Acetaminophen dosing of humans results in blood transcriptome and metabolome changes consistent with impaired oxidative phosphorylation†
Potential conflict of interest: Nothing to report.
fax: 919-316-4771
Abstract
The diagnosis and management of drug-induced liver injury (DILI) is hindered by the limited utility of traditional clinical chemistries. It has recently been shown that hepatotoxicants can produce compound-specific changes in the peripheral blood (PB) transcriptome in rodents, suggesting that the blood transcriptome might provide new biomarkers of DILI. To investigate in humans, we used DNA microarrays as well as serum metabolomic methods to characterize changes in the transcriptome and metabolome in serial PB samples obtained from six healthy adults treated with a 4-g bolus dose of acetaminophen (APAP) and from three receiving placebo. Treatment did not cause liver injury as assessed by traditional liver chemistries. However, 48 hours after exposure, treated subjects showed marked down-regulation of genes involved in oxidative phosphorylation/mitochondrial function that was not observed in the placebos (P < 1.66E-19). The magnitude of down-regulation was positively correlated with the percent of APAP converted to the reactive metabolite N-acetyl-p-benzoquinone-imide (NAPQI) (r= 0.739;P= 0.058). In addition, unbiased analysis of the serum metabolome revealed an increase in serum lactate from 24 to 72 hours postdosing in the treated subjects alone (P< 0.005). Similar PB transcriptome changes were observed in human overdose patients and rats receiving toxic doses. Conclusion: The single 4-g APAP dose produced a transcriptome signature in PB cells characterized by down-regulation of oxidative phosphorylation genes accompanied by increased serum lactate. Similar gene expression changes were observed in rats and several patients after consuming hepatotoxic doses of APAP. The timing of the changes and the correlation with NAPQI production are consistent with mechanisms known to underlie APAP hepatoxicity. These studies support the further exploration of the blood transcriptome for biomarkers of DILI. (HEPATOLOGY 2010.)
In the United States, drug-induced liver injury (DILI) is the most commonly identifiable cause of acute liver failure and is the major reason behind regulatory actions on drugs, including failure to approve for marketing, restrictions on labeled indications, and removal from the marketplace.1, 2 One reason why DILI remains problematic is that its diagnosis is generally one of exclusion, requiring costly and prolonged patient evaluation. In addition, the currently available liver chemistries, such as serum alanine aminotransferase (ALT), do not reliably distinguish between mild and transient DILI, which is of no consequence for the patient who can continue to receive the drug safely, versus DILI that will progress to life-threatening injury if drug therapy is not promptly stopped.3 In addition, currently available tests generally cannot distinguish which specific drug is causing the DILI in patients on multiple drug therapy. What is clearly needed are better biomarkers of DILI to help clinicians, as well as provide more meaningful liver safety data in clinical trials of new drugs.
We believe that the peripheral blood (PB) transcriptome may contain information that could address the shortcomings of currently available DILI diagnostic tools. Support for the PB transcriptomic approach comes from several recent findings. In an in-life rat study of eight hepatoxicants, we recently demonstrated that PB cell gene expression can be successfully utilized to detect the presence and severity of toxic responses in the liver.4 In fact, these studies suggested that PB transcriptomic data might be more sensitive to liver injury than traditional clinical tests and therefore able to detect DILI earlier. In addition, the pattern of PB cell transcriptomic response varied across toxicants, indicating the existence of “signatures” that could be useful in identifying the specific drug responsible for DILI. With specific respect to acetaminophen (APAP), the most common identifiable causative agent of acute liver failure in the US, we have shown that in rats treated with toxic doses, PB transcriptomic signatures, particularly in immune and inflammatory pathways, outperform traditional histological or clinical chemistry markers in detecting DILI. Furthermore, by probing human whole blood transcriptomic data from clinical overdose patients with human orthologs of this rat PB signature, we were also able to differentiate these patients from nonexposed individuals.5
The hypothesis tested in the current study was that a supratherapeutic but not overtly toxic APAP dose would result in readily detectable changes in the human PB transcriptome and that these changes would be qualitatively similar to changes we have demonstrated in rats and humans after toxic doses of APAP.5
Abbreviations
ALT, alanine aminotransferase; APAP, acetaminophen; AUC, area under the curve; CBC, complete blood counts; CYP2E1, cytochrome P4502E1; DEGs, differentially expressed genes; DILI, drug-induced liver injury; GSA, gene set analysis; GSH, glutathione; IPA, ingenuity pathways analysis; NAPQI, N-acetyl-p-benzoquinone-imide; PB, peripheral blood.
Patients and Methods
Subject Selection.
Subjects were healthy volunteers from 18-55 years old weighing 55 to 85 kg and not taking any over-the-counter or prescription medications. The protocol was approved by the University of North Carolina-Chapel Hill (UNC-CH) Institutional Review Board. Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the a priori Internal Review Board's approval. APAP history use was recorded and no subjects had taken APAP within a month of enrollment. Subjects were excluded if they had abnormal liver tests on screening or a history of chronic liver disease. Nine subjects were enrolled for 7 days each as inpatients in the General Clinical Research Center at the UNC Hospital. Human overdose subject descriptions have been reported.5
Diet.
Subjects were placed on a defined liquid diet to assure uniform nutritional intake. The protein source was soy, the fat source was safflower oil of known composition, and the carbohydrate source was cane or beet sugar. Other ingredients included Metamucil to provide fiber and vanilla. The overall macronutrient composition was 15% of total calories from protein, 30% from fat, and 55% from carbohydrate. Subject's daily calorie intake, divided into five consistently timed meals per day, was based on the formula 35 kcal/kg actual body weight. On day 4 the subjects were fasting until 2 hours after receiving APAP. Weight was monitored daily and calories adjusted to maintain body weight.
Sample Collections.
On the morning of the fourth day, six subjects received a single dose of 4 g of APAP administered as eight, 500-mg capsules, whereas three received placebo pills. Blood was collected at 6 AM on each of the clinical days for ALT measurement. PB, 7.5 mL, was drawn into PAXgene (PreAnalytiX/Qiagen, Hilden, Germany) blood RNA collection tubes (3 tubes at 2.5 mL) immediately before the first dose and at 6, 18, 24, 48, 72, and 96 hours postdosing. Samples were mixed and allowed to remain at room temperature for 2 hours, then frozen at −20°C until RNA isolation. Blood was also collected at 6 AM on each of the clinical days for measurement of clinical chemistries and complete blood counts (CBCs), performed by the UNC Hospital clinical laboratories. Serum was collected and frozen at −80°C predose and at the following times postdose: 30 minutes, 60 minutes, 90 minutes, 2, 3, 4, 5, 6, 8, and 12 hours. Upon study completion, APAP and metabolites were assayed in the serum by high-performance liquid chromatography (HPLC).6 In order to measure APAP metabolite excretion, urine was also collected for 24 hours postdosing and stored at −20°C with ascorbic acid (1 g/L).
RNA Isolation.
RNA was isolated utilizing the PAXgene blood RNA isolation kit (PreAnalytiX/Qiagen) according to the manufacturer's protocol, including the optional on-column DNase digestion. RNA quality was assessed with an Agilent Bioanalyzer (Palo Alto, CA) and only samples with intact 18S and 28S ribosomal RNA peaks were used for microarray analysis.
Microarray Analysis.
Gene expression profiling was conducted using Agilent Human 1A(V2) oligo arrays with ≈20,000 genes represented. Each sample was hybridized against a human universal RNA control (Stratagene, La Jolla, CA). Then 500 ng of total RNA was amplified and labeled using the Agilent Low RNA Input Fluorescent Linear Amplification Kit according to the manufacturer's protocol. For each two-color comparison, 750 ng of each Cy3- (universal control) and Cy5-labeled (sample) cRNA were mixed and fragmented using the Agilent In Situ Hybridization Kit protocol. Hybridizations were performed for 17 hours in a rotating hybridization oven according to the Agilent 60-mer oligo microarray processing protocol prior to washing and scanning with an Agilent Scanner (Agilent Technologies, Wilmington, DE). Details of microarray analysis of APAP-treated rats have been reported.5
Reverse-Transcription Polymerase Chain Reaction (RT-PCR).
Relative abundance of five nuclear DNA encoded differentially expressed genes (DEGs) and two mitochondrial DNA encoded genes not found on the Agilent chip was measured with RT-PCR utilizing 18S ribosomal RNA as the endogenous control. Reagents were obtained from Applied Biosystems (ABI, Foster City, CA). The ABI gene assay product numbers were: ATP5L: Hs00758883_s1; ATP5H: Hs01046892_gH; NDUFA1: Hs00244980_m1; NDUFA4: Hs00800172_s1; COX5A: Hs00362067_m1; MT-ND4: Hs02596876_g1; MT-RNR2: Hs02596860_s1.
Metabolomics Analysis.
Serum (300 μL) was added to 300 μL of a D2O solution containing 5 mM formate for concentration and chemical shift reference. The solution was vortexed and transferred to 5-mm nuclear magnetic resonance (NMR) tubes. Samples were kept on ice until analysis. NMR analyses were performed on a Varian Inova 600 MHz NMR using a 5-mm pulsed field gradient, inverse detection probe (Varian, Palo Alto, CA). The spectra were acquired with 256 transients and took ≈28 minutes per sample. The spectra were collected using the Carr-Purcell-Meiboom-Gill pulse sequence. The pulse sequence included a 2-second water presaturation period followed by a 100-ms spin echo sequence. An additional 3-second relaxation delay period was used to ensure quantitative results. A sweep width of 6,300 Hz was acquired with 16K data points with an acquisition time of 1.3 seconds. Data were processed using the ACD 1D NMR Processor software (v. 9, Advanced Chemistry Development, Toronto, Canada). A 0.1 Hz exponential line broadening was applied and peak phasing and baseline correction were applied. The spectra were integrated using the ACD Intelligent binning protocol. Statistical analysis of the binned NMR data was performed using SimcaP+ (v. 10, Umetrics, Umea, Sweden). An unbiased analysis of the metabolite perturbations was performed using principal components analysis with Pareto scaling applied to the input data.
A targeted metabolite profiling was carried out using the Chenomx NMR Suite program (Alberta, Canada). All spectra were imported into the Chenomx software and concentrations of 21 metabolites were determined. Absolute concentrations were determined based on the 5-mM formate used in sample preparation. Reference deconvolution was applied to the spectrum based on formate peak shape.
To gauge the significance of metabolite changes, measurements from 0 hours to 96 hours postdose were used to estimate the area under the curve (AUC). AUC Testing allows pooling of the data across time for a single test of differences in trend. The R package PK was utilized to estimate metabolite AUC for each sample. A t test was then performed to test for differences in AUC between cases and controls.
Gene Expression Data Analysis.
Microarray data were obtained using Agilent's Feature Extraction software (v. 7.5), using defaults for all parameters. The Feature Extraction Software performs error modeling before data are loaded into a database system. Images and GEML files were exported from the Agilent Feature Extraction software and deposited into Rosetta Resolver (v. 5.0, build 5.0.0.2.48) (Rosetta Biosoftware, Kirkland, WA). Rosetta Resolver combines data hybridizations using an error-weighted average that adjusts for additive and multiplicative noise.7 The resultant universal control profiles were then exported as normalized log ratios, median centered across subjects and utilized for further statistical analyses by the R-project software.8 Principal component analysis was performed to investigate the presence of experimental artifacts. The first component of variation was defined by sample ethnicity, and this component was removed to produce an adjusted dataset that did not contain an ethnicity bias.9 The resultant ratio profiles from both the ethnically unadjusted and adjusted datasets were analyzed for differential gene expression. First, a two-tailed t test was utilized comparing universal control profiles with time-matched sham controls and statistically significant DEGs were identified at the P < 0.05 confidence level. DEGs from both datasets were then analyzed with ingenuity pathways analysis (IPA) (Ingenuity Systems, www.ingenuity.com). Canonical pathways analysis identified the pathways from the IPA library of canonical pathways that were most significant to the dataset. The significance of the association between the dataset and the canonical pathway was measured in 2 ways: 1) A ratio of the number of genes from the dataset that map to the pathway divided by the total number of genes that map to the canonical pathway was obtained. 2) Benjamini-Hochberg testing corrected P-values were used to determine the probability that association between genes in the dataset and the canonical pathway is explained by chance alone. To increase our confidence in the IPA canonical pathway analysis, we utilized the more stringent gene set analysis (GSA) methodology on both the adjusted and unadjusted datasets comparing the cases to controls at each timepoint.12 For the five human overdose subjects, universal control profiles were normalized to five ethnically and gender-matched controls. A one-way analysis of variance (ANOVA) analysis with a Bonferroni multiple test correction was performed to identify DEGs. For the rat study, DEGs were identified using the Rosetta Resolver error-weighted P-value (P < 0.05).7
Results
Clinical
All subjects completed the inpatient study and there were no adverse events. Subject characteristics are listed in Table 1. Serum ALT levels are shown in Fig. 1. No subject had statistically significant increases in serum ALT or other liver enzymes or significant changes in CBCs during the study.
| Subject | Age | Gender | Ethnicity | Weight (kgs) | ALT |
|---|---|---|---|---|---|
| 1 | 45 | M | AA | 81 | 31 |
| 2 | 26 | F | AA | 73 | 26 |
| 3 | 28 | M | AA | 60 | 19 |
| 4 | 27 | F | AA | 78 | 24 |
| 5 | 22 | M | C | 78 | 36 |
| 6 | 39 | F | C | 55 | 23 |
| 7* | 36 | F | C | 66 | 28 |
| 8* | 22 | F | A | 55 | 27 |
| 9* | 24 | M | C | 54 | 38 |
- Abbreviations: M, Male; F, Female; AA=African-American, C=Caucasian, A=Asian. ALT=Serum alanine aminotransferase (IU/L) upon entry into clinic. * indicated placebo treatment.
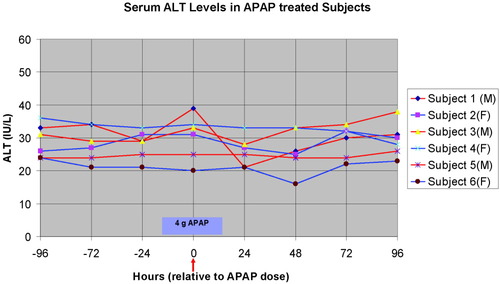
Serum ALT levels of six subjects receiving APAP at the beginning of clinical day 4. Blood was drawn for serum chemistries at approximately 6 AM each day. Red arrow indicates time of administration of the single 4-g APAP bolus following the blood draw on the fourth day. M, male; F, female.
APAP Pharmacokinetics
Peak serum APAP concentration and time to peak concentration varied among subjects (Fig. 2). Time to peak concentration was most rapid in Subject 5 at 30 minutes after dosing and the highest peak concentration was reached by Subject 6 at 62.4 μg/mL at 60 minutes after dosing. Subject 6 also had the lowest body weight (Table 1).
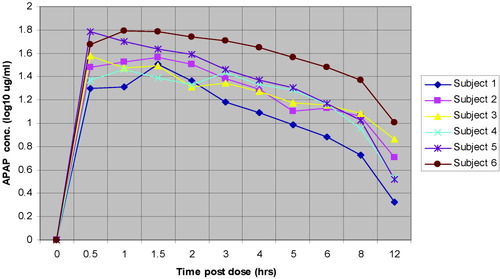
Serum APAP concentrations (log10 μg/mL) for treated subjects through 12 hours postdosing.
Gene Expression Changes
Supratherapeutic Dose Subjects.
Genes were found to be differentially expressed at all timepoints examined following APAP dosing in both the ethnically unadjusted and ethnically adjusted data, but only the 48-hour timepoint gave consistent changes in similar genes in all APAP-treated subjects. In the ethnically unadjusted dataset at 48 hours, there were 1,404 DEGs when all treated subjects were compared to all placebos, whereas the ethnically-adjusted dataset had 795 DEGs (Supporting Table 1). Pathway analysis results are shown in Table 2. IPA analysis of all identified DEGs at 48 hours from the unadjusted datasets revealed enrichment of genes in the oxidative phosphorylation (P < 1.44E-07), mitochondrial function (P < 0.0042), ubiquinone biosynthesis (P < 0.0295), protein ubiquination (P < 0.0001), and nucleotide excision repair (P < 0.0044) canonical pathways at 48 hours. Common genes in the first three pathways largely contributed to their significance. No other timepoint in the unadjusted or adjusted dataset demonstrated consistent significant cross-patient differential expression in any IPA pathway. Of the 35 genes identified in the oxidative phosphorylation pathway, all were down-regulated relative to the placebos. Because of the commonality of genes in these pathways, the mitochondrial function and ubiquinone pathways were, with a few exceptions, also down-regulated. When the ethnically adjusted dataset was analyzed the APAP-treated subjects demonstrated appreciably increased significance for effects on mitochondrial function (P < 0.0002, 21 genes) and ubiquinone biosynthesis pathways (P < 0.0014, 12 genes), and similar significance for the oxidative phosphorylation pathway (P < 2.75E-07, 26 genes) (Supporting Table 2). Conversely, both the nucleotide excision repair and protein ubiquination pathways were no longer significant. GSA confirmed much of the IPA analysis, with oxidative phosphorylation (P < 1.98E-07), mitochondrial function (P < 2.85E-07), ubiquinone biosynthesis (P < 6.88E-06), and nucleotide excision repair (P < 0.0003), showing significance in the unadjusted dataset. In addition, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling (P < 0.0189) and antigen signaling (P < 8.42E-11) pathways were also identified as significant. As with IPA analysis, GSA analysis of the adjusted data revealed marked increase in the significance of the oxidative phosphorylation (P < 1.66E-19), mitochondrial function (P < 3.89E-09), and ubiquinone biosynthesis (P < 9.06E-09) pathways. The nucleotide excision repair and PTEN signaling decreased. Chemokine signaling was identified as significant in the adjusted dataset alone. Therefore, focusing on the overlap between IPA and GSA, genes in the oxidative phosphorylation, mitochondrial function, and ubiquinone biosynthesis were significantly down-regulated in the ethnically unadjusted dataset at 48 hours, whereas adjusting for ethnicity only increased the significance for these pathways. As in the unadjusted data, the significance of these pathways was driven by a shared core of down-regulated genes. All of these genes are found in the mitochondrial oxidative phosphorylation Complex I (nicotinamide adenine dinucleotide [NADH] dehydrogenase, NADH CoQ oxidoreductase). Nucleotide excision repair and protein ubiquination, because of decreased significance when the data were adjusted for ethnicity bias, appear to be more related to ethnic ancestry than APAP treatment. A hierarchical cluster of the down-regulated oxidative phosphorylation genes in the adjusted dataset is presented in Fig. 3A.
| Canonical Pathway | IPA | GSA | ||
|---|---|---|---|---|
| Unadjusted for ethnicity | Adjusted for ethnicity | Unadjusted for ethnicity | Adjusted for ethnicity | |
| Oxidative Phosphorylation | 1.44E–07 | 2.75E–07 | 1.98E–07 | 1.66E–19 |
| Mitochondrial Function | 0.0042 | 0.0002 | 2.85E–07 | 3.89E–09 |
| Ubiquinone Biosynthesis | 0.0295 | 0.0014 | 6.88E–06 | 9.06E–10 |
| Antigen Presentation Pathway | 8.42E–11 | 0.00175 | ||
| Nucleotide Excision Repair | 0.0044 | 0.6324 | 0.0003 | 0.0047 |
| Protein Ubiquination | 0.0001 | 0.1079 | ||
| PTEN signaling | 0.8188 | 0.0189 | 0.4922 | |
| Chemokine Signaling | 0.8535 | 0.5074 | 0.0245 | |
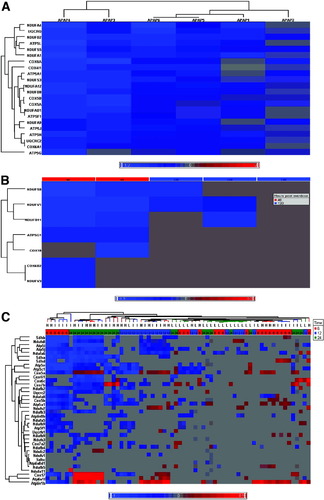
Hierarchical clusters of differentially expressed oxidative phosphorylation genes in blood samples. Log2-calibrated ratios were clustered using commercial software (Partek, St. Louis, MO) using an agglomerative method and average linkage clustering. Shade of color in the heat map represents the extent of differential expression: red, up-regulation; blue, down-regulation. Areas of lightest gray indicate no statistical significance at the given condition of time and dose. (A) Clinical subjects: 48 hours after single 4-g APAP dose. (B) Human overdose subjects. (C) APAP-treated rats. Horizontal experiment dendogram is labeled with dose (H = 2,500 mg/kg; I = 1,500 mg/kg; L = 150 mg/kg) and colored by time (red = 6 hours; blue = 12 hours; green = 24 hours).
Overdose Subjects.
Comparison of the human overdose subjects with five matched controls revealed a similar but muted oxidative phosphorylation down-regulation response in the two overdose subjects whose blood was collected ≈48 hours after APAP ingestion (six and five genes, respectively) (Fig. 3B). This is the same timepoint when down-regulation of oxidative phosphorylation genes was observed in the subjects who received the supratherapeutic dose. Of the remaining three subjects, all had their blood collected ≈120 hours after overdose. One had no change in the expression of oxidative phosphorylation genes. However, the other two had three down-regulated oxidative phosphorylation genes, all of which were also down-regulated in the two 48-hour subjects.
Rats.
In rats dosed with APAP, there was a general time- and dose-dependent down-regulation trend for oxidative phosphorylation genes (Fig. 3C). Overall, there was notable down-regulation of oxidative phosphorylation genes in the PB of animals treated at 24 hours with 2,500 mg/kg or 1,500 mg/kg APAP, when there was clear evidence of liver injury.5 There was a similar but less prominent down-regulation of oxidative phosphorylation genes at 12 hours in the 1,500 and 2,500 mg/kg dose animals. However, the most extensive down-regulation occurred in samples from animals 6 hours after treatment with the toxic 1,500 and 2,500 mg/kg doses, a time prior to any evidence of liver injury.
RT-PCR.
RT-PCR analysis confirmed the down-regulation of five selected nuclear encoded oxidative phosphorylation genes (ATP5H, ATP5L, COX5A, NDUFA1, NDUFA4) in the 4-g dose human clinical samples (Supporting Fig. 1). In addition, four mitochondrial DNA encoded genes that were not on the Agilent 1vA2 chip were also down-regulated. Two are involved in oxidative phosphorylation (MTND3, MTATP) and two encode transfer RNAs (MTRNR1, MTRNR2).
Correlation of the Ratio of Genes Down-regulated in the Mitochondrial Function Pathway and Excreted APAP/Mercapturate/Cysteine Conjugates.
The urinary elimination of APAP and metabolites during the 24 hours after dosing is shown in Table 3. The breakdown products of the reactive APAP metabolite N-acetyl-p-benzoquinone-imide (NAPQI) (the sum of the mercapturate and cysteine conjugates) varied substantially. In the ethnically adjusted dataset there was a positive correlation across the six treated subjects between their urinary production of mercapturate and cysteine conjugate and the ratio of genes down-regulated in the mitochondrial function pathway as reported by IPA (r = 0.739; P = 0.58) for each individual treated subject (Fig. 4).
| Glucuronide | Sulfate | Free APAP | Mercapturate | Cysteine | Sum | |
|---|---|---|---|---|---|---|
| Subject1 | 59.427 | 30.252 | 3.830 | 5.530 | 0.962 | 100 |
| Subject2 | 52.972 | 33.485 | 5.460 | 5.649 | 2.434 | 100 |
| Subject3 | 47.358 | 38.051 | 5.397 | 6.871 | 2.323 | 100 |
| Subject4 | 54.986 | 31.581 | 4.372 | 8.736 | 0.326 | 100 |
| Subject5 | 59.212 | 29.150 | 5.139 | 4.049 | 2.449 | 100 |
| Subject6 | 42.723 | 38.718 | 5.317 | 9.146 | 4.096 | 100 |
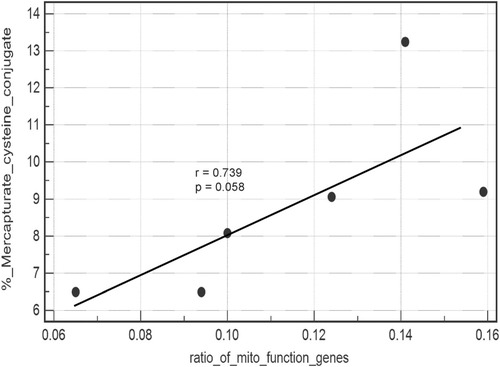
Regression analysis: toxic APAP conjugates versus the ratio of down-regulated mitochondrial function genes. Regression analysis of a scatterplot of mercapturate/cysteine APAP conjugates (as a percent of total urinary excreted metabolites) and the ratio of genes in the mitochondrial function pathway down-regulated in each treated subject. Each data point represents one of the six treated subjects.
Metabolomics.
The binned NMR data were analyzed by principal components analysis to search for metabolic perturbations in an unbiased manner. The results showed significant segregation of dosed versus control samples and highlighted lactate levels as being significantly altered in subjects after APAP dosing. To follow up on this result, targeted quantitative profiling of selected metabolites was performed. Lactate concentrations along with 20 more readily identifiable metabolites were determined using the Chenomx NMR database. No statistically significant perturbations were observed in any of the metabolites except for lactate. The lactate trend test indicates a significant increase in lactate abundance in cases relative to controls (P < 0.005). A time course graph of targeted profile metabolite concentrations can be seen in Fig. 5A,B. A sharp increase appears at 6 hours after dose. Lactate levels appear highly variable at 18 hours, then show a consistent rise from 24-72 hours before dropping back to basal levels at 96 hours after dose. These changes in lactate concentration were not observed in controls.
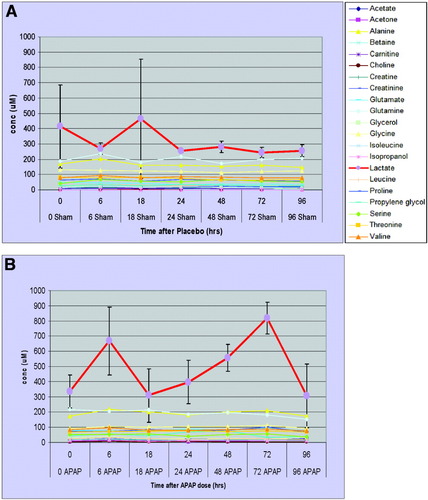
Serum metabolome time course profiles. Targeted metabolite profiles: time course of 21 metabolites measured in placebo (A) and APAP-treated (B) subjects' serum. Lactate increases (APAP versus placebo) are seen consistently after 24 hours. All other metabolites showed no significant changes over time.
Discussion
Consistent with our hypothesis, we were able to identify changes in the transcriptome of PB cells in subjects treated with a single dose of APAP that did not produce liver injury as detected by currently available liver chemistries. Furthermore, these observations are consistent with whole blood transcriptome changes observed in rats and humans exposed to overtly hepatotoxic doses of APAP.
Our observations indicate a distinct putative PB transcriptomic signature for a subtoxic dose in humans. Specifically, we observed down-regulation of multiple nuclear DNA encoded and four mitochondrial DNA encoded genes for proteins located in mitochondria, particularly those associated with oxidative phosphorylation. Although this phenomenon was seen most clearly when using the power of pooling the six clinical replicates, we did see this response in individual subjects. Moreover, directed analysis of data from our rat and human overdose subjects revealed a similar effect on oxidative phosphorylation genes. In rats, we found a dose-dependent down-regulation of oxidative phosphorylation genes at toxic doses of APAP at 12 and 24 hours, when liver injury had occurred. In addition, a subgroup of animals treated with toxic doses showed strong down-regulation at 6 hours, when there was no indication of liver injury. Of the five human overdose subjects, only two had their blood collected 48 hours after APAP ingestion and each showed clear evidence of down-regulation of six oxidative phosphorylation genes. Two of the remaining subjects had down-regulation in a total of three of the genes that were also down-regulated in the 48-hour subjects. Clearly, more data are needed, but the limited amount at our disposal is consistent with our observations in the supratherapeutic subjects.
Because we measured thousands of messenger RNAs (mRNAs) in only six treated subjects of differing ethnicity, false discovery is a concern. However, several lines of evidence support that the changes observed were real. First, the significance of the canonical pathway changes using stringent false discovery rate parameters was even stronger after making appropriate adjustments to the data for ethnicity. Second, these changes were not observed in any of our three placebo patients. Third, down-regulation of oxidative phosphorylation genes was temporally associated with a rise in serum lactate when the pooled data from APAP-treated and placebos was compared, as would be expected during functional impairment of oxidative phosphorylation. This is therefore an example of the power of “metabolomic anchoring” of transcriptomic data. Fourth, there was a positive correlation among the individual treated subjects between the extent of down-regulation of genes associated with mitochondrial function and the production of APAP mercapturate and cysteine conjugates in the urine, an accepted quantitative measure of conversion of APAP to its toxic metabolite, NAPQI. Finally, as discussed below, there are plausible biological mechanisms that could account for the observed changes. Also worthy of note is the absence of changes in CBCs in any of the patients during the course of the study. This is important because any such changes could contribute strongly to differential gene expression changes. In aggregate, these observations solidify our conclusion that a nonovertly toxic dose of APAP can produce down-regulation of oxidative phosphorylation genes in PB.
It may be important that, although all complexes of the oxidative phosphorylation pathway were affected, genes of complex I of the oxidative phosphorylation pathway were most consistently down-regulated. Among the complexes of the oxidative phosphorylation chain, complex I dysfunction has been especially linked with lactic acidosis, whereas complex III has been implicated as a sensor of hypoxia and activator of hypoxia inducible factors.10-13 Impairment in complex 1 function may therefore account in part for the observed increase in serum lactate. We cannot rule out other tissues as the source of the increased serum lactate.
It is tempting to speculate that the down-regulation of oxidative phosphorylation genes we observed reflects APAP toxic effects on lymphocytes. Mitochondria are known to be a primary target for APAP toxicity in hepatocytes through production of NAPQI, which is chiefly produced in the liver by cytochrome P4502E1 (CYP2E1). NAPQI causes depletion of mitochondrial glutathione (GSH) and resulting oxidative stress,14 and covalently binds to mitochondrial proteins.15 Because lymphocytes contain detectable amounts of CYP2E1 mRNA and protein,16, 17 NAPQI could be produced within lymphocytes and target the lymphocyte mitochondria. Further support for possible mitochondrial toxicity in lymphocytes is our RT-PCR results that demonstrate down-regulation of two mitochondrial DNA encoded genes (MT-RNR1, MTRNR2) that are not involved in oxidative phosphorylation. However, it is also possible that APAP is metabolized to NAPQI in the liver and then released into the serum, resulting in damage to circulating PB leukocytes. On the other hand, mitochondrial toxicity alone is unlikely to explain our findings because some of the down-regulated mRNAs involved in oxidative phosphorylation are products of nuclear and not mitochondrial gene transcription. It may therefore be relevant that APAP has been shown to induce caspase-dependent apoptosis in cultured primary lymphocytes with no evidence of formation of NAPQI-bound proteins.18, 19 However, in this study we did not detect significant changes in apoptotic pathways across all patients.
Another possible explanation for down-regulation of both mitochondrial and nuclear genes could involve an adaptive metabolic strategy by the leukocytes. Activation of granulocytes, monocytes, and T lymphocytes, as would be expected to occur during overt liver injury, results in a metabolic shift from reliance on oxidative phosphorylation for energy production to aerobic glycolysis.20-22 Although our observation of down-regulated oxidative phosphorylation genes would be entirely consistent with this hypothesis, we did not see consistent up-regulation of genes involved in glycolysis.
It should be noted that a link between the transcriptome changes and APAP toxicity is suggested by the timing of the changes relative to dose administration. Down-regulation of the oxidative phosphorylation pathway and sustained increase in serum lactate were both observed 48 hours postdosing. Although we cannot specifically attribute the increase in lactate to any particular organ or cell type, this timing is consistent with the onset of overt liver injury in clinical overdose cases where abnormal liver chemistries typically do not appear until 24 to 48 hours after ingestion.23 These observations are consistent with the PB transcriptome changes being at least associated with some mild liver stress, but presumably they would represent an early, harmless transitory stage in the process.
As a final note, it is unclear whether the tightly controlled clinical environment and dietary intake incorporated in this study were important in detecting these changes. However, environmental factors such as diet and exercise have been shown to significantly influence PB gene expression.24, 25
In summary, we have demonstrated down-regulation of mitochondrial genes, most prominently in the oxidative phosphorylation pathway, in PB cells after a single supratherapeutic but not overtly toxic APAP dose. The gene expression changes are supported by our metabolomic finding of a concurrent increase in serum lactate. The basis for these changes are unclear, but they are consistent with known mechanisms underlying APAP liver injury and support our earlier rat work suggesting that certain blood transcripts might provide earlier detection of potential DILI. Further studies will be needed to determine if there are blood transcriptome “signatures” that could be used to both diagnose DILI and potentially identify specific culprit drugs.




