in the enterohepatic circulation of the small fraction of norUDCA that is conjugated with taurine, if true for humans, would mean that administration of norUDCA results in the continuing hepatocyte transport of both norUDCA and TnorUDCA, each of which has distinct therapeutic properties in animal models of acute and chronic cholestasis.
C23-nordihydroxy bile acids such as norUDCA have a higher choleretic potential than their C24 homologs and markedly stimulate biliary HCO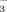 secretion presumably at least in part via a mechanism termed “cholehepatic shunting.”8, 34 The apical hepatocyte export pump for unconjugated norUDCA has not been defined so far, but the bile salt export pump, Bsep, appears as the most likely candidate8 because it also transports unconjugated UDCA in polarized cells.35 According to the cholehepatic shunt hypothesis, non-conjugated bile acids in bile like norUDCA which are sufficiently hydrophobic to be membrane-permeable in their protonated form undergo cholangiocellular absorption. Continuous withdrawal of protons from bile by cholangiocellular resorption of protonated bile acids may lead to a steady rise in HCO
secretion presumably at least in part via a mechanism termed “cholehepatic shunting.”8, 34 The apical hepatocyte export pump for unconjugated norUDCA has not been defined so far, but the bile salt export pump, Bsep, appears as the most likely candidate8 because it also transports unconjugated UDCA in polarized cells.35 According to the cholehepatic shunt hypothesis, non-conjugated bile acids in bile like norUDCA which are sufficiently hydrophobic to be membrane-permeable in their protonated form undergo cholangiocellular absorption. Continuous withdrawal of protons from bile by cholangiocellular resorption of protonated bile acids may lead to a steady rise in HCO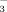 anions in bile and HCO
anions in bile and HCO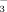 -rich choleresis. Protonated bile acids are believed to be transferred back into the hepatic circulation via the peribiliary plexus, where they are supposed to be taken up by hepatocytes, secreted into bile again and reabsorbed by cholangiocytes in their protonated form thereby generating more HCO
-rich choleresis. Protonated bile acids are believed to be transferred back into the hepatic circulation via the peribiliary plexus, where they are supposed to be taken up by hepatocytes, secreted into bile again and reabsorbed by cholangiocytes in their protonated form thereby generating more HCO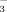 for a HCO
for a HCO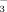 -rich choleresis.8, 34 The biliary levels of norUDCA were markedly lower than those of UDCA in line with the assumption of an effective cholehepatic cycle of norUDCA. An accumulation of norUDCA in liver tissue during the present short-term experiments was not observed. Still, cholehepatic shunting with effective biliary reabsorption most likely explains the low rate of biliary secretion of norUDCA when hepatovenous efflux of norUDCA as an inverse measure of basolateral norUDCA uptake tended to be only slightly higher than that of UDCA in the present experiments.
-rich choleresis.8, 34 The biliary levels of norUDCA were markedly lower than those of UDCA in line with the assumption of an effective cholehepatic cycle of norUDCA. An accumulation of norUDCA in liver tissue during the present short-term experiments was not observed. Still, cholehepatic shunting with effective biliary reabsorption most likely explains the low rate of biliary secretion of norUDCA when hepatovenous efflux of norUDCA as an inverse measure of basolateral norUDCA uptake tended to be only slightly higher than that of UDCA in the present experiments.
The choleretic effect of TUDCA at the level of the cholangiocyte has been partly unravelled in experimental settings. TUDCA administration increased biliary ATP levels in IPRL by hepatocellular and/or cholangiocellular ATP secretion.36 Recent data in mice indicated that UDCA may stimulate cholangiocellular ATP release into bile by a CFTR-dependent mechanism defective in Cftr−/− mice.37 ATP release may induce purinergic activation of cholangiocytes via apical purinergic P2Y receptors and CFTR-dependent stimulation of chloride secretion in a Ca++/PKCα/PI3K-dependent way.37 This complex sequence of events finally leads to enhanced cholangiocyte HCO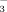 secretion putatively mediated by the Cl−/HCO
secretion putatively mediated by the Cl−/HCO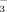 exchanger, AE2, after exposure to TUDCA. Whether TUDCA also stimulates apical targeting and insertion of AE2 and other key channels and transporters by Ca++/PKCα-dependent mechanisms as shown in cholestatic hepatocytes in the past,7 remains to be further elucidated. It is unclear at present whether norUDCA affects cholangiocellular ATP release and activates signaling cascades in cholangiocytes as TUDCA does. Notably, recent data indicate that norUDCA directly stimulates fluid secretion in isolated bile duct units in a HCO
exchanger, AE2, after exposure to TUDCA. Whether TUDCA also stimulates apical targeting and insertion of AE2 and other key channels and transporters by Ca++/PKCα-dependent mechanisms as shown in cholestatic hepatocytes in the past,7 remains to be further elucidated. It is unclear at present whether norUDCA affects cholangiocellular ATP release and activates signaling cascades in cholangiocytes as TUDCA does. Notably, recent data indicate that norUDCA directly stimulates fluid secretion in isolated bile duct units in a HCO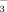 -dependent fashion to a higher extent than UDCA.10 Continuous HCO
-dependent fashion to a higher extent than UDCA.10 Continuous HCO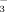 secretion may maintain an alkaline pH near the apical surface of hepatocytes and cholangiocytes to prevent the uncontrolled membrane permeation of protonated glycine-conjugated or unconjugated bile acids. and may be regarded as an essential protective mechanism of cells exposed to millimolar levels of bile acids.38
NorUDCA effectively stimulates biliary HCO
secretion may maintain an alkaline pH near the apical surface of hepatocytes and cholangiocytes to prevent the uncontrolled membrane permeation of protonated glycine-conjugated or unconjugated bile acids. and may be regarded as an essential protective mechanism of cells exposed to millimolar levels of bile acids.38
NorUDCA effectively stimulates biliary HCO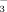 secretion8 and, thereby, strengthens this biliary HCO
secretion8 and, thereby, strengthens this biliary HCO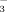 umbrella38 in mice, rats, and humans. It may be this mechanism which turns norUDCA to an attractive candidate for treatment of various cholangiopathies.38
umbrella38 in mice, rats, and humans. It may be this mechanism which turns norUDCA to an attractive candidate for treatment of various cholangiopathies.38
Acute exposure of rat livers to the hydrophobic bile acid TLCA is a well-established model of hepatocellular cholestasis12-14, 16 and is characterized by retrieval of apical transport proteins from the canalicular membrane in a PI3K-dependent and putatively protein kinase C ε (PKCε)-dependent way14 whereas continuous exposure to LCA via the oral route may induce a destructive cholangitis with segmental bile duct obstruction in mice15 and finally bile acid–induced cirrhosis, as described more than 50 years ago.39
Potentially toxic bile acids such as GCDCA or TLCA induce or aggravate cholestasis when accumulating in the liver. In addition, they are potent apoptotic stimuli and induce apoptosis by both death receptor-dependent and death receptor–independent mechanisms, involving mitochondrial signaling pathways.18 TUDCA has been shown in the past to exert potent antiapoptotic activity in hepatic and nonhepatic cells.7, 19, 20 Our study indicates that TnorUDCA, like TUDCA, has antiapoptotic properties. More detailed studies are needed to further unravel the molecular mechanisms which mediate this antiapoptotic effect.
In conclusion, taurine conjugation is essential for norUDCA to exert anticholestatic effects in experimental hepatocellular cholestasis. In TLCA-induced hepatocellular cholestasis in IPRL, norUDCA is ineffective and TnorUDCA is moderately effective in showing anticholestatic properties when compared to TUDCA. Because TUDCA stimulates impaired secretion by activation of complex signaling pathways at the level of the hepatocyte in this experimental model7, 11 and norUDCA induces hypercholeresis putatively via different molecular mechanisms such as cholehepatic shunting at the level of the cholangiocyte,8, 10 it is tempting to speculate that combined therapy with UDCA and norUDCA may be superior to UDCA or norUDCA monotherapy in biliary disorders in which hepatocyte and cholangiocyte dysfunction contribute to disease progression and liver failure.




