Vitamin E for nonalcoholic steatohepatitis: Ready for prime time?†
Potential conflict of interest: Nothing to report.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-1685. (Reprinted with permission.)
Abstract
BACKGROUND: Nonalcoholic steatohepatitis is a common liver disease that can progress to cirrhosis. Currently, there is no established treatment for this disease. METHODS: We randomly assigned 247 adults with nonalcoholic steatohepatitis and without diabetes to receive pioglitazone at a dose of 30 mg daily (80 subjects), vitamin E at a dose of 800 IU daily (84 subjects), or placebo (83 subjects), for 96 weeks. The primary outcome was an improvement in histologic features of nonalcoholic steatohepatitis, as assessed with the use of a composite of standardized scores for steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis. Given the two planned primary comparisons, P values of less than 0.025 were considered to indicate statistical significance. RESULTS: Vitamin E therapy, as compared with placebo, was associated with a significantly higher rate of improvement in nonalcoholic steatohepatitis (43% vs. 19%, P=0.001), but the difference in the rate of improvement with pioglitazone as compared with placebo was not significant (34% and 19%, respectively; P=0.04). Serum alanine and aspartate aminotransferase levels were reduced with vitamin E and with pioglitazone, as compared with placebo (P<0.001 for both comparisons), and both agents were associated with reductions in hepatic steatosis (P=0.005 for vitamin E and P<0.001 for pioglitazone) and lobular inflammation (P=0.02 for vitamin E and P=0.004 for pioglitazone) but not with improvement in fibrosis scores (P=0.24 for vitamin E and P=0.12 for pioglitazone). Subjects who received pioglitazone gained more weight than did those who received vitamin E or placebo; the rates of other side effects were similar among the three groups. CONCLUSIONS: Vitamin E was superior to placebo for the treatment of nonalcoholic steatohepatitis in adults without diabetes. There was no benefit of pioglitazone over placebo for the primary outcome; however, significant benefits of pioglitazone were observed for some of the secondary outcomes. (ClinicalTrials.gov number, NCT00063622.) 2010 Massachusetts Medical Society
Comment
Nonalcoholic steatohepatitis (NASH) is a frequent condition and often progresses to cirrhosis and further complications. These facts are unchallenged, but a pressing issue remains: identification of an effective therapy. Patients are poorly served when, after liver biopsy and the histological confirmation of a NASH diagnosis, they learn that no recognized treatment will follow. Notwithstanding the essential, therapeutic measures of weight loss and increased exercise, it is still unknown to what extent a given patient will respond to lifestyle changes and whether certain lifestyles are even amenable to change.
In 2006, Belfort et al.1 published a controlled study that enrolled 55 NASH patients with impaired glucose tolerance or type 2 diabetes. Patients received either pioglitazone (45 mg daily) or placebo for 6 months. Pioglitazone lowered the serum aminotransferase concentrations, improved glycemic control and hepatic insulin sensitivity, and decreased the fat content in the liver. Since then, several randomized controlled trials have confirmed that glitazones improve relevant endpoints in NASH.2-4 However, the future of glitazones in the treatment of NASH is questionable because glitazones have been associated with cardiovascular events and cause weight gain.5 Cardiovascular events are leading causes of death among NASH patients, who inevitably struggle to lose weight.
Because glitazones, which improve the metabolic aspects of NASH, do not provide optimal treatment, alternative drugs that affect other pathophysiological aspects of the disease have been investigated (Fig. 1). Ursodeoxycholic acid (UDCA) was an obvious candidate because of its safety and well-documented cytoprotective properties. However, in two randomized controlled trials, NASH patients who were treated with UDCA monotherapy did not fare better than those in a placebo group.6, 7
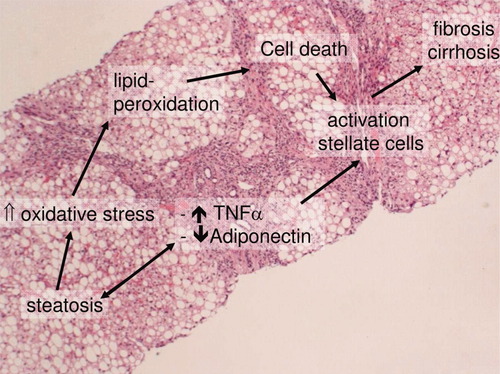
Key pathophysiological events in the progression of NASH toward cirrhosis. Abbreviation: TNF-α, tumor necrosis factor α.
Vitamin E is a lipophilic antioxidant that blocks the propagation of lipid peroxidation. In two randomized controlled trials, a beneficial effect of vitamin E in NASH patients was found, but in the trials, vitamin E was administered in combination with pioglitazone3 or UDCA.7 These positive results justified the testing of vitamin E as monotherapy and were the basis for the Pioglitazone or Vitamin E for NASH Study (PIVENS). This NASH Clinical Research Network study compared a placebo, pioglitazone, and vitamin E.8 The inclusion criteria required steatohepatitis with a nonalcoholic fatty liver disease (NAFLD) activity score of 5 or higher or a score of 4 plus the diagnostic consensus of two pathologists. Moreover, a score of at least 1 for hepatocellular ballooning was required. The primary outcome was defined as an improvement in the histological findings: (1) an improvement of 1 or more points in the hepatocellular ballooning score, (2) no increase in the fibrosis score, and (3) a decrease in the activity score to less than 3 or a decrease of 2 points with at least a 1-point decrease in either the lobular inflammation or steatosis score. The trial was designed to attain a power of 90% to detect an absolute difference in the rate of improvement in NASH of 26 percentage points. The study was not designed to compare vitamin E and pioglitazone. The primary outcome was reached in 19% of the patients assigned to the placebo, in 43% of the patients assigned to vitamin E (P = 0.001), and in 34% of the patients assigned to pioglitazone (P = 0.04). The mean change in the histological NAFLD activity score was −0.5 in the placebo group, −1.9 in the vitamin E group (P < 0.001), and −1.9 in the pioglitazone group (P < 0.001). The two treatments had similar effects on aspartate aminotransferase and alanine aminotransferase. Pioglitazone improved insulin resistance and consequently stimulated weight gain. Neither drug affected the quality of life. This trial shows clearly that vitamin E is just as potent as pioglitazone in the improvement of histological lesions and aminotransferases levels. Pioglitazone is superior to vitamin E in the improvement of insulin sensitivity, but its drawback is weight gain.
What is the mechanism of action of vitamin E? The prevailing view is that it acts as a fat-soluble antioxidant that stops the propagation of lipid oxidation and the production of reactive oxygen species, actions that depend on continuous recycling by L-ascorbic acid. Yet vitamin E has also been shown to modulate the activity of signaling enzymes, and there really is no confirmation that effects in vivo are attributable to its properties as an antioxidant.9, 10 The eight natural analogues of vitamin E (α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol, α-tocotrienol, β-tocotrienol, γ-tocotrienol, and δ-tocotrienol) have equal antioxidant potency and yet individually lead to type-specific cellular outcomes. Several signaling enzymes are reportedly affected by vitamin E, although experimentation with different cell types has produced conflicting results (Table 1). Liver cells have not yet been tested. Vitamin E's role as an essential cofactor may require its phosphorylation to α-tocopherol phosphate or its oxidation to α-tocopherol quinine, which is the cofactor bound to the mitochondrial long chain fatty acid desaturase.11
| Inhibition | Reference | Activation | Reference |
|---|---|---|---|
| Protein kinase C α | 17 | Protein phosphatase 2A | 18 |
| Protein kinase C δ | 19 | Diacylglycerol kinase | 20 |
| Phospholipase A2 | 21 | Phospholipase A2 | 22 |
| Protein kinase B | 23 | ||
| Tyrosine kinase 2 | 24 | ||
| Cyclooxygenase 2 | 25 | ||
| Lipoxygenase | 26 |
Despite its sound design, the PIVENS trial is not without weaknesses. First, the Belfort trial used a dose of 45 mg, whereas the PIVENS trial used a dose of 30 mg, a choice that was not justified. The Belfort trial enrolled patients with impaired glucose tolerance or type 2 diabetes, whereas the PIVENS trial excluded those patients. It is therefore unknown whether vitamin E is beneficial in patients with NASH and type 2 diabetes, and it is unknown whether pioglitazone (45 mg daily) would have been more appropriate. Second, the histological diagnosis of NASH relied on the NAFLD activity score, although this score was originally proposed only for grading and not for diagnostic purposes.12 Third, the primary outcome was a histological improvement; although this is appropriate, it remains subject to random sampling, and its translation into a clinical benefit is not confirmed. Fourth, the authors avoided a comparison of the two treatments, and the question arises why there was a significant difference in the proportion of patients who stopped treatment before 96 weeks [7% (6/84) in the vitamin E group versus 18% (14/80) in the pioglitazone group, P < 0.034]. Fifth, it is questionable whether the vitamin E dose of 800 IU/day is the most effective. A meta-analysis found that the intake of high-dosage vitamin E supplements (≥400 IU/day) may increase mortality.13 Much higher doses have been used in trials testing neuroprotective effects (up to 5000 IU/day) without evidence of deleterious effects.14 It has been estimated that 11% of US adults consume 400 IU or more of vitamin E daily from supplements.15 Finally, an assessment of important biomarkers such as serum adiponectin levels and circulating levels of cytokeratin 18 fragments is missing.
This trial provides food for thought. Will all patients with NASH or only a fraction respond? How can we identify eventual nonresponders? How important is measuring vitamin E concentrations in NASH patients? Is vitamin E monotherapy effective, or is a combination better? We tested UDCA in combination with vitamin E and found that the combination improved histological parameters7 in addition to circulating levels of adiponectin.16 Moreover, the combination reduced the levels of apoptotic markers.16 It would be interesting to learn whether the patients in the vitamin E group of the PIVENS trial experienced similar changes.




