Anti–tumor necrosis factor α treatment promotes apoptosis and prevents liver regeneration in a transgenic mouse model of chronic hepatitis C†
Potential conflict of interest: Nothing to report.
Abstract
Tumor necrosis factor α (TNFα) has been implicated in a variety of inflammatory diseases, and anti-TNFα has been shown to improve therapy when added to standard of care in chronic hepatitis C virus (HCV) infection. In addition, patients with chronic HCV have increased serum levels of TNFα and the macrophage-attracting chemokine (C-C motif) ligand 2 (CCL2). A mouse model of chronic HCV with hepatic nonstructural (NS) 3/4A protein expression mimics the human infection through a reduced response to double-stranded RNA and cleavage of the T cell protein tyrosine phosphatase. The mice also display a resistance to TNFα in vivo. We therefore analyzed the relationship between NS3/4A and TNFα. Wild-type and NS3/4A-transgenic (Tg) mice were treated with TNFα/D-galactosamine (D-galN), acting through the TNF receptor 1 on hepatocytes and macrophages, or lipopolysaccharide (LPS)/D-galN, acting through Toll-like receptor 4 on sinusoidal endothelial cells, macrophages, and dendritic cells. Mice were analyzed for hepatic signaling, liver damage, TNFα, and CCL2. Similar to HCV-infected humans, NS3/4A-Tg mice displayed elevated basal levels of TNFα and CCL2. Treatment of NS3/4A-Tg mice with TNFα/D-galN or LPS/D-galN led to increased hepatic nuclear factor kappa B (NFκB) activation, increased TNFα and CCL2 levels, decreased apoptosis, and increased hepatocyte regeneration. Importantly, blocking NFκB activation (bortezomib) or administering anti-TNFα (infliximab) 4 hours after LPS/D-galN injection reversed the resistance of NS3/4A-Tg mice to TNFα-induced liver injury. Conclusion: Resistance to TNFα seen in NS3/4A-Tg mice is explained by a hepatoprotective effect of NFκB and TNFα. Hence, anti-TNFα agents block these effects and are antiviral by promoting hepatocyte apoptosis and preventing hepatocyte regeneration. (HEPATOLOGY 2010;.)
Hepatitis C virus (HCV) is a main cause of chronic hepatitis worldwide, with a significant proportion of infected patients developing liver fibrosis, liver cirrhosis, hepatocellular carcinoma, and/or liver failure. An estimated 170 million people are currently infected with HCV. The actual therapies based on interferon (IFN) and ribavirin can cure only approximately 55% of the treated patients depending on viral genotype.1
The HCV genome consists of a 9.4-kb linear, single-stranded, positive-sense RNA molecule coding for 10 structural and nonstructural (NS) proteins (core, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B). As a persistent virus, HCV has evolved mechanisms both to use and control cellular molecules or pathways required for the viral life cycle and to evade elimination by innate and adaptive immunity. The primary evasion strategies of HCV are the capability to undergo mutational escape and the ability to modulate both intracellular and intercellular signaling.2 The proteolytic activity of the NS3/4A complex is responsible for the cleavage of the precursor polyprotein translated from the HCV genome. However, the protease activity is also required for HCV-mediated interference with retinoic acid–inducible gene I, Toll-like-receptor (TLR) 3, and epidermal growth factor/Akt signaling by cleaving CARD adaptor–inducing IFNβ,3, 4 Toll/interleukin-1 receptor domain–containing adaptor–inducing IFNβ,5 and T cell protein tyrosine phosphatase (TC-PTP).6
By studying Tg mice, we have noted that NS3/4A may affect cells other than hepatocytes, and that these effects seem to converge around tumor necrosis factor α (TNFα). Three observations suggest, quite unexpectedly, that TNFα may actually be a factor that promotes viral replication and persistent HCV infection. First, the levels of both TNFα and chemokine (C-C motif) ligand 2 (CCL2) have been found to be increased in the blood and/or liver of patients with chronic hepatitis C compared with healthy individuals.7, 8 Second, it has been found that anti-TNFα compounds are beneficial as an add-on to IFNα and ribavirin standard of care (SOC) therapy.9 Third, the plant extract silymarin, which is in use as alternative therapy for chronic liver diseases, including hepatitis C, blocks TNFα, IFNγ, and interleukin (IL)-2 production as well as nuclear factor kappa B (NFκB) transcriptional activation.10 We have observed that transgenic (Tg) mice expressing hepatic NS3/4A seem to have an altered hepatic immunity related to TNFα, reflected in a reduced sensitivity to TNFα and lipopolysaccharide (LPS).11 Macrophages are a potent source of TNFα and are, together with dendritic cells, the major hepatic populations expressing the LPS ligand TLR4. The key transcription factor for the production of TNFα is NFκB. A central factor responsible for macrophage activation and recruitment of monocytes and macrophages in the liver is the chemokine CCL2.12 Taken together, these findings suggest that TNFα may exert unexpected effects during HCV infection.
In this study, we aimed to understand the relationship between NS3/4A and TNFα, because this may help explain the beneficial effects of agents blocking TNFα in therapies for HCV. Indeed, this study shows that treatment of NS3/4A-Tg mice with TNFα/D-galN or LPS/D-galN results in increased intrahepatic NFκB activation and enhanced levels of TNFα in both serum and liver. This was paralleled by a reduction in the number of apoptotic cells and a decrease in the amount of cleaved caspase-3. By inhibiting NFκB or by blocking TNFα, we could reverse the protective effects of NS3/4A. Thus, NS3/4A improves hepatocyte survival and liver regeneration by enhancing NFκB activation that causes an increase in hepatoprotective TNFα, which is likely to promote viral infection. Therefore, anti-TNFα most likely exerts its beneficial effects in HCV therapy by preventing hepatocyte regeneration and promoting hepatocyte apoptosis.
Abbreviations
CCL2, chemokine (C-C motif) ligand 2; D-galN, D-galactosamine; ELISA, enzyme-linked immunosorbent assay; HCV, hepatitis C virus; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; NFκB, nuclear factor kappa B; NS, nonstructural; SOC, standard of care; TC-PTP, T cell protein tyrosine phosphatase; Tg, transgenic; TLR, Toll-like receptor; TNFα, tumor necrosis factor α; TRIF, Toll/interleukin-1 receptor domain–containing adaptor inducing IFNβ; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling; WT, wild-type.
Materials and Methods
Mice.
C57BL/6xCBA mice transgenic for full-length HCV genotype 1a NS3/4A were bred and housed at The Karolinska Institute, Division of Comparative Medicine, Clinical Research Center. All animals were analyzed for presence of the genomic NS3/4A transgene as described.11 Wild-type (WT) C57BL/6xCBA mice were purchased from Charles River (Sulzfeld, Germany). All animal experiments followed the guidelines for animal work at The Karolinska Institute and were approved by The Karolinska Institute ethics committee.
Induction of Liver Damage in Mice.
Liver injury was induced by intraperitoneal injection of recombinant murine TNFα (15 ng/g mouse; Roche, Mannheim, Germany) in combination with D-galN (1 mg/g mouse; Sigma, St. Louis, MO) or LPS (5 ng/g mouse; Sigma)/D-galN (1 mg/g mouse). To block the p38MAPK pathway, 25 μg/g mouse SB203580 hydrochloride (Calbiochem, Schwalbach, Germany) in sterile dH2O was given intraorally 30 minutes before TNFα/D-galN application. Bortezomib 1 μg/g mouse (Velcade; Millenium Pharmaceuticals, Cambridge, MA) was injected 1 hour before LPS/D-galN application intravenously, whereas infliximab 30 μg/g mouse (Remicade; Schering-Plough, Stockholm, Sweden) was given intraperitoneally 4 hours after LPS/D-galN treatment. Liver injury was monitored using serum alanine aminotransferase levels or survival in groups consisting of 5 to 10 male mice.
Samples from Murine Livers.
Six- to 12-week-old male mice were treated at different time points and either monitored for survival or bled for serum analysis and sacrificed for organ removal. Serum for cytokine analysis was collected by way of retro-orbital bleeding of isofluorane-anesthetized mice followed by centrifugation of the blood. For organ removal, mice were sacrificed by cervical dislocation and the liver was perfused through the portal vein with cold phosphate-buffered saline containing 1 mM Na3VO4 until the liver turned pale. Whole cell extracts and nuclear extracts were performed as described.6
Histology and Immunohistochemistry.
Liver tissue was incubated in 4% zink paraformaldhehyde solution (HistoLab, Gothenburg, Sweden) overnight. Paraform aldhehyde–fixed liver samples were paraffin-embedded and liver sections of 4 μm thickness were prepared. For analysis, slides were deparaffinated and rehydrated according to standard procedures. Liver sections were stained for immunohistochemistry with cleaved caspase-3 (Cell Signaling Technology, Beverly, MA), F4/80 antigen (AbD Serotec, Oxford, UK), Ki67 (Abcam, Cambridge, UK), NFκB p65 (Thermo Fisher Scientific, Fremont, CA), and TNFα (R&D Systems, Abingdon, UK) antibody as the primary antibody and the respective peroxidase-conjugated IMPRESS immunoglobulin (Vector, Burlingame, CA) as a secondary antibody. A Betazoid-containing substrate solution (Biocare, Concord, CA) was used for detection. Nuclei were stained with Mayer's HTX solution (HistoLab) diluted 1:10 in water. Cellular apoptosis and necrosis were measured using a terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay according to the manufacturer's protocol (ApoTag peroxidase in situ apoptosis detection kit; Chemicon, Billerica, MA). Liver sections were further stained with a standard hematoxylin-eosin staining.
SDS-PAGE and Western Blot Analysis.
For running and blotting of protein gels the XCell SureLock Mini-Cell system and XCell II Blot Module from Invitrogen (Carlsbad, CA) were used and the procedure followed the guidelines stated by the manufacturer. Antibodies against NFκB p65, IκBα, and cleaved caspase-3 (Asp175) were purchased from Cell Signaling Technology, the secondary antibodies from Dako (Glostrup, Denmark).
Enzyme-Linked Immunosorbent Assay.
Mouse monocyte chemoattractant protein 1/CCL2 and mouse TNFα enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioLegend (San Diego, CA). ELISAs were performed according to the manufacturer's instructions.
Statistical Analysis.
The statistical analysis of the number of TUNEL-positive hepatocytes and the number of dividing hepatocyte nuclei in the respective liver sections was performed by way of semiquantitative counting using a light microscope (Zeiss, Jena, Germany) equipped with an ocular grid at a magnification of ×400. Forty high-power fields equal to 10 mm2 for 3 to 4 individual mice per time point and group were evaluated. The mean values for each group/time point were compared by way of Mann-Whitney U test and analysis of variance using InStat 3 software. The statistical analysis of the survival experiments was performed using the Wilcoxon test.
Results
Increased NFκB Activation in NS3/4A-Tg Mice.
We have reported that mice with liver-specific expression of NS3/4A have a reduced sensitivity to liver damage induced by CCl4, LPS/D-galN, and TNFα/D-galN.11 A common characteristic shared by these three liver toxic stimuli is that TNFα is involved in liver injury, suggesting that NS3/4A interferes with one or more steps of TNFα-mediated apoptosis/necrosis. TNFα signaling is characterized by simultaneous activation of both FADD- and caspase-8–dependent proapoptotic pathways and the NFκB pathway, which can inhibit the TNFα-induced cell death process. Thus, we decided to analyze the activation status of NFκB in naïve as well as TNFα/D-galN–treated NS3/4A-Tg mice and the respective non-Tg mice.
The hepatic activation of NFκB is significantly enhanced after injection with TNFα/D-galN in mice with liver-specific expression of NS3/4A (Fig. 1). The TNFα-induced activation of NFκB demonstrated by a time-dependent decrease in the amount of cytoplasmic NFκB paralleled by a corresponding increase in the amount of nuclear NFκB was much more pronounced in NS3/4A-Tg mice compared with non-Tg mice (Fig. 1A). The nuclear translocation of NFκB induced by degradation of the endogenous NFκB inhibitor IκB could already be detected 30 minutes after TNFα/D-galN administration and was still present 240 minutes after the start of the treatment (Fig. 1A and data not shown). A similar NS3/4A-mediated increase in NFκB activation was also evident when NS3/4A-Tg and the corresponding WT mice were treated with LPS/D-galN (Fig. 3C and data not shown).
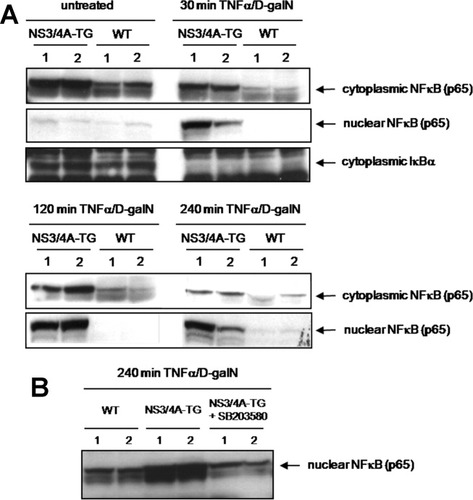
Increased NFκB activation in the livers of NS3/4A-Tg mice after TNFα/D-galN administration. (A) WT mice or NS3/4A-Tg mice were treated for the indicated time points with 15 ng/g mouse TNFα and 1 mg/g mouse D-galN. Cytoplasmic and nuclear cell extracts were prepared from mouse livers and analyzed by way of western blotting. Antibodies specifically raised against the respective proteins were used for detection of NFκB (p65) and IκBα. (B) A group of NS3/4A-Tg mice was pretreated intraorally with 25 μg/g mouse of the p38MAPK inhibitor SB203580 30 minutes prior to TNFα/D-galN administration.
Because we had shown that the NS3/4A-mediated protection toward TNFα-induced liver damage was p38MAPK-dependent, we analyzed the effect of the p38MAPK inhibitor SB203580 on TNFα/D-galN-induced NFκB activation. Interestingly, pretreatment of NS3/4A-Tg mice with SB203580 before injection of TNFα/D-galN resulted in a reduction of nuclear NFκB levels to the levels in WT mice (Fig. 1B), suggesting a role of p38MAPK in the NS3/4A-mediated increase in NFκB activation and implying that these pathways may be connected.
Reduced Apoptosis in NS3/4A-Tg Mice.
TNFα-induced apoptosis is mediated by the induction of caspase cleavage, with caspase-3 as the executioner caspase. Thus, we analyzed the level of cleaved caspase-3 after treatment with TNFα/D-galN or LPS/D-galN in both NS3/4A-Tg and WT mice using western blot and immunohistochemical analysis. The appearance of the bands representing cleaved caspase-3 was prominent in cytoplasmic liver samples 120 minutes after TNFα/D-galN treatment in WT mice but only after 480 minutes in NS3/4A-Tg mice to a much lower degree (Fig. 2A). This could be confirmed in liver sections from LPS/D-galN–treated mice using immunohistochemistry. Whereas a significant staining for cleaved caspase-3 was visible in WT mice 6 hours after application of LPS/D-galN, only a weak staining for cleaved caspase-3 was evident in NS3/4A-Tg mice (Fig. 2B).
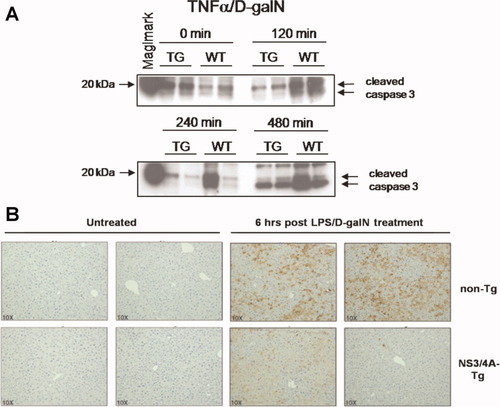
Decreased levels of cleaved caspase-3 in the livers of NS3/4A-Tg mice after application of TNFα/D-galN or LPS/D-galN. (A) WT mice or NS3/4A-Tg mice (TG) were treated for the indicated time points with 15 ng/g mouse TNFα and 1 mg/g mouse D-galN. Cytoplasmic cell extracts were prepared from the mouse livers and analyzed by way of western blotting. An antibody detecting endogenous levels of the large fragment (17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175 was used. (B) WT mice or NS3/4A-Tg mice were left untreated or treated for 6 hours with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Paraffin-embedded liver slices were performed for immunohistochemistry and stained for cleaved caspase-3. Two examples per condition are shown (magnification ×10).
Furthermore, we analyzed the degree of apoptosis and necrosis in murine liver samples taken at different time points after LPS/D-galN administration through TUNEL staining. Again, liver sections from WT mice taken 6 hours after LPS/D-galN injection had a significantly (P < 0,0001) higher number of TUNEL-positive cells per 10 mm2 of liver compared with NS3/4A-Tg mice (Fig. 3A, upper right). Additionally, WT mice showed extensive and severe changes in the liver parenchyma with a high infiltration of inflammatory cells 6 hours after LPS/D-galN application (Fig. 3A, lower right).
Thus, NS3/4A-Tg mice are less sensitive to TNFα-induced apoptosis compared with WT mice. These antiapoptotic effects might be exerted by the NS3/4A-related increase in NFκB activation.
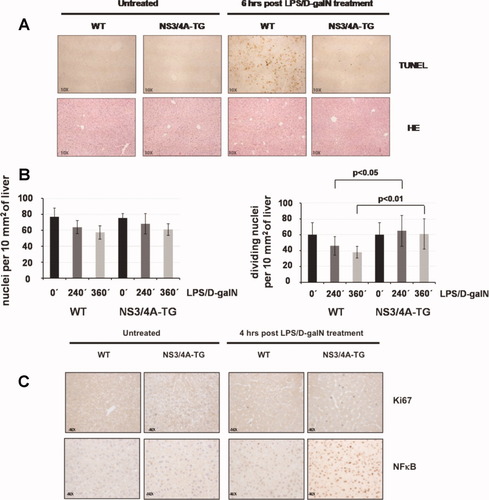
Decreased apoptosis paralleled by increased hepatocyte regeneration in the livers of NS3/4A-Tg mice after treatment with LPS/D-galN. (A) WT mice or NS3/4A-Tg mice were left untreated or treated for 6 hours with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Paraffin-embedded liver slices were prepared for immunohistochemistry and stained for apoptotic cells by way of TUNEL assay (upper part) or with hematoxylin-eosin (lower part) (magnification ×10). (B) Hematoxylin-eosin–stained liver sections from WT or NS3/4A-Tg mice treated for the indicated time points (0, 240, or 360 minutes) with LPS/D-galN [as in (A)] were used to determine the total number of hepatocyte nuclei per 10 mm2 of liver (left) or the number of dividing hepatocyte nuclei per 10 mm2 of liver (right). A Mann-Whitney U test was performed for statistical analysis. (C) WT mice or NS3/4A-Tg mice were left untreated or treated for 4 hours with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Paraffin-embedded sections from the respective livers were stained for Ki67 (upper part) or NFκB (p65) (lower part) (magnification ×40).
Enhanced Hepatocyte Regeneration in NS3/4A-Tg Mice.
Activated NFκB is known both to protect hepatocytes from apoptosis and to promote liver regeneration.13 We therefore determined the total number of hepatocyte nuclei and the number of dividing hepatocyte nuclei per 10 mm2 of liver in hematoxylin-eosin–stained liver sections from WT and NS3/4A-Tg mice left untreated or treated with LPS/D-galN. The total number of hepatocyte nuclei decreased during LPS/D-galN treatment in both WT and NS3/4A-Tg mice, with a more pronounced reduction in WT mice, compared with NS3/4A-Tg mice illustrating LPS/D-galN–mediated liver injury (Fig. 3B, left). In order to investigate liver regeneration, the amount of actively dividing hepatocyte nuclei was determined. Interestingly, this showed that, whereas the number of dividing hepatocyte nuclei decreased in WT mice, NS3/4A-Tg mice revealed an increase in the number of dividing hepatocyte nuclei during LPS/D-galN treatment (Fig. 3B, right). Furthermore, after staining for the proliferation marker Ki67, liver sections from NS3/4A-Tg mice treated with LPS/D-galN had a significantly higher number of Ki67-positive nuclei compared with WT mice treated the same way (19.20 ± 2.49 versus 5.60 ± 1.65 positive nuclei per 10 mm2 of liver; P < 0.0001 [Mann-Whitney]) (Fig. 3C).
Thus, NS3/4A not only exerts antiapoptotic effects but also promotes hepatocyte regeneration, most likely by increasing NFκB activation.
Elevated Serum and Liver TNFα Levels in NS3/4A-Tg Mice.
NFκB regulates the transcription of an exceptionally large number of genes, many of which participate in immune and inflammatory responses, including TNFα, IL-1α, and IL-6.14 Because TNFα is not only an important proinflammatory cytokine but also a key factor in inducing liver regeneration,15 we investigated the levels of TNFα in WT and NS3/4A-Tg mice after administration of LPS/D-galN. Interestingly, we detected higher levels of TNFα in both serum and livers of NS3/4A-Tg mice with a peak at around 60 minutes after LPS/D-galN injection (Fig. 4). Although the differences in serum levels of TNFα were only evident between 30 and 240 minutes after LPS/D-galN administration, the hepatic levels of TNFα in NS3/4A-Tg mice were elevated already before treatment and remained high throughout (Fig. 4). The comparison of liver sections stained with F4/80 antigen and TNFα reveals that the majority of the cells producing TNFα seem to be macrophages (Fig. 4B). Furthermore, the LPS/D-galN–mediated expression of TNFα, which is significantly higher in NS3/4A-Tg mice compared with WT mice (39.60 ± 5.17 versus 18.60 ± 2.76 positive cells per 10 mm2 of liver, P < 0.0001), is NFκB-dependent, because pretreatment with the NFκB inhibitor bortezomib was able to block LPS-induced TNFα expression (decrease from 39.60 ± 5.17 to 19.90 ± 4.53 positive cells per 10 mm2 of liver, P < 0.0001) (Fig. 4B). This suggests that the increased activation of NFκB in NS3/4A-Tg livers results in an increase in intrahepatic TNFα levels produced mainly by macrophages. It is therefore probable that we are observing NFκB- and TNFα-mediated hepatoprotective effects that cause decreased apoptosis and improved liver regeneration, which explains the increased resistance to TNFα/LPS-mediated liver damage in NS3/4A-Tg mice.
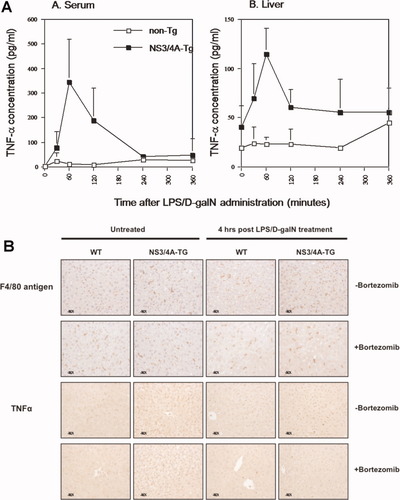
Elevated TNFα levels in both sera and livers of NS3/4A-Tg mice. (A) WT mice (non-Tg) or NS3/4A-Tg mice were treated for 0 to 360 minutes with LPS/D-galN as above. At the indicated time points (six mice per group), the mice were either bled for serum analysis or sacrificed, after which liver perfusion was undertaken to obtain liver samples. The liver samples were used for the preparation of whole cell extracts in which the concentration of murine TNFα was determined by ELISA. (B) WT mice or NS3/4A-Tg mice were intravenously injected with NaCl (control) or 1 μg/g mouse bortezomib for 1 hour and then sacrificed or treated for 4 hours with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Paraffin-embedded sections from the respective livers were stained for F4/80 antigen (upper part) or TNFα (lower part) (magnification ×40).
Blocking NFκB Activation Reverses NS3/4A-Mediated Resistance Toward TNFα-Induced Liver Damage.
To confirm the hepatoprotective role of NFκB in the resistance of NS3/4A-Tg mice toward LPS/D-galN–induced liver damage, we pretreated NS3/4A-Tg and WT mice with the proteasome inhibitor bortezomib, which blocks NFκB activation by inhibiting the degradation of the natural NFκB inhibitor IκB. The efficient blockade of NFκB nuclear translocation in response to LPS/D-galN in bortezomib-pretreated NS3/4A-Tg mice is shown in Fig. 5A. If the elevated activation of NFκB seen in the NS3/4A Tg mice is a key to the observed liver protective effects, then blocking of NFκB activation should reverse them. Indeed, bortezomib pretreatment resulted in an almost complete block of NS3/4A-mediated resistance whereby the NS3/4A-Tg mice became as sensitive to LPS/D-galN as WT mice (Fig. 5B). Thus, the increase in NFκB activation seems to play a key role in the resistance toward TNFα-induced liver damage of NS3/4A-Tg mice.
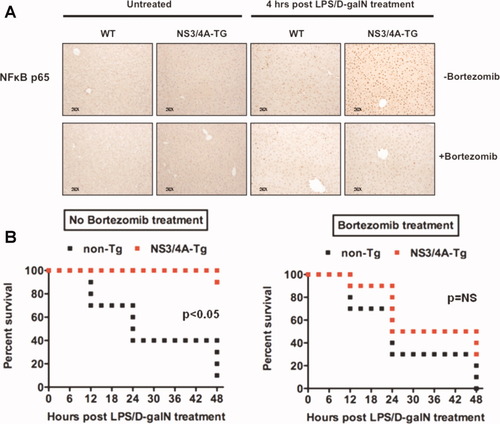
Inhibition of NFκB activation impedes resistance to LPS/D-galN in NS3/4A-Tg mice. (A) WT mice or NS3/4A-Tg mice were pretreated with NaCl as a control or 1 μg/g mouse bortezomib for 1 hour and then sacrificed or treated for 4 hours with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Paraffin-embedded sections from the respective livers were stained for NFκB (p65) (magnification ×20). (B) WT (non-Tg) or NS3/4A-Tg mice (10 mice per treatment group) were intravenously injected with NaCl (left) or 1 μg/g mouse bortezomib (right) 1 hour before application of 5 ng/g mouse LPS and 1 mg/g mouse D-galN. The survival of mice was followed over a period of 48 hours. A Wilcoxon test was used for statistical analysis. NS, not significant.
Blocking TNFα in the Regeneration Phase Reverses NS3/4A-Mediated Resistance Toward TNFα-Induced Liver Damage.
After having shown that inhibition of NFκB activation is able to reverse the NS3/4A-mediated resistance toward TNFα-induced liver damage, we decided to study the role of TNFα in survival after LPS/D-galN treatment by blocking the action of TNFα with anti-TNFα antibodies (infliximab). To test the potency of infliximab in our mouse model, we pretreated NS3/4A-Tg and WT mice with infliximab before TNFα/D-galN treatment. As expected, all mice survived because the induction of TNFα-induced liver damage was blocked (data not shown).
We wanted to test our hypothesis that TNFα plays a dual role in LPS/D-galN-induced liver injury, first acting as a proapoptotic mediator of liver damage, and later as an important hepatoprotective factor. We therefore injected infliximab 4 hours after LPS/D-galN injection. Interestingly, a late administration of anti-TNFα indeed resulted in a loss of NS3/4A-mediated resistance (Fig. 6). This indicates that the sustained increase in intrahepatic TNFα levels seen in NS3/4A-Tg mice mediates the hepatoprotective effects of TNFα (Fig. 4).
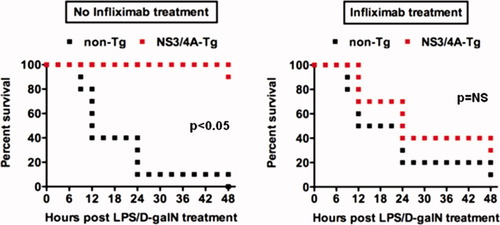
Inhibition of TNFα signaling in the regeneration phase breaks tolerance toward LPS/D-galN in NS3/4A-Tg mice. WT (non-Tg) or NS3/4A-Tg mice (10 mice per treatment group) were first treated with 5 ng/g mouse LPS and 1 mg/g mouse D-galN and after 4 hours intraperitoneally injected with NaCl (left) or 30 μg/g mouse infliximab (right). The survival of mice was followed over a period of 48 hours. A Wilcoxon test was used for statistical analysis. NS, not significant.
Enhanced CCL2 Levels in Livers of NS3/4A-Tg Mice.
LPS is sensed in the liver mainly by TLR4 expressed on the surface of Kupffer cells, which are the liver-specific macrophages, and liver sinusoidal endothelial cells. In response to TLR4, these cells release proinflammatory cytokines such as TNFα. In human hepatitis, intrahepatic macrophages are the main producers of TNFα. We therefore investigated the expression levels of CCL2 (monocyte chemoattractant protein 1), which represents the main chemokine involved in intrahepatic activation and recruitment of monocytes/macrophages. We found that CCL2 protein levels were enhanced both in untreated and LPS/D-galN-treated livers of NS3/4A-Tg mice as compared to the corresponding WT mice (Fig. 7). This was paralleled by a higher number of F4/80 antigen-positive cells in LPS/D-galN-treated livers of NS3/4A-Tg mice as compared to WT mice (43.70 ± 5.83 versus 28.50 ± 3.37 positive cells per 10 mm2 of liver, P < 0,0001, Mann-Whitney; Fig. 4B) which may be due to increased CCL2-mediated recruitment of macrophages to the liver.
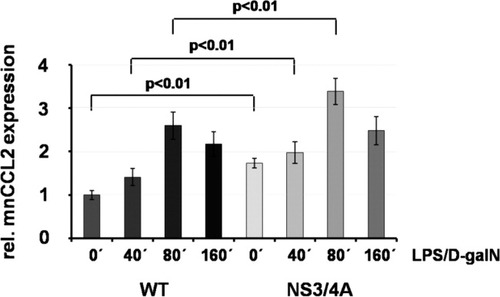
Enhanced basal and LPS/D-galN–induced CCL2 expression in NS3/4A-Tg mice. WT mice or NS3/4A-Tg mice (NS3/4A) (four mice per time point, respectively) were treated for the indicated time points (0 to 160 minutes) with 5 ng/g mouse LPS and 1 mg/g mouse D-galN. Livers were perfused and used for the preparation of whole cell extracts in which the concentration of murine CCL2 was determined by way of ELISA. The value representing the untreated control was set to 1, and the data are presented in relation to the control value. A Mann-Whitney U test was used for statistical analysis.
Thus, the NS3/4A-mediated resistance to LPS/D-galN and TNFα/D-galN in our NS3/4A-Tg mice may be caused by increased CCL2 expression resulting in induction of TNFα production and subsequent NFκB activation, which provokes a paracrine loop with further release of TNFα and activation of NFκB.
Discussion
It is well known that patients with chronic HCV infection have increased serum levels of TNFα, a potent proinflammatory factor with a broad spectrum of effects. A major concern in patients with chronic HCV and rheumatoid arthritis has been that the effective block of TNFα conferred by the new class of anti-TNFα agents should have deleterious effects on the HCV infection; however, this has not been the case. On the contrary, when anti-TNFα compounds are added to SOC therapy in patients with chronic HCV, treatment results improve.9 This highly unexpected finding suggests that TNFα has effects that actually promote the viral infection. We therefore used our NS3/4A-Tg mouse model, which we have shown has a reduced sensitivity to TNFα, to elucidate these issues further.
We have shown that the NS3/4A complex exerts protective effects toward hepatotoxic stimuli such as LPS/D-galN, TNFα/D-galN, and CCl4 in NS3/4A-Tg mice. All of these compounds induce liver injury, at least in part, through TNFα. Interestingly, pretreatment with the p38MAPK inhibitor SB203580 restored the sensitivity toward TNFα/D-galN.11 We then demonstrated that the TNFα/D-galN- or LPS/D-galN–mediated activation of the transcription factor NFκB is much stronger in NS3/4A-Tg mice than in WT mice. This enhanced NFκB activation could be blocked by pretreatment with the p38MAPK inhibitor SB203580, corroborating the important role of p38MAPK for the NS3/4A-mediated resistance toward TNFα-induced liver damage.
The NFκB polypeptide is a dimer with p50:p65 as its most common form and has diverse functions in regulation of cell survival, activation of innate and adaptive immune responses, and maintenance of liver homeostasis.16 The relevance of NFκB in liver physiology is supported by the finding that mice with a p65 deletion die mid-embryonically because of extensive liver apoptosis.17 NFκB is rapidly activated by exposure to proinflammatory stimuli such as TNFα, LPS, or IL-1β and targets for antiapoptotic genes such as A1/Bfl-1, A-20, Bcl-XL, c-IAP, FHC, c-Flip, Gadd45β, SOD2, and XIAP. Moreover, liver regeneration after partial hepatectomy is characterized by rapid NFκB activation, followed by an NFκB-dependent promotion of hepatocyte proliferation and protection of hepatocytes from apoptosis.13 Thus, the potent NFκB activation after LPS/D-galN or TNFα/D-galN treatment in NS3/4A-Tg mice may contribute to both the observed decrease in cleaved caspase-3 and apoptosis, and the observed increase in hepatocyte regeneration. The relevance of the increased NFκB activation for the resistance of NS3/4A-Tg mice toward TNFα-induced liver damage was confirmed by pretreating mice with the NFκB inhibitor bortezomib, which resulted in an almost complete loss of NS3/4A-mediated protection.
Activation of NFκB is also increased in the livers of chronically HCV-infected patients compared with controls.18 Furthermore, hepatic messenger RNA levels of the NFκB component p65 were inversely correlated with apoptosis,18 which is in line with our observation that the increase in NFκB activation seen in NS3/4A-Tg mice is associated with a lower number of apoptotic cells. This should not be surprising, because the HCV-infected liver is generally characterized by an increase in inflammation and immune cells that produce NFκB.
We further demonstrated that TNFα levels in the serum of NS3/4A-Tg mice are increased after LPS/D-galN treatment and that the intrahepatic levels of TNFα were elevated both basally and after LPS/D-galN treatment. Although NFκB activation is induced by TNFα binding to TNF receptor 1/2, NFκB is able to promote the expression of TNFα thus supporting a positive feedback loop. Using TNF receptor 1 knockout mice, it has been shown that TNFα plays a dominant role in promoting liver regeneration.15 TNFα regulates the proliferative response after liver injury by inducing the secretion of IL-6 and transforming growth factor α and sensitizing hepatocytes for signaling mediated by hepatocyte growth factor and epidermal growth factor receptor ligands.19 Interestingly, NS3/4A has been shown to mediate immunomodulatory effects by triggering NFκB activation and TNFα production in monocytes in the absence of the intact virus.20
The importance of elevated TNFα levels for the resistance of NS3/4A-Tg mice toward TNFα-induced liver damage was confirmed by treating mice with the TNFα inhibitor infliximab. Blocking the effects of TNFα during the regeneration phase by injecting NS3/4A-Tg mice with infliximab 4 hours after LPS/D-galN treatment resulted in the abolishment of NS3/4A-mediated protective effects, whereby the NS3/4A-Tg mice displayed the same susceptibility toward LPS/D-galN as WT mice.
Elevated TNFα levels have been well documented in the sera and livers of patients with chronic hepatitis C.7, 21 Interestingly, TNFα levels return to normal when patients are successfully treated.7 Our data suggest that NS3/4A may also play a role in the elevated levels of TNFα in humans. We have shown recently that the phosphatase TC-PTP is a substrate of the NS3/4A protease, resulting in down-regulation of TC-PTP protein expression in both NS3/4A-Tg mice and patients infected with HCV.6 Because TC-PTP knockout mice are characterized by a dramatic increase of TNFα levels in the liver,22 the NS3/4A-mediated cleavage of TC-PTP may help to explain the molecular basis for the elevated TNFα levels in both humans and Tg mice. Furthermore, TC-PTP was shown to suppress epidermal growth factor receptor signaling and TNFα-mediated IL-6 production, thus playing an important role in liver regeneration.23, 24 It should be further noted that TNFα is able to switch from inflammatory to anti-inflammatory functions depending on the time and context.25
Because intrahepatic macrophages are the main producers of TNFα in the liver, we investigated the intrahepatic level of relevant chemokines and found that CCL2 protein levels are enhanced both in untreated and LPS/D-galN–treated livers of NS3/4A-Tg mice. CCL2 triggers chemotaxis and transendothelial migration of monocytes/macrophages and is involved in the activation of macrophages by interacting with the membrane CC chemokine receptor 2 (CCR2).12 Thus, the enhanced intrahepatic levels of CCL2 may contribute to the elevated levels of TNFα present in the livers of NS3/4A-Tg mice by recruiting TNFα-producing macrophages to the liver. The observation that blocking of p38MAPK activity was able to restore the sensitivity toward TNFα/D-galN may possibly be explained at least in part by reports showing that treatment with p38MAPK inhibitors, such as SB203580, resulted in a significant reduction of CCL2 expression.26, 27 This should be studied further.
Overall, the data from the current study clarify two previous observations. First, the NS3/4A-mediated resistance to LPS/D-galN and TNFα/D-galN in our NS3/4A-Tg mice can now be explained by an increase in CCL2 expression inducing TNFα production and NFκB activation, thus resulting in a paracrine loop with further release of hepatoprotective TNFα and activation of NFκB. More importantly, we can now propose an explanation for the beneficial role of anti-TNFα therapy as an add-on to SOC therapy in chronic HCV.9 By blocking TNFα, the hepatoprotective effects are lost and the hepatocytes are rendered more sensitive to apoptosis; at the same time, regeneration is prevented. During HCV infection, a resistance to apoptosis and promotion of regeneration is most likely important for maintaining the balance of the chronic infection. Although IFNα in combination with ribavirin blocks HCV replication and induces apoptosis, the virus can still replicate slowly during the second phase of viremia decline. This second phase has been proposed to reflect the elimination of infected cells. If anti-TNFα blocks the antiapoptotic and regenerative effects induced by TNFα, the clearance in the second phase should be improved. This fits very well with the observed beneficial effect seen when anti-TNFα is added to SOC therapy.
In conclusion, our data suggest that HCV benefits from increased levels of TNFα in vivo. NS3/4A somehow promotes NFκB activation and TNFα production, possibly through the cleavage of TC-PTP. This in turn prevents apoptosis and supports regeneration. The HCV-mediated induction of this signaling loop makes infected cells survive longer and provides new uninfected cells when the infected ones eventually die. Subsequently, the beneficial effects of blocking TNFα in the therapy of chronic hepatitis C might be explained by promoting hepatocyte apoptosis and by preventing hepatocyte regeneration, which blunts the infection.
Acknowledgements
We thank M. Bjon-Holm for excellent technical assistance and Millenium Pharmaceuticals for kindly providing us with bortezomib (Velcade).




