Treatment response in patients with autoimmune hepatitis†
Potential conflict of interest: Nothing to report
We congratulate Manns and colleagues1. on their comprehensive review of and guidelines for the treatment of autoimmune hepatitis (AIH). The importance of complete biochemical remission, which is defined as normalization of aminotransferases and immunoglobulin G (IgG)/gamma-globulins, is underlined as the ideal treatment endpoint and as the goal of initial therapy. Notably, normalization of only aminotransferases is still being used as a definition of biochemical remission.2. We and others have previously shown that elevated levels of aminotransferases, IgG/gamma-globulins, or both may indicate histological activity, and this in turn indicates an increased risk of disease relapse and progression.3.,4. Therefore, complete biochemical remission as a surrogate parameter for histological remission should be achieved with as few side effects as possible. In addition, recent studies have suggested that a fast response to treatment may be associated with a better outcome.5.,6.
With the two treatment algorithms proposed in the guidelines, adults rarely achieve resolution of their laboratory and liver tissue abnormalities in less than 12 months, and a complete response rate of only 11% has been reported with 6 months of treatment.6. This is supported by the recent and so far largest controlled treatment trial for AIH, which compared prednisone (40 mg daily) as the initial therapy to budesonide, each in combination with azathioprine.2. Here, prednisone was able to induce biochemical remission (defined as normalization of aminotransferases) with 6 months of treatment in only 39% of patients. Patients with cirrhosis were excluded from this trial. We are concerned by this rather low biochemical response rate, which may be associated with a poorer outcome,5.,6. and we are also worried that a prednisone maintenance dose of 20 mg or less until remission, as stated in the guidelines, is associated with considerable long-term steroid side effects.
We therefore suggest a more individualized treatment regimen that has been reported to result in excellent long-term prognosis.7. This approach includes an initial dose of prednisolone of 1 mg/kg of body weight, which is rapidly tapered within the next 3 months to a maintenance dose of 5 to 10 mg/day. This treatment is combined from the beginning with azathioprine at a dose of 1 to 1.5 mg/kg of body weight, unless severe hyperbilirubinemia is present. We have reviewed our current experience and report data from 92 patients with AIH for whom complete laboratory follow-up data at months 0, 1, 3, and 6 are available (Fig. 1). Patients with cirrhosis were excluded from this analysis in order to allow a better comparison with the recently published treatment trial.2. With this approach, a reduction of both aminotransferases and IgG/gamma-globulins to less than 2 times the upper limit of normal within 6 months of treatment was not reached by only 10% of the patients. Normalization of aminotransferases was achieved in 77%, and normalization of both aminotransferases and IgG/gamma-globulins was achieved in 64% at 6 months. At our center, patients with a complete biochemical response are taken off prednisolone after 1 year of treatment, and they are maintained on azathioprine until histological remission occurs.
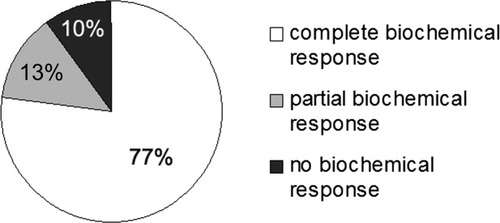
Biochemical response after 6 months of treatment in 92 patients with noncirrhotic AIH. Complete response is defined as normalization of aminotransferases and IgG/gamma-globulins, partial response is defined as a reduction of aminotransferases and IgG/gamma-globulins to less than 2 times the upper limit of normal, and no response is defined as none of the above.
Because of the high and fast response rates, we strongly believe that this regimen spares patients long-term steroid exposure, reduces long-term side effects, and helps to achieve histological remission and therefore should improve the prognosis of our patients. The liver transplantation–free survival rate in this cohort of patients was 100% after a mean observation time of 60 months.
References
Christoph Schramm M.D.*, Christina Weiler-Normann M.D.*, Christiane Wiegard M.D.*, Stefanie Hellweg*, Susanne Müller*, Ansgar W. Lohse M.D.*, * Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.




