Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C†
Potential conflict of interest: Dr. Färkkilä is on the speakers' bureau of Roche. Dr. Hjerrild received grants from Roche.
Abstract
The Major Depression Inventory (MDI) was used to estimate the value of routine medical interviews in diagnosing major depression among patients receiving peginterferon alfa-2a and ribavirin therapy for chronic hepatitis C virus (HCV) infection (n = 325). According to criteria from the MDI and Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 19 patients (6%) had major depression at baseline. An additional 114 (37%) developed depression while on HCV combination therapy, with baseline MDI score and female sex independently predicting the emergence of major depression during treatment in a multivariate analysis. Only 36 (32%) of the 114 patients developing major depression according to MDI/DSM-IV criteria were correctly diagnosed during routine medical interviews. The emergence of major depression frequently led to premature discontinuation of peginterferon/ribavirin therapy, and an on-treatment MDI score increment exceeding 30 points (i.e., a validated marker of idiopathic DSM-IV major depression) was correlated with impaired outcome of HCV therapy (P = 0.02). This difference was even more pronounced among patients with an on-treatment increase in MDI score greater than 35 points (P = 0.003). Conclusion: We conclude that (1) depressive symptoms among patients undergoing HCV therapy are commonly overlooked by routine clinical interviews, (2) the emergence of depression compromises the outcome of HCV therapy, and (3) the MDI scale may be useful in identifying patients at risk for treatment-induced depression. (HEPATOLOGY 2010;)
The current standard-of-care for patients with chronic hepatitis C virus (HCV) infection is weekly injections of pegylated interferon alpha (peginterferon) in combination with daily oral ribavirin for 24-48 weeks.1-3 This therapy leads to major depression and other psychiatric complications in a sizable fraction of patients,4-6 which may compromise treatment compliance and frequently leads to premature discontinuation of antiviral therapy.7, 8
The psychiatric evaluation of patients with HCV is commonly performed by clinicians with limited psychiatric training,9 and thus to facilitate the assessment of depressive symptoms, several self-report instruments are available.10-12 The Major Depression Inventory (MDI), which quantifies depressive symptoms of depression using a self-reported scale,13 was recently demonstrated to correctly diagnose depression with a total score of 20-26 points indicating mild depression, 27-35 points indicating moderate depression, and >35 points indicating major or severe DSM-IV depression.14 For the present study, we compared self-reported MDI scores with psychiatric symptoms recorded by the clinician during repeated medical interviews. Our results emphasize that the current practice of interviews severely underestimates the frequency of major depression, and that the emergence of depressive symptoms, as evaluated by the MDI score, is a significant risk factor for poor outcome of HCV therapy.
Abbreviations:
CRF, clinical report form; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; HCV, hepatitis C virus; MDI, Major Depression Inventory; SVR, sustained viral response.
Patients and Methods
Patient Characteristics and Study Design.
A randomized open-label study (the NORDynamIC trial) was carried out in 31 medical centers in Denmark, Finland, Norway, and Sweden from February 2004 to November 2005. The primary trial endpoint was the sustained viral response (SVR), defined as absence of HCV RNA in plasma at 24 weeks after the completion of therapy as measured by Cobas AmpliPrep/COBAS TaqMan HCV Test (Roche Diagnostics, Branchburg, NJ) in patients infected with HCV genotype 2 or 3 who received 12 weeks versus 24 weeks peginterferon alfa-2a at 180 μg/week plus ribavirin 800 mg/day, as previously reported.15 Patients with clinically significant psychiatric illness that limited the use of peginterferon and ribavirin therapy, as judged by the treating physician, were excluded from the study.
Assessment of Depressive Symptoms.
The MDI was used to diagnose major depression according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV),16 as well as to measure the severity of depression by the total score (range: 0 [no depression] to 50 points [extreme depression]).14 The MDI questionnaire was completed by the patients at baseline before the initiation of HCV therapy and every second week while on treatment and continuing until the 8th week after the completion of peginterferon/ribavirin therapy. Throughout the study, health personnel were blinded with regards to the results of the self-rating scales. Management of neuropsychiatric complaints, including depressive symptoms, was based on clinical judgment after a routine medical interview lasting approximately 20-30 minutes as part of the biweekly outpatient consultation. After the completion of the trial, the results of the MDI questionnaires were disclosed. Clinical Report Forms (CRFs), including information regarding results of routine medical interview and the prescription of psychotropic medications, were then reviewed.
Statistics.
The chi-squared test and the Fisher's exact test were used for comparison of relative frequencies. For analysis of paired data, the Wilcoxon signed test was used, and differences between groups of unpaired observations were analyzed by the Mann-Whitney U test. Categorical data modeling (CATMOD) in the SAS data handling system was used for multivariate analysis.
Ethical Considerations.
Written, informed consent was obtained from each participating patient. Ethics committees in each participating country approved the study.
Clinical Trial Registration.
This trial has been registered at the National Institutes of Health trial registry (ClinicalTrials.gov identifier: NCT00143000).
Results
Of the 382 patients enrolled in the NORDynamIC trial, 325 (85%) completed the MDI questionnaires (Table 1). The therapeutic outcome in terms of SVR was similar for those who completed the MDI questionnaires and those who did not (data not shown). Thirty-two patients (10%) reported a past episode of depression, and antidepressants were prescribed to 25 patients (8%) prior to initiation of HCV therapy (20 patients received long-term therapy and 5 patients received antidepressants resulting from pre-emptive treatment).
| Characteristic | Value |
|---|---|
| Mean SD age (years) | 42 ± 11 |
| Sex (M/F) | 186/139 |
| HCV genotype (2/3) | 93/232 |
| Cirrhosis (Ishak score ≥5), n (%)* | 42 (13) |
| History of intravenous drug use, n (%) | 234 (72) |
| Increased alcohol consumption (>10 drinks weekly), n (%) | 20 (7) |
| Substance dependence treatment, n (%) | 18 (6) |
| Past episode of depression, n (%) | 32 (10) |
| Antidepressants, n (%) | 25 (8) |
- * Liver biopsy performed in 296 (91%) patients.
Prevalence of Major Depression at Onset and During Therapy.
Peginterferon and ribavirin combination therapy was given to 168 (52%) patients for 12 weeks and to 157 (48%) patients for 24 weeks. Nineteen of the 325 (6%) patients had major depression at baseline as evaluated by the MDI, and an additional 114 (35%) patients developed major depression during HCV therapy. A total of 130 (40%) patients had a MDI score below 20 points throughout therapy, which suggested lack of depression. During the first 12 weeks of HCV therapy, 100 (33%) patients—50 in each treatment arm—developed major depression (Fig. 1). In the 24-week arm, an additional 14 (9%) patients developed major depression during the following 12 weeks of therapy. The mean end-of-therapy MDI scores were 18 points in the 12-week arm and 16 points in the 24-week arm. The mean MDI score and the major depression prevalence declined toward baseline levels over the 8 weeks following cessation of therapy.
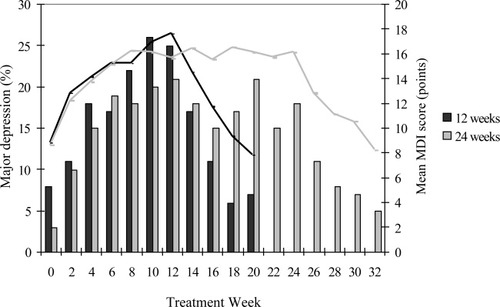
Biweekly major depression prevalence (bars) and mean MDI scores (lines) in the 12 weeks (black) and 24 weeks (gray) peginterferon alfa-2a plus ribavirin treatment arms.
Risk Factors.
Among the 306 patients (174 men and 132 women) without major depression at baseline, a significantly higher proportion of women (46%) developed major depression (31%) (P < 0.01; Table 2). MDI scores at baseline were significantly higher in the 114 patients who developed major depression than in the 192 patients who did not (9.6 [95% confidence interval {CI}, 2-23] points versus 6.1 [95% CI, 0-17] points, respectively; P < 0.001). Multivariate regression analysis identified MDI score at baseline and female sex as significant independent predictors of subsequent development of major depression (P < 0.01; odds ratio for MDI score 2.39 [CI, 1.41-4.05] and 1.96 for female sex [CI, 1.19-3.20]), whereas no significant association was observed regarding age, country of origin, history of depression, previous drug abuse, current substance dependence treatment, or liver cirrhosis.
| Characteristic | Patients With Major Depression n = 114 | Patients Without Major Depression n = 192 | P Value |
|---|---|---|---|
| Age (years), mean ± standard deviation | 42 ±10 | 42 ± 10 | NS |
| Sex (M/F) | 53/61 | 121/71 | <0.01 |
| MDI score at baseline (point) | 9.6 | 6.1 | <0.001 |
- MDI, Major Depression Inventory; NS, not significant.
Reported Symptoms.
Of the 114 patients developing major depression according to the MDI scale, depression was diagnosed by means of routine medical interview in 36 (32%) patients and in 21 (11%) of the 211 patients without major depression according to MDI. Among the remaining 78 (68%) patients with major depression identified by the MDI but not reported in the CRF, 63 (81%) patients had documented symptoms consistent with depression such as sadness, irritability, anger, anxiety, emotional instability, fatigue, and changes in sleep and appetite. The medical interviews recorded fatigue and sleep disturbance in approximately one-third of patients, regardless of whether they were depressed or not (Fig. 2). In contrast, the MDI scoring demonstrated large differences in fatigue and sleep disturbance between depressed (95% and 78%, respectively) and nondepressed patients (19% and 5%). The presence of fatigue or sleep disturbance as evaluated by the MDI score had positive predictive values for the development of major depression during HCV therapy of 73% and 93%, respectively.
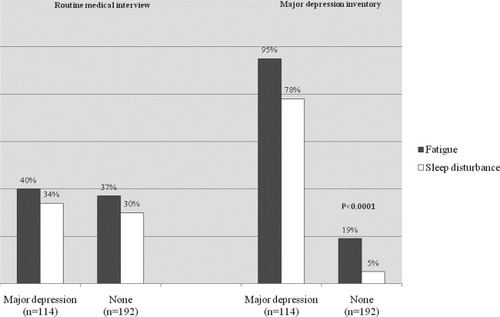
Frequency of fatigue and sleep disturbance being reported during routine medical interview in comparison to the same symptoms being rated “present all the time” or “most of the time” in the MDI in the 114 patients with major depression versus in the 192 patients without major depression.
Antidepressant Medication.
Antidepressants were prescribed to 47 (15%) patients: 25 patients prior to initiation of peginterferon and ribavirin therapy and 22 patients during therapy. Women initiated antidepressant medication more frequently than men (29 [22%] versus 18 [10%]; P < 0.05). Nine (3%) patients were referred to a psychiatrist for further evaluation, and one patient was admitted to a psychiatric ward with severe depression and suicidal ideation. In total, 31 (27%) patients with major depression were provided with antidepressant medication (16 patients prior to initiating HCV therapy and 15 patients during HCV therapy) as compared with 17 (9%) patients who had no major depression. Fourteen (93%) of the 15 patients treated after initiating HCV therapy were no longer rated as having major depression 4 weeks after the initiation of antidepressive medication, as compared with 22 (27%) of 83 patients with major depression who did not receive antidepressants (P < 0.0001; Fig. 3). In the former group, the mean MDI score declined from 36.0 (95% CI, 6-37) points to 17.1 (95% CI, 3-21) points as compared with a decline from 32.6 (95% CI, 7-35) points to 28.9 (95% CI, 6-31) points in patients not provided with antidepressant medication (P < 0.0001; Fig. 3).
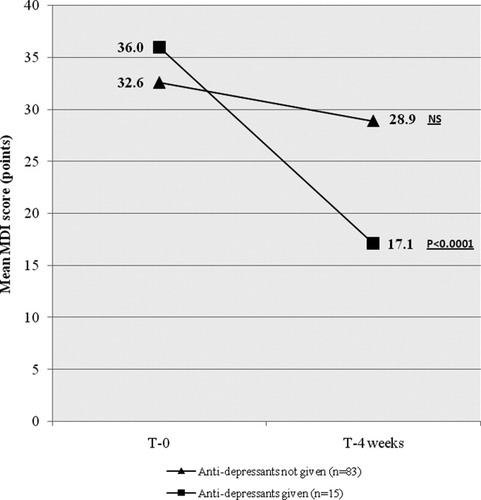
Changes in the MDI scores during a 4-week obervation period in patients with major depression who were given antidepressants compared to patients with major depression who were not given antidepressants.
Impact of Emerging Depression on Outcome of HCV Therapy.
Peginterferon and ribavirin combination therapy was prematurely discontinued in 32 (10%) of the 325 patients. The discontinuation rate was significantly higher among the 114 patients with MDI-rated major depression than among the 192 patients without (18% [n = 21] versus 6% [n = 11]; P < 0.001). With one exception, the 21 patients with major depression who discontinued HCV therapy had not initiated antidepressant medication.
Mean MDI score at baseline was similar in the patients achieving SVR as compared to those who did not (7.9 points and 8.4 points, respectively). In contrast, 7 of the 33 (21%) patients not achieving SVR had an increase in the MDI score during HCV combination therapy exceeding 30 points as compared to baseline as opposed to 9 of 124 (7%) patients with SVR (P = 0.02; Fig. 4), indicating that an increase in MDI exceeding 30 points above baseline (i.e., a validated marker of primary or idiopathic major depression13), rather than the absolute MDI score at any given time-point, may adversely affect the outcome of HCV therapy. This difference was even more pronounced among patients with an on-treatment increase in MDI score greater than 35 points, which was noted among 5 of 33 (15%) patients in the non-SVR group versus 3 of 124 (2%) patients in the SVR group (P = 0.003). A nonsignificant trend toward a greater mean increase in MDI score during HCV therapy was observed in the non-SVR group than in the SVR group (18.5 versus 15.4 points), especially in female patients (18.6 versus 13.3 points).
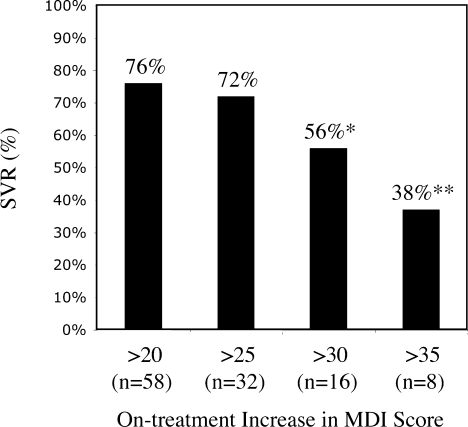
Proportion of patients achieving SVR grouped according to increase in MDI score as compared to the pretreatment, baseline score (*P = 0.02 and **P = 0.003).
Discussion
Assessment of psychiatric symptoms by use of the MDI scale during antiviral treatment for chronic hepatitis C revealed the emergence of major depression in approximately one-third of patients. However, only 30% of these patients were identified by means of routine medical interviews. Emotional complaints, such as feeling sad or being low in spirit, and vegetative symptoms, such as fatigue and sleep disturbance, were underreported (or were not interpreted as signs of depression) during routine clinical consultations. Therefore, a self-report instrument such as the MDI scale may be a useful complementary tool for health providers with limited psychiatric training to identify patients at risk for depression during HCV therapy.
Our results confirm the high frequency of depression during combination therapy for HCV as well as the relatively early onset, typically occurring within the first 3 months of therapy.5, 6, 17-19 However, 14% of the patients in the 24-week treatment arm developed major depression during the last 3 months of therapy, indicating that depression may arise at any time throughout HCV therapy. The MDI scores returned to baseline level within 8 weeks after cessation of therapy, thus emphasizing the reversible nature of interferon-induced depression.20
Earlier studies show that antidepressive treatment may optimize the outcome of HCV therapy, in particular by reducing the risk of early treatment discontinuation,6, 7, 21 and that recognition and treatment of pre-existing psychiatric illness prior to the initiation of HCV antiviral therapy yields improved tolerability and response.22 However, our study is, to the best of our knowledge, the first to report that the emergence of therapy-induced major depression is associated with a reduced likelihood of achieving SVR. We thus observed a gradual decline in the rates of SVR with increasing MDI score and that patients with an increase in MDI scores exceeding 30 points as compared to baseline had significantly poorer outcome of antiviral therapy, underlining the importance of identifying and treating patients with emerging depression during HCV therapy. This cutoff increment of an increase in MDI score greater than 30 was chosen because patients with idiopathic DSM-IV major depression have a mean MDI score of 38 points at the time of diagnosis and 8 points at remission.13
Antidepressant medication was initiated in only 27% of the 114 patients with major depression in this study, whereas 25% of the depressed patients were provided with hypnotics and anxiolytics without the use of antidepressants. Hence, almost half of the patients with major depression did not receive any psychotropic treatment. As expected, use of antidepressants in this study was followed by a significant reduction of the MDI score and resolution of symptoms, but the study was not powered to clarify whether the use of antidepressants affected the outcome of HCV therapy.
Our study also comprised an analysis of risk factors associated with the development of depression during HCV therapy. In accordance with previous studies,5, 17, 23 depressive symptoms at baseline were associated with higher risk of major depression. A history of depression was not a risk factor in our study, although divergent observations in this regard have previously been reported.5, 6, 24 Similarly, age was not a risk factor, in accordance with previous reports5, 6 but in contrast to the observations reported by Horikawa et al.18 Previous studies have yielded conflicting data with respect to female sex as a risk factor for development of interferon-induced depression.4, 6, 25 In our study, female sex was found to be an independent predictor of the emergence of major depression during HCV therapy, with women more frequently reporting depressive symptoms as captured in the CRF, and more frequently initiating antidepressant medication. The MDI scoring also identified two symptoms, fatigue and sleep disturbance, which strikingly heralded emergence of depression: more than 70% of patients reporting fatigue and more than 90% of those reporting sleep disturbances developed major depression. Notably, the medical interviews failed to establish fatigue or sleep disturbance as risk factors for emerging depression.
In conclusion, our results imply that the development of major depression in patients undergoing HCV therapy is commonly overlooked by means of routine clinical examination, and that pronounced symptoms of depression may negatively affect the outcome of antiviral therapy. Self-reporting scales such as the MDI instrument may help identify patients with emerging depression and thus improve the outcome of HCV therapy.
Acknowledgements
We thank Ove Aaskoven for statistical assistance and Kerstin Fredricson Oversø, D.Sc. (Pharm), for her contribution in the manuscript review process.




