MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2
Potential conflict of interest: Nothing to report.
Abstract
MicroRNAs (miRNAs) are small, noncoding RNAs that can act as oncogenes or tumor suppressors in human cancer. Our previous study showed that miR-125b was a prognostic indicator for patients with hepatocellular carcinoma (HCC), but its functions and exact mechanisms in hepatic carcinogenesis are still unknown. Here we demonstrate that miR-125b suppressed HCC cell growth in vitro and in vivo. Moreover, miR-125b increased p21Cip1/Waf1 expression and arrested cell cycle at G1 to S transition. In addition, miR-125b inhibited HCC cell migration and invasion. Further studies revealed that LIN28B was a downstream target of miR-125b in HCC cells as miR-125b bound directly to the 3′ untranslated region of LIN28B, thus reducing both the messenger RNA and protein levels of LIN28B. Silencing of LIN28B recapitulated the effects of miR-125b overexpression, whereas enforced expression of LIN28B reversed the suppressive effects of miR-125b. Conclusion: These findings indicate that miR-125b exerts tumor-suppressive effects in hepatic carcinogenesis through the suppression of oncogene LIN28B expression and suggest a therapeutic application of miR-125b in HCC. (HEPATOLOGY 2010)
MicroRNAs (miRNAs) are small, noncoding RNA molecules that negatively regulate gene expression, mainly through direct interaction with the 3′ untranslated region (3′-UTR) of corresponding target messenger RNAs (mRNAs).1 After binding to target mRNAs, miRNAs form a complex with them and reduce their protein levels, either by degrading the mRNA or by suppressing the translation of the target gene.2 It has been reported that miRNAs can posttranscriptionally regulate ≈30% of human genes, suggesting that miRNAs may have pivotal roles in physiological and pathological processes, including human carcinogenesis.3 Over the past 5 years, emerging evidence has demonstrated that miRNAs are crucial for the initiation, promotion, and progression of human cancers. For example, miR-15a and miR-16-1 were first investigated in tumorigenesis and found to be frequently translocated or deleted in chronic lymphocytic leukemia.4 It has been reported that AAV-mediated miR-26a had therapeutic effects in vivo in a murine liver cancer model.5 Recently, Trang et al.6 found that loss of tumor suppressor let-7 could facilitate the progression of lung tumors in mice, and exogenous delivery of let-7 into established lung tumors in mice remarkably inhibited tumor growth. These findings suggest that tumor-suppressive miRNAs can be delivered in vivo to suppress tumor growth, thus providing a new strategy for cancer therapy.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and is the third most common cause of cancer-related death.7 China alone accounts for more than 50% of HCC incidence in the world.8 The molecular pathogenesis of HCC is complicated and poorly understood. Although previous studies have suggested that many protein-coding genes are involved in the development and progression of HCC,9 the roles and potential mechanisms of miRNAs in HCC are largely unexplored. In a previous report, our miRNA profiling result showed that 84 miRNAs were differentially expressed in HCC versus nontumorous liver tissues, and only miR-125b expression was associated with patients' survival.10 Recent studies have demonstrated that miR-125b is dysregulated in multiple types of cancer, including breast,11 oral,12 bladder,13 and anaplastic thyroid carcinomas.14 These findings indicate that miR-125b may function importantly in human carcinogenesis. However, the possible roles and mechanisms of miR-125b in human HCC are still not well established.
In this study, we found that expression of miR-125b was suppressed in about 70% of primary HCCs and was highly associated with Ki-67 expression. miR-125b could inhibit cell proliferation, cell cycle progression and metastasis of HCC cells. Moreover, the oncogene LIN28B was identified as a direct and functional target for miR-125b in hepatic carcinogenesis.
Abbreviations
3′-UTR, 3′ untranslated region; HCC, hepatocellular carcinoma; miRNA, microRNA; mRNA, messenger RNA; PCR, polymerase chain reaction; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; siRNA, small interfering RNA.
Materials and Methods
RNA Isolation, Reverse-Transcription, and Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was extracted with TRIzol reagent (Invitrogen, CA). Complementary DNA synthesis was performed using a PrimeScript RT reagent kit (Takara, Dalian, China). The primers used are listed in Supporting Table 1. For the detection of mature miR-125b, RNA was reverse-transcribed using a specific reverse-transcription primer (Applied Biosystems, CA). The expression of miR-125b was quantified by way of quantitative reverse-transcription polymerase chain reaction (RT-PCR) using TaqMan microRNA assays (Applied Biosystems).
Transfection of miR-125b Inhibitor or Small Interfering RNA Against LIN28B.
Cells were transfected with miR-125b inhibitor (Ribobio, Guangzhou, China) or small interfering RNA (siRNA) against LIN28B (Invitrogen, Shanghai, China) using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, CA). For proliferation assays, cells were trypsinized 24 hours after transfection. For migration, invasion, cell cycle, and western blot assays, cells were collected 48 hours after transfection.
Cell Proliferation and Colony Formation Assays.
The cell proliferation was determined by way of WST-8 staining with Cell Counting Kit-8 (Dojinodo, Shanghai, China) according to the manufacturer's instructions. For colony formation assays, 500 cells were plated onto six-well plates and incubated at 37°C for 2 weeks. Cells were then stained with crystal violet, and the numbers of colonies per well were counted.
Fluorescence-Activated Cell Sorting Analysis.
Cells were fixed into 70% ethanol at −20°C for 24 hours, stained with 50 μg/mL propidium iodide (Kaiji, NanJing, China), and analyzed using FACSCaliber (BD Bioscience, MA). The results were analyzed using ModFit software (BD Bioscience).
In Vitro Migration and Invasion Assays.
Cells in serum-free medium were placed into the upper chamber of the insert (BD Bioscience) with or without matrigel. After several hours of incubation, cells remaining in the upper chamber or on the upper membrane were carefully removed. Cells adhering to the lower membrane were stained with 0.1% crystal violet and 20% methanol, imaged, and counted using an IX71 inverted microscope (Olympus, Tokyo, Japan).
Tumor Formation in Nude Mice.
Huh-7 cells stably expressing vector or miR-125b or SK-Hep-1 cells transfected with antagomir-125b or negative control were subcutaneously injected into 6- to 8-week-old nude mice. After 4 weeks, the mice were sacrificed and the tumors were weighed. Mice were manipulated and housed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Luciferase Assay.
HEK293T cells were plated into 96-well plates with 70% confluence 24 hours before transfection. A mixture of 50 ng pLUC-UTR, 100 ng pWPXL-miR-125b, and 10 ng Renilla were transfected into HEK293T cells using Lipofectamine 2000. Firefly and Renilla luciferase activities were measured using a dual-luciferase reporter system (Promega, Madison, WI).
Statistical Analysis.
miR-125b expression in primary HCCs and corresponding nontumorous livers was compared using a Wilcoxon signed-rank test. The correlation between miR-125b and Ki-67 was determined by way of Spearman correlation test. Clinicopathological correlations were preformed with a Fisher's exact test in SPSS17. For cell line models, the data were subjected to a two-tailed Student t test. P < 0.05 was considered statistically significant.
Results
Expression of miR-125b Is Frequently Down-Regulated and Inversely Correlated with Ki-67 Expression in Human HCCs.
Our previous miRNA expression profiling study indicated that miR-125b was commonly down-regulated in human HCC and that underexpression of miR-125b was associated with poor prognosis. However, the functional role of miR-125b in human HCC remains elusive. In this study, we sought to confirm the miR-125b down-regulation in an independent primary HCC cohort. The expression level of mature miR-125b in 54 pairs of snap-frozen primary HCC and their corresponding nontumorous liver specimens was examined by way of qRT-PCR and normalized against an endogenous control (U6 RNA). We found that the median miR-125b expression level in primary HCCs was three-fold lower than that of the nontumorous livers (median expression = 0.194 and 0.606, respectively), and overall miR-125b was significantly down-regulated in primary HCC samples (P < 0.0001 [Wilcoxon signed-rank test]) (Fig. 1A). When comparing paired primary HCCs with their corresponding nontumorous livers, down-regulation of miR-125b (more than two-fold [i.e., log2 (fold change) < −1]) was observed in 38 (70%) cases, indicating that down-regulation of miR-125b was a frequent event in human HCC (Fig. 1B). miR-125b expression was inversely correlated with Ki-67 expression in our clinical samples (P < 0.001, r = −0.3852 [Spearman correlation]), suggesting that miR-125b may play a role in controlling cell proliferation (Fig. 1C). However, we found no significant correlation between miR-125b and other clinicopathological features (Supporting Table 2).
miR-125b Inhibits HCC Cell Growth In Vitro and In Vivo.
Because the expression of miR-125b was inversely related to Ki-67 level in HCC, we sought to determine whether miR-125b might affect the proliferation rate of HCC cells. According to the expression level of miR-125b in HepG2 and Huh-7 cells (Supporting Fig. 1A), both of which have a very low basal level of miR-125b, these two liver cancer cell lines were selected to establish miR-125b stably expressing cells. Both HepG2 cells and Huh-7 cells were infected with the lentivirus containing miR-125b expression sequence, and the expression of miR-125b was confirmed by way of real-time PCR (Supporting Fig. 1B). As indicated in Fig. 2A,B, the cell proliferation rates of HepG2 and Huh-7 infected with lenti–miR-125b were significantly decreased when compared with those of the vector-infected cells. Because the expression of miR-125b was very low in HCC cell lines, we selected the adenocarcinoma cell line SK-Hep-1, which has a relatively high level of miR-125b, to estimate the effects of miR-125b inhibition. In contrast, silencing of miR-125b with transfection of miR-125b inhibitor in SK-Hep-1 cells could increase cell proliferation rates compared with the negative controls (Fig. 2C). Moreover, colony formation assay showed that enforced expression of miR-125b resulted in a more than 50% decrease in colony numbers in HepG2 and Huh-7 cells expressing miR-125b compared with the vector controls (Supporting Fig. 2A,B). Additionally, transfection of miR-125b inhibitor into SK-Hep-1 cells significantly increased the colony numbers of SK-Hep-1 cells when compared with negative controls (Supporting Fig. 2C). To further determine the effect of miR-125b on HCC cell growth in vivo, the Huh-7 cells stably expressing miR-125b or vector control were subcutaneously injected into nude mice. After 4 weeks, the mice were sacrificed and the tumors were weighed. The results showed that the tumor weight of the miR-125b stably expressing Huh-7 cells was significantly lower than that of the vector control cells (P = 0.0017) (Fig. 2D). Conversely, after the inhibition of miR-125b, the tumor weight of SK-Hep-1 cells was significantly higher than that of the negative controls (P = 0.0201) (Fig. 2E). Taken together, these results indicated that miR-125b inhibited HCC cell proliferation both in vitro and in vivo.
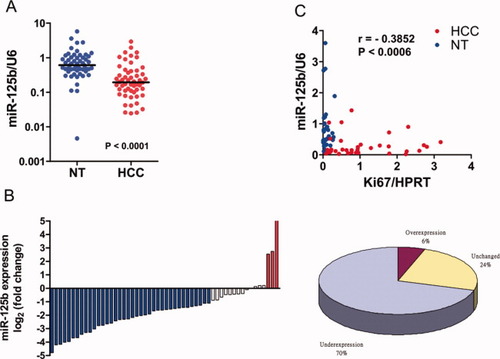
Expression of miR-125b was often down-regulated and inversely correlated with ki-67 expression in HCC. (A,B) Expression of mature miR-125b in 54 pairs of primary HCC and their corresponding nontumorous livers. Expression level of miR-125b was determined by way of qRT-PCR and normalized against an endogenous control (U6 RNA). Data were analyzed using a ΔΔCt approach and expressed as either miR-125b/U6 ratio [−2ΔCt(miR-125b-U6)] or log2 fold change (ΔΔCt [nontumorous/HCC]). miR-125b expression in HCC and nontumorous (NT) liver were compared by way of Wilcoxon signed-rank test. Significant down-regulation of miR-125b in paired HCC/nontumorous samples was defined as log2 fold change < −1 (i.e., two-fold). (C) miR-125b expression was correlated with Ki-67 level data available in 38 paired HCC and nontumorous liver samples using Spearman correlation. An inverse correlation between miR-125b and Ki67 expression was observed (P = 0.0006, r = −0.3852).
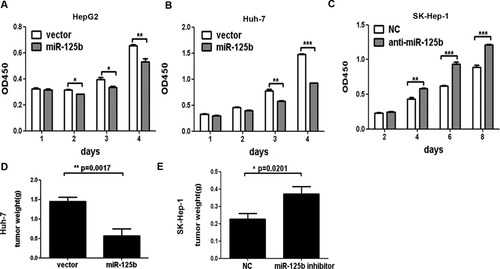
miR-125b inhibited HCC cell growth in vitro and in vivo. (A-C) The growth of HCC cells was inhibited by miR-125b in vitro. The growth of cells with miR-125b overexpression or inhibition was determined as described in Materials and Methods. *P < 0.05; **P < 0.01; ***P < 0.001. (D,E) miR-125b suppressed tumor growth in vivo. (D) Tumor weight of Huh-7 cells with vector or miR-125b transfection. (E) Tumor weight of SK-Hep-1 cells transfected with negative control (NC) or mir-125b inhibitor.
miR-125b Induces Cell Cycle Arrest of HCC Cells at G1/S Transition.
Given that miR-125b obviously inhibited HCC cell proliferation in vitro and in vivo, we next sought to determine whether miR-125b has any impact on cell cycle progression of HCC cells. The cell cycle distribution of HepG2 and Huh-7 cells showed that the cell number at G1 phase was increased in miR-125b–expressing cells compared with vector control (P = 0.040 and 0.004, respectively), whereas the cell population at S phase reduced sharply (P = 0.0151 and 0.0304, respectively) (Fig. 3A,B). In contrast, the cell cycle distribution analysis of SK-Hep-1 cells after transfection of miR-125b inhibitor showed that silencing of miR-125b could noticeably increase the cells at S phase, when compared with negative control (Fig. 3C). To further investigate the possible molecular mechanisms for miR-125b-induced G1 arrest, we detected several cell cycle regulatory proteins controlling G1/S transition (including cyclin D1, CDK6, CDC25A, cyclin E1, E2F1, Rb, p53, p21Cip1/Waf1, etc) after infection with lenti-miR-125b or transfection with miR-125b inhibitor. The results demonstrate that p21Cip1/Waf1, an important cell cycle inhibitor for G1/S transition, was markedly up-regulated by miR-125b overexpression, whereas the expression of p21Cip1/Waf1 was noticeably decreased by silencing of miR-125b (Fig. 3D). To further investigate the role of p21Cip1/Waf1 in the G1 arrest induced by miR-125b, we knocked down the expression of p21Cip1/Waf1 in the miR-125b–expressing cells by siRNA against p21Cip1/Waf1 (Supporting Fig. 3A). The results showed that the G1 arrest induced by miR-125b was abrogated by the knockdown of p21Cip1/Waf1 expression (Supporting Fig. 3B). Taken together, these results indicate that miR-125b blocks the G1/S transition and thus arrests the cell cycle at G1 phase of HCC cells possibly through up-regulation of p21Cip1/Waf1.
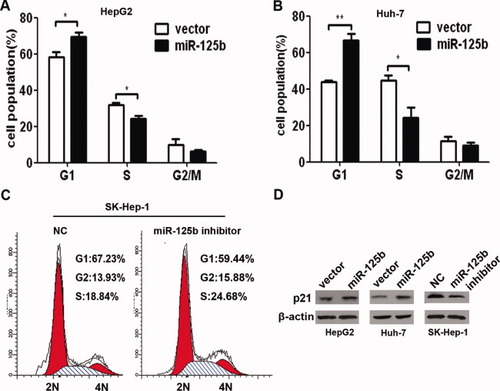
miR-125b induced cell cycle arrest of HCC cells at G1/S transition. (A,B) miR-125b induced G1 arrest of HCC cells. HepG2 or Huh-7 cells stably expressing miR-125b or vector control were analyzed by way of fluorescence-activated cell sorting. *P < 0.05; **P <0.01. (C) Silencing of miR-125b with transfection of miR-125b inhibitors promoted the entry of SK-Hep1 cells into S phase. (D) Western blot analysis of p21Cip1/Waf1 in miR-125b overexpressed or suppressed liver cancer cells.
miR-125b Impairs the Migratory and Invasive Abilities of HCC Cells.
It has been reported that miR-125b can impair the migration and invasion of breast cancer cells in vitro,15 thus prompting us to determine whether miR-125b could also inhibit HCC cell migration and invasion. As shown in Fig. 4A and Supporting Fig. 4A, Huh-7 cells stably expressing miR-125b displayed a significant reduction in migration ability compared with Huh-7 cells stably expressing vector control (P = 0.0355). Moreover, transwell invasion assay with matrigel coating demonstrated that enhanced expression of miR-125b significantly impaired the invasion ability of Huh-7 cells when compared with control cells (P < 0.0001) (Fig. 4B and Supporting Fig. 4B). It is worthy to note that the incubation time for migration and invasion assays were 4 hours and 16 hours, respectively, and at those time points, the cell growth of Huh-7 cells was not affected by miR-125b. So the inhibitory effects on cell migration and invasion were not caused by reduction of the cell numbers. Furthermore, silencing of miR-125b in SK-Hep-1 cells markedly promoted SK-Hep-1 cell migration (Fig. 4C and Supporting Fig. 4C) and invasion (Fig. 4D and Supporting Fig. 4D).
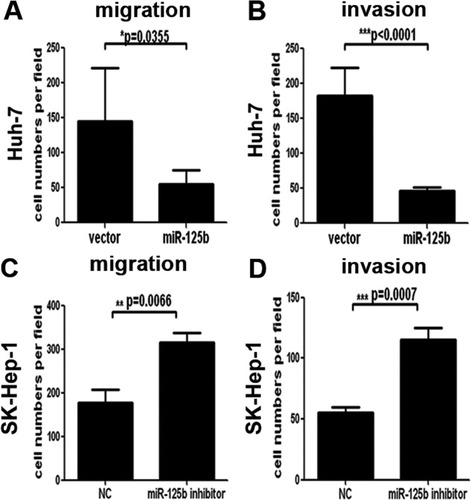
miR-125b impaired the migratory and invasive abilities of HCC cells. (A,B) Migration (A) and invasion (B) assays of Huh-7 cells infected with lenti–miR-125b or vector. (C,D) miR-125b silencing promoted the migration (C) and invasion (D) of SK-Hep-1 cells. Forty-eight hours after transfection of miR-125b inhibitor or negative control (NC), the migration and invasion of SK-Hep1 cells was measured by way of transwell assays.
Oncogene LIN28B Was a Direct Downstream Target for miR-125b.
MicroRNA usually exerts its functions by suppressing the expression of target mRNAs, so we next searched for the target genes of miR-125b in HCC. According to the prediction of TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/), and miRanda (microrna.org and miRbase), we performed real-time PCR to screen the candidate growth regulatory genes that could be suppressed by miR-125b. We found that overexpression of miR-125b in both Huh-7 and HepG2 cells reduced the mRNA level of LIN28B greater than 50% (P = 0.005 and P < 0.001 respectively) (Fig. 5A). Further semi–qRT-PCR experiments showed similar results (Fig. 5B). In addition, western blot analysis indicated that enforced expression of miR-125b significantly inhibited endogenous LIN28B protein expression (Fig. 5C). Furthermore, after transfection of miR-125b inhibitor in SK-Hep-1 cells, the expression of LIN28B was remarkably increased (Supporting Fig. 5A).
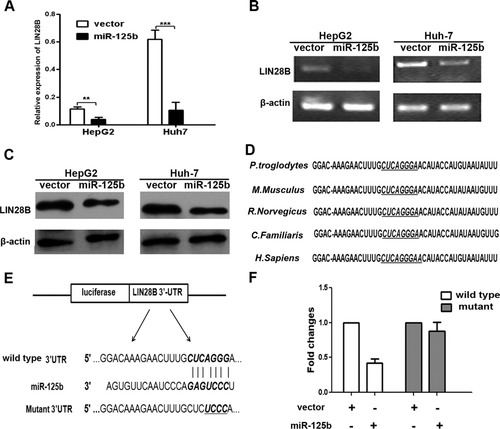
LIN28B was a direct downstream target for miR-125b in HCC cells. (A) The expression level of LIN28B mRNA was down-regulated by miR-125b using real-time PCR assays. (B) Expression level of LIN28B mRNA in miR-125b stably expressing cells or vector control cells by semiquantitative PCR analysis. β-Actin was used as an internal control. (C) Western blot results of endogenous LIN28B protein in HepG2 and Huh-7 cells infected with lenti–miR-125b or vector control. (D) Potential binding site of miR-125b on LIN28B 3′-UTR in different species. The seed sequence is underlined and italicized. (E) Sketch of the construction of wild-type or mutant pGL3-LIN28B 3′-UTR vectors. The mutant binding site is underlined and italicized. (F) Relative luciferase activity analyses. The pGL3, pGL3-wild-LIN28B 3′-UTR or pGL3-mutant-LIN28B 3′-UTR was transfected into 293T cells with pWPXL or pWPXL-miR-125b. Renilla luciferase vector was used as an internal control. The relative luciferase activity of pWPXL group was set as 1.
TargetScan analysis indicated that LIN28B contains one miR-125b binding site on its 3′-UTR, and the sequence of the binding site is highly conserved across different species (chimpanzee, mouse, rat, dog, and human) (Fig. 5D). Therefore, we constructed vectors containing wild-type or mutant 3′-UTR of LIN28B directly fused to the downstream of the firefly luciferase gene (Fig. 5E). The wild-type or mutant vector was cotransfected into HEK-293T cells with miR-125b expression construct or vector control. The transfection efficacy was normalized by cotransfection with Renilla reporter vector. As shown in Fig. 5F, miR-125b significantly decreased the relative luciferase activity of wild-type LIN28B 3′-UTR (more than 50%), whereas the reduction of the luciferase activity with mutant LIN28B 3′-UTR was not as sharp as that observed in the wild-type counterpart, suggesting that miR-125b could directly bind to the 3′-UTR of LIN28B. Taken together, these findings indicate that LIN28B is a direct downstream target for miR-125b in HCC cells.
Knockdown of LIN28B Recapitulates the Effects of miR-125b on HCC Cells.
LIN28B was first identified as a homolog of LIN28 in HCC16 and facilitated the cell transformation in vitro.17 LIN28B protein was up-regulated in poorly differentiated HCCs, but the effects of LIN28B on HCC cells have not yet been well characterized. To explore the functions of LIN28B, two specific siRNAs against LIN28B mRNA were synthesized. As shown in Fig. 6A, both siRNAs remarkably reduced the expression of LIN28B protein. Cell proliferation assays showed that both si-LIN28B-1 and si-LIN28B-2 significantly inhibited the proliferation of Huh-7 cells (Fig. 6B). Furthermore, we investigated the effect of si-LIN28B on cell cycle progression by way of fluorescence-activated cell sorting analysis. The results showed that both si-LIN28B-1 and si-LIN28B-2 blocked G1/S transition and retained Huh-7 cells at G1 phase (P = 0.0476 and 0.0221, respectively) (Fig. 6C). The suppression of proliferation and blockade of cell cycle progression by si-LIN28B-1 and si-LIN28B-2 mimicked the phenotype induced by enforced expression of miR-125b in HCC cells.
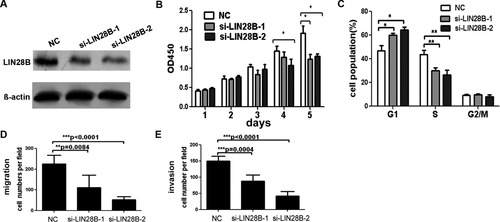
LIN28B silencing recapitulated the effects of miR-125b on HCC cells. (A) The protein level of LIN28B was detected by western blot assays after transfection with si-LIN28B-1 and si-LIN28B-2. (B) The cell proliferation of Huh-7 cells after siRNA transfection was determined as described in Materials and Methods. (C) Cell cycle analysis of Huh-7 cells after transfection with si-LIN28B or negative control. *P < 0.05; **P < 0.01. (D,E) Migration (D) and invasion (E) assays of Huh-7 cells after transfection with si-LIN28B or negative control (NC). Statistical results of three independent experiments are shown.
We next investigated the effect of si-LIN28B on the migration and invasion of Huh-7 cells. Remarkably, transwell assay without matrigel coating showed that si-LIN28B-1 elicited an inhibitory effect on Huh-7 cell migration compared with the control group (Fig. 6D). Si-LIN28B-2 showed a greater inhibitory effect than si-LIN28B-1, because si-LIN28B-2 had a better knockdown of the LIN28B protein level. Transwell assay with matrigel coating showed that si-LIN28B-1 and si-LIN28B-2 significantly reduced the invasion ability of HCC cells (Fig. 6E). Together, our results indicate that reduction of LIN28B through siRNA interference has similar effects on the HCC cells to those induced by miR-125b, suggesting that LIN28B may act as a downstream functional mediator for miR-125b.
Reintroduction of LIN28B Abrogated miR- 125b–Induced Growth Arrest and Metastasis Suppression.
If LIN28B indeed acts as a functional target of miR-125b, reintroduction of LIN28B into miR-125b–expressing cells should be able to antagonize the effects of miR-125b. To test the hypothesis, we first constructed a lentiviral expression vector of LIN28B without the 3′-UTR and infected miR-125b–expressing cells. As shown in Supporting Fig. 7A, the expression of LIN28B was recovered after LIN28B lentivirus infection. Interestingly, cell proliferation assay demonstrated that reintroduction of LIN28B enhanced the proliferation of miR-125b–expressing cells (Fig. 7A). Moreover, the inhibition of miR-125b on the colony formation was also antagonized by enforced expression of LIN28B (Supporting Fig. 7B). Furthermore, enforced expression of LIN28B significantly counteracted the G1 arrest induced by miR-125b (Fig. 7B). In addition, in vitro migration and invasion assays showed that enforced expression of LIN28B rescued the migration and invasion suppression induced by miR-125b (Fig. 7C,D). It is noteworthy that the LIN28B overexpressing cells displayed a phenotype of faster growth and increased aggressiveness compared with the other cells due to higher expression of LIN28B in these cells. Taken together, these findings show that LIN28B reintroduction could abrogate miR-125b–induced cell growth arrest and metastasis suppression, suggesting that LIN28B is a functional mediator for miR-125b in HCC cells.
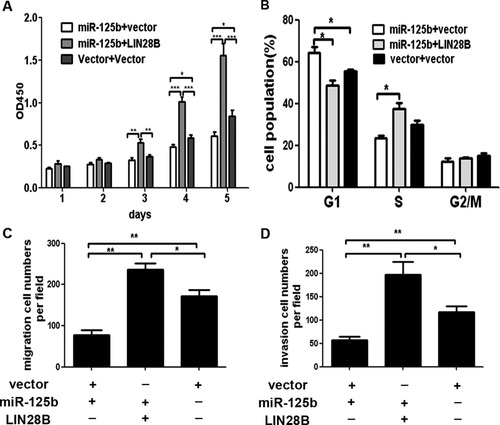
Reintroduction of LIN28B abrogated miR-125b–induced growth arrest and metastasis suppression of HCC cells. Huh-7 cells stably expressing miR-125b or vector were infected with lenti-LIN28B or corresponding vector. The following experiments were performed with the above cells. (A) The cell proliferation analyses. (B) Fluores cence-activated cell sorting assays for cell cycle distribution. (C) Migration assays. (D) Invasion assays. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
The survival and prognosis of HCC are poor partly due to relapse and metastasis, resistance to existing tumor therapies, and liver failure after resection. Therefore, clarification of the molecular pathogenesis of HCC is crucial for developing effective intervention and therapy strategies to improve the outcome of patients with this disease. In the present study, we found that the underexpression of miR-125b in ≈70% of HCC samples and the expression of miR-125b are inversely correlated with Ki-67, a cell proliferation index, suggesting that miR-125b might negatively control HCC cell growth. Indeed, our results showed that miR-125b could significantly suppress HCC cell proliferation in vitro and in vivo. In addition, our results demonstrated that miR-125b is a potential metastasis suppressor for HCC, because overexpression of miR-125b can significantly inhibit HCC cell migration and invasion. Altogether, the suppressive effects of miR-125b on HCC cell growth and metastasis might contribute to the poor prognosis of HCC patients with low expression of miR-125b.10
The mechanistic insight into the inhibitory effects of miR-125b on cell proliferation indicated that miR-125b can induce cell cycle arrest at the G1/S transition of HCC cells, suggesting that down-regulation of miR-125b in HCC may facilitate the cancer cells to divide and grow quickly. Furthermore, we found that miR-125b can markedly increase the expression of p21Cip1/Waf1 protein, a cyclin-dependent kinase inhibitor for the G1/S transition. It is noteworthy that although p53 has been reported to be a downstream target for miR-125b,18 the expression of p53 is not altered by miR-125b in human HCC cells (Supporting Fig. 8), suggesting that the induction of p21Cip1/Waf1 by miR-125b is mediated in a p53-independent manner. Because miR-125b induced the expression of p21Cip1/Waf1 and LIN28B acted as a downstream target of miR-125b, we speculated that LIN28B may regulate the expression of p21Cip1/Waf1. Therefore, we detected the expression of p21Cip1/Waf1 after the inhibition of LIN28B and found that the expression of p21Cip1/Waf1 was up-regulated by LIN28B silencing (Supporting Fig. 6A). Furthermore, knockdown of p21Cip1/Waf1 can antagonize the effect of G1 arrest induced by LIN28B silencing (Supporting Fig. 6B,C). These results indicate that LIN28B increases HCC cell growth, possibly through down-regulation of p21Cip1/Waf1 expression. Recently, LIN28B was reported to promote proliferation and metastasis of HCC cells through regulation of c-myc and E-cadherin.19 In the current study, our results confirmed that knockdown of LIN28B can decrease the expression of c-myc but can increase the expression of E-cadherin (Supporting Fig. 5C). Interestingly, miR-125b overexpression can up-regulate E-cadherin expression and down-regulate c-myc expression (Supporting Fig. 5C). Taken together, these results suggest that miR-125b regulates LIN28B and its downstream molecules (including p21Cip1/Waf1, c-myc, and E-cadherin) to suppress HCC cell proliferation and metastasis.
It is noteworthy that miR-125b may behave differentially in different types of human cancer, because miR-125b is underexpressed in HCC,10 breast cancer,11 prostate carcinoma,20 oral cancer,12 bladder cancer,13 and lung cancer,21 whereas it is up-regulated in glioma cancer,22 gastric cancer,23 leukemia,24 and urothelial carcinoma.25 Correspondingly, the effect of miR-125b on cell proliferation is seen in different cancer cells, which may be due to the distinct target genes for miR-125b in different types of cancer. The exploration of the target genes of miR-125b led to the identification of LIN28B as a direct and functional downstream mediator for miR-125b in HCC, whereas several reported target genes were unchanged by miR-125b overexpression (HER2,15 HER3,15 p53,18 and smo26) (Supporting Fig. 8). In Caenorhabditis elegans, miR-125b ortholog lin-4 can regulate the expression of LIN28a,27 a homologue of LIN28B. It has since been proven that miR-125 can repress the expression of LIN28a in mammals.28, 29 However, the interaction between miR-125b and LIN28B has not been reported. The binding site of miR-125b on the 3′-UTR of LIN28B is conserved across various species, including Caenorhabditis elegans, suggesting that the interaction between miR-125b and LIN28B may have an important function during evolution. LIN28B was first identified as a homologue of LIN28a in HCC.16 The expression of LIN28B is up-regulated in HCC, epithelial ovarian cancer,30 chronic myeloid leukemia, colon cancer, breast cancer, lung cancer, and cervical cancer.17 It is intriguing that LIN28B can be a prognosis predictor for epithelial ovarian cancer and is associated with the advanced disease and poor outcomes of HCC.17 However, the mechanism of LIN28B overexpression in human cancer has not yet been characterized. Although there are rare amplifications and translocations in some tumors,17 ours is the first evidence to support that overexpression of LIN28B in HCC may result from underexpression of a specific miRNA molecule (miR-125b).
LIN28B belongs to a highly conserved family that contains a cold shock motif and two zinc finger domains. It has been demonstrated that LIN28B can bind to the loop region of let-7 and inhibit the processing of let-7.31-33 LIN28B activation suppressed the expression of let-7 and promoted the proliferation induced by myc activation.34 In the present study, we found that expression of let-7 was up-regulated after reduction of LIN28B by exogenous miR-125b (Supporting Fig. 5B). Meanwhile, it has been reported that LIN28B is involved in the inactivation of the Raf kinase inhibitory protein signal pathway and promotes the migration and invasion of breast cancer cells.35 Therefore, the activation of Raf kinase inhibitory protein signal might contribute to the migration and invasion inhibition mediated by miR-125b. Recently, LIN28B was found to promote the transformation of cells and to be universally overexpressed in tumor samples.17 As for HCC, 66% of tumors had a high level of LIN28B and the expression of LIN28B was associated with the tumor stage. Consistent with our observations, Wang et al.19 recently reported that LIN28B can markedly promote the proliferation and metastasis of HCC cells.
In conclusion, our results show that miR-125b is underexpressed in most cases of HCC and is inversely related to cell proliferation index in HCC. miR-125b can suppress cell growth, induce cell cycle arrest at G1 phase, and inhibit migration and invasion of HCC cells. These tumor-suppressive functions of miR-125b are mediated by the target gene LIN28B, a potential oncogene in HCC. These findings facilitate a better understanding of the molecular pathogenesis of HCC and suggest that miR-125b might be a candidate for the treatment of HCC.
Acknowledgements
We thank Didier Trono (School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) for providing pWPXL, psPAX2, and pMD2.G lentivirus plasmids.




