Major histocompatibility complex class I–related chains A and B (MIC A/B): A novel role in nonalcoholic steatohepatitis†
Potential conflict of interest: Nothing to report.
Abstract
Stress-induced soluble major histocompatibility complex class I–related chains A/B (MIC A/B) are increased in chronic liver diseases and hepatocellular malignancy. We investigated the impact of these molecules on liver injury, apoptosis, and fibrosis in nonalcoholic steatohepatitis (NASH). Blood and liver tissue were obtained from 40 patients with NASH undergoing bariatric surgery for obesity. The control group consisted of 10 healthy individuals. We also investigated 10 patients with nonalcoholic fatty liver (NAFL). Polymerase chain reaction was used to measure messenger RNA (mRNA) transcripts of MIC A/B, natural killer cell receptor G2D (NKG2D), CD95/Fas, and tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–death receptor 5 (DR5). Apoptosis was quantified by way of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) (intrahepatic) and M30/M65 (systemic). Liver injury was assessed histopathologically and serologically (alanine aminotransferase/aspartate aminotransferase). Fibrosis was identified by Sirius red staining, quantitative morphometry, and α-smooth muscle actin and collagen 1α transcripts. Compared with controls, patients with NASH revealed significant increases in (1) NKG2D mRNA (13.1-fold) and MIC A/B mRNA (3.6-fold and 15.8-fold, respectively); (2) TRAIL–DR5 and CD95/Fas mRNA (2.7-fold and 3.6-fold, respectively); (3) TUNEL-positive hepatocytes (4.0-fold); and (4) M30 and M65 levels (4.6-fold and 3.4-fold, respectively). We found relevant correlations between MIC protein expression rates and NAS and fibrosis stages. In contrast, NKG2D and MIC A/B transcripts were attenuated in patients with NAFL compared with NASH. Histopathologically, NASH patients revealed increased NAS scores, an accumulation of natural killer cells, and 2.7-fold increased hepatic fibrosis by quantitative morphometry. Conclusion: Our findings suggest an important role for MIC A/B in liver injury. Therapeutic intervention aimed at reducing MIC A/B levels may beneficially affect the progression of NASH. (HEPATOLOGY 2009.)
With the increasing prevalence of the metabolic syndrome in western and westernized countries, the diagnosis of nonalcoholic fatty liver disease (NAFLD) has greatly increased in clinical practice. NAFLD is the most common cause of elevated liver enzymes and probably the most common liver disease in these countries, with an overall prevalence of up to 30%.1, 2 Hepatocellular apoptosis is a prominent feature of liver injury in the pathogenesis of nonalcoholic steatohepatitis (NASH).3
Recently, soluble forms of major histocompatibility complex class I–related chains A and B (MIC A/B) were reported to be increased in the sera of patients with chronic liver disease and hepatocellular malignancy.4-7 As membrane glycoproteins, MIC A/B indicate cellular stress by presenting peptides derived from cytosolic proteins for recognition by circulating cytotoxic natural killer (NK) cells.8, 9 There are several receptors on NK cells engaged in activating the signal transduction that leads to enhanced NK-mediated cytolysis. One of these, natural killer cell receptor G2D (NKG2D), is expressed on virtually all NK cells and recognizes MIC A/B ligands.10-14 We hypothesize that, as generic signals of tissue distress, expression of MIC A/B may be triggered during the progression of NASH, which has not yet been explored.
As components of the innate immune system in the liver, NK cells are involved in several processes of liver injury. For example, in hepatitis B virus transgenic mice mimicking human hepatitis B surface antigen carriers, increased susceptibility to liver injury was related to enhanced interaction between NKG2D and stress-induced ligands.15 Likewise, recent reports demonstrated a critical role for NKG2D in peripheral blood and intrahepatic lymphocytes in patients with chronic viral hepatitis B and C infection. These patients displayed significantly increased NKG2D expression resulting in the stimulation of intrahepatic CD8+ T cells.16
We thus aimed at investigating the role of these stress-induced ligands on liver injury, apoptosis, and hepatic fibrosis in patients with NASH undergoing bariatric surgery for obesity. To address this subject, the data of 40 morbidly obese patients (body mass index >40 kg/m2) with biopsy-proven NASH, as well as that of 10 patients with NAFL, were analyzed and compared with normal liver samples.
Abbreviations
DR5, death receptor 5; ELISA, enzyme-linked immunosorbent assay; MIC A/B, major histocompatibility complex class I–related chains A/B; mRNA, messenger RNA; NAFL, nonalcoholic fatty liver; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; NK, natural killer; NKG2D, natural killer cell receptor G2D; qrt-PCR, quantitative real-time polymerase chain reaction; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
Patients and Methods
Patients.
All enrolled patients were physically examined and a complete set of laboratory parameters was obtained. Individuals aged <18 years and >65 years with different liver pathologies (infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus), history of organ transplantation, history of malignancy within the previous 5 years, alcohol or drug abuse within the previous year, autoimmunity, genetic disorders, and therapy with immunosuppressive or cytotoxic agents were excluded. Indication for bariatric surgery was made according to National Institutes of Health guidelines (body mass index ≥40 kg/m2, plus comorbidities). Ultrasonographic examination of the liver was performed and biopsies were harvested from all 40 morbidly obese patients undergoing bariatric surgery. The surgeon's choice (for example, adjustable gastric band, Roux-Y, or gastric bypass surgery) was based on the current guidelines adjusted to the patient's clinical status and comorbidities as well as on the clinical expertise. All patients were informed about the additional risks of a wedge liver biopsy during the bariatric procedure. Blood and liver samples were obtained from consented liver transplantation donators used as healthy controls. In addition, to further explore the role of MIC A/B, we also investigated a cohort of 10 patients with NAFL (that is, as a benign form of NAFLD with only simple steatosis). Specimens were split, with one piece stored in 4% formalin solution (Roth, Karlsruhe, Germany) for subsequent histological examination and the other piece stored in RNA-preserving agent (RNAlater; Ambion Applied Biosystems, Darmstadt, Germany) to determine the expression of selected genes.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the University Hospital of Essen. All patients provided written informed consent before enrollment. The patients' baseline characteristics are given in Table 1.
| Healthy Controls (n = 10) | Patients with NAFL (n = 10) | Patients with NASH (n = 40) | |
|---|---|---|---|
| Female:Male | 3:7 | 8:2 | 31:9 |
| Age (years) | 26 ± 7.6 | 39 ± 3.4 | 43 ± 10.5 |
| Weight (kg) | 69.7 ± 16.1 | 132.8 ± 7.5 | 164 ± 17.4 |
| Height (cm) | 171.4 ± 11.5 | 170 ± 8 | 174.7 ± 11.5 |
| BMI (kg/m2) | 22.5 ± 1.3 | 45.7 ± 2.1 | 59.6 ± 4.6 |
| Aspartate aminotransferase (U/L) | 24.5 ± 1.7 | 21.9 ± 3.2 | 79 ± 11.3 |
| Alanine aminotransferase (U/L) | 28.7 ± 2.8 | 20.3 ± 7.1 | 87.3 ± 17.2 |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.2 |
| Serum cholesterol (mg/dL) | 141.3 ± 17.3 | 184.8 ± 10.5 | 189.8 ± 39.7 |
| HbA1c (%) | 3.1 ± 0.8 | 4.9 ± 1.8 | 5.8 ± 1.2 |
| Diabetes mellitus | None | 10% | 17.1% |
| Arterial hypertension | None | 20% | 31.4% |
- Values are expressed as the mean standard ± error.
Histological Analysis.
Hematoxylin-eosin staining was performed according to standard techniques. Samples were investigated and quantified according to NAFLD activity score (NAS).17 Steatosis (0–3), hepatocellular ballooning (0–2), and lobular inflammation (0–2) were quantified, respectively. NAS of ≥5 or ≥4 with at least one score for ballooning was defined as NASH. Extent of liver fibrosis was assessed using the modified METAVIR criteria.18
Quantitative Real-Time Polymerase Chain Reaction.
Liver tissue was homogenized with a blade homogenizer (IKA, Staufen, Germany) according to standard laboratory procedures. Total RNA was isolated with the RNeasy mini kit (Qiagen, Hilden, Germany) following the protocol using spin technology. Having spectrophotometrically assured the samples' purities and adjusted their concentrations, 2 μg of each RNA sample was filled up to a total volume of 100 μL with RNAse-free water. Reverse transcription was performed with the Quanti Tect RT kit (Qiagen) according to the manufacturer's instructions. Quantitative real-time polymerase chain reaction (qrt-PCR) of complementary DNA was performed using the iCycler iQ thermal cycler (Bio-Rad, Hercules, CA) with real-time detection system software 3.0a and Genex software (Bio-Rad) in 30 μL reactions containing 15 μL Quanti Tect Sybr Green master mix (Qiagen), 5 μL complementary DNA, 1 μL forward primer, 1 μL reverse primer (at 10 pmol/μL each), and 8 μL aqua dest. Amplification was performed for 15 minutes at 95°C, followed by 40 cycles of 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C. Melting curve data were collected from 95°C to 55°C, at −0.5°C steps for 10 seconds each. Relative gene expressions were calculated from the threshold cycles in relation to housekeeping gene, to untreated controls or healthy donors, respectively.
Hypoxanthine-phosphoribosyltransferase 1 primers were used to normalize the samples. PCR primers (all obtained from Eurofins MWG Operon, Ebersberg, Germany) were as follows: hypoxanthine-phosphoribosyltransferase 1, 5′-GAC-CAG-TCA-ACA-GGG-GAC-AT-3′ (forward) and 5′-CTT-GCG-ACC-TTG-ACC-ATC-TT-3′ (reverse); MIC A, 5′-GTA-TTG-GGA-CCG-GAA-CAC-AC-3′ (forward) and 5′-ATG-CTC-TGG-AGG-GTG-TGA-GA-3′ (reverse); MIC B, 5′-TGC-CAT-GAA-GAC-CAA-GAC-AC-3′ (forward) and 5′-GGG-GCA-CTG-TTC-TCC-TGA-T-3′ (reverse); NKG2D, 5′-TTC-AGA-TAT-CCC-CAA-GGC-TG-3′ (forward) and 5′-TGA-TCT-GCT-GGC-CTT-CTC-TT-3′ (reverse); tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)–death receptor 5 (DR5), 5′-CAC-TGG-AAT-GAC-CTC-CTT-TTC-3′ (forward) and 5′-CTT-CCG-GCA-CAT-CTC-AGG-3′ (reverse); CD95/Fas, 5′-CAA-AGC-CCA-TTT-TTC-TTC-CA-3′ (forward) and 5′-TTT-GGT-TTA-CAT-CTG-CAC-TTG-G-3′ (reverse); collagen 1α (I), 5′-AAC-AGC-CGC-TTC-ACC-TAC-AG-3′ (forward) and 5′-GGA-GGT-CTT-GGT-GGT-TTG-GT-3′ (reverse); and α-small muscle actin, 5′-TTC-GTT-ACT-ACT-GCT-GAG-CGT-GAG-A-3′ (forward) and 5′-AAG-GAT-GGC-TGG-AAC-AGG-GTC-3′ (reverse).
Enzyme-Linked Immunosorbent Assays.
CD95/Fas and Fas ligand concentrations were determined by sandwich enzyme-linked immunosorbent assay (ELISA) methods. Plates precoated with human Fas/TNF RSF6 and human Fas ligand/TNF SF6 monoclonal antibodies (Quantikine ELISA kit, R&D Systems, Wiesbaden, Germany) were blocked by adding 15% bovine serum albumin, washed, and incubated with the standard or patients' sera. Patients' sera were diluted 1:3 in 7.5% bovine serum albumin. Absorbance was measured at 450 nm.
Apoptosis.
Cell death markers M30 (for apoptosis) and M65 (for overall cell death) were assessed both in the sera of patients and healthy controls using the M30 (Apoptosense) and M65 ELISA kit (both from Peviva, Bromma, Sweden) following the manufacturer's instructions. Whereas M30 is a cytokeratin-18 neo-epitope only exposed upon apoptotic cleavage by activated caspase-3,19 M65 reflects total cleaved and uncleaved cytokeratin-18.
MIC A/B are induced upon cellular distress conditions such as DNA damage, malignant transformation, or intracellular infection.20-23 Therefore, sections were counterstained with 4′,6-diamidino-2-phenylindole–containing ProLong antifade reagent (Invitrogen, Karlsruhe, Germany), and apoptotic hepatocytes were quantitated by way of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, which enzymatically labels free 3′ OH ends of damaged DNA with a fluorescently labeled nucleotide as described.24 Cells displaying TUNEL-labeled fluorescent nuclei were quantified by counting the number of positive cells per high-power field. A total of 10 high-power fields were analyzed for each patient with excitation and emission wavelengths of 380 and 430 nm, respectively, using an inverted laser scanning confocal microscope (LSM 510, Carl Zeiss Micro-Imaging, Göttingen, Germany) equipped with a ×40 NA 1.4 lens and LSM 510 imaging software. Data are expressed as the number of TUNEL-positive cells per 10 high-power fields.
Immunohistochemistry for Hepatic NK Cells and MIC A/B.
Tissue sections were preincubated with Block-ace (Dako Cytomotion Inc., Carpinteria, CA) for 10 minutes at 37°C to block nonspecific binding of the primary antibody. Endogenous peroxidase activity was blocked with 0.3% H2O2and 0.1% NaN3in distilled water for 10 minutes at room temperature. Sections were then incubated overnight with 1:500 diluted biotin-conjugated anti-CD57 primary antibody (Invitrogen, Karlsruhe, Germany) for hepatic NK cells and with MIC A/B–specific monoclonal antibodies (AbD Serotec, Münster, Germany), rinsed three times with phosphate-buffered saline, and incubated with avidin-biotin peroxidase complexes (Vector Laboratories, Burlingame, CA). Histochemical development was achieved by employing the 3,3′-diaminobenzidine substrate kit (Vector Laboratories). Finally, sections were counterstained for 3 minutes with hematoxylin and coverslipped with mounting medium for light microscopy.
Sirius Red Staining for Liver Fibrosis.
We proceeded according to Arteel et al.25 Direct red 80 and Fast green FCF (color index 42053) were obtained from Sigma-Aldrich Diagnostics (Taufkirchen, Germany). Red-stained collagen fibers were quantified in liver sections by digital image analysis as described.26
Statistical Analysis.
All data represent the measurements of three separate experiments and are expressed as the mean ± standard error unless otherwise indicated. Statistical analyses were performed using SAS version 8.2 software (SAS Institute, Cary, NC). Qualitative characteristics were analyzed using the χ2 test or Fisher's exact test as appropriate. Correlation coefficients reported are rank (Spearman) correlations. Statistical significance was assumed at P < 0.05. This explorative analysis performed no adjustment for multiple testing. Descriptive statistics were computed for all variables and included means and standard deviations or medians. Differences between concentrations were evaluated statistically by one-way analysis of variance, repeated-measures analysis of variance, or paired Student t test.
Results
Hepatic NK Cells and NKG2D, MIC A/B Transcripts Are Increased in NASH.
We initially sought to investigate the role of hepatic NK cells in patients with NASH following bariatric surgery through immunohistochemical analysis. As depicted in Fig. 1A, an increase of this cell type was observed in patients with NASH, whereas NK cell numbers were significantly lower in patients with NAFL (13.7 ± 0.9 versus 5.5 ± 0.8 NK cells/10 hpf, p = 0.001). In contrast, almost no NK cells were observed in controls (Fig. 1A). Likewise, qrt-PCR also demonstrated a significant 13.1-fold increase in NKG2D messenger RNA (mRNA) expression as compared with healthy controls (P < 0.01, Fig. 1B). Interestingly, NAFL patients revealed lower NKG2D mRNA transcription levels than patients with NASH (9.0 ± 5.1 for NAFL vs. 13.1 ± 1.8 for NASH, p = 0.27, n.s. = not significant). In addition, MIC A/B mRNAs were increased in the livers of patients with NASH versus control livers by 3.6- and 15.8-fold (P < 0.001, Fig. 1C). We observed a significant reduction of MIC B levels in individuals with NAFL as compared to patients with NASH whereas transcripts for MIC A were almost identical in both groups. Next, the tissue distribution of MIC A/B was examined by immunohistochemistry. The diffuse cellular staining pattern of MIC A/B in patients with NASH in Fig. 1C is no artifact but rather typical of this kind of stain.27 In contrast, control hepatocytes were negative while individuals with NAFL weakly expressed MIC A/B.
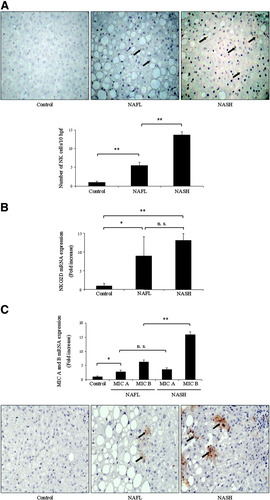
MIC A/B play an important role during hepatic inflammation in patients with NASH. (A) Immunohistochemistry revealed an increase of hepatic NK cells (marked with arrows) in patients with NASH. Only few of these cells were observed in healthy controls and in patients with NAFL. qrt-PCR also demonstrated significantly elevated mRNA expression of the receptor NKG2D in NASH. (B) Likewise, qrt-PCR data also demonstrated increased mRNA levels for MIC A/B in the livers of patients with NASH. (C) Tissue distribution of MIC A/B was examined microscopically and revealed a diffuse cellular staining pattern (arrows) in NASH, whereas hepatocytes from control livers were negative to the staining. However, staining was weak in the NAFL group. Representative photomicrographs of liver sections are shown.
TRAIL–DR5 and CD95/Fas Transcripts Are Increased in NASH.
Hepatic NK cells play a critical role in TRAIL- and Fas-mediated liver injury. The liver harbors many NK cells,28 and approximately 30% to 40% of these constitutively express TRAIL.29 We thus questioned whether the expression of TRAIL–DR5 and CD95/Fas mRNAs is increased in NASH livers. Indeed, a 2.7-fold increase in TRAIL–DR5 and a 3.6-fold increase in CD95/Fas mRNA were observed in patients with NASH compared with controls (Fig. 2A). TRAIL–DR5 mRNA was significantly less increased in individuals with NAFL compared with patients with NASH (1.1 ± 0.1 versus 2.7 ± 0.2, P = 0.01). In contrast, CD95/Fas transcripts were also reduced in the NAFL group, but this difference was not significant when compared with patients with NASH (2.5 ± 0.5 versus 3.6 ± 0.9; P = 0.16). Up-regulation of CD95/Fas in patients with NASH was further confirmed through ELISA and demonstrated a significant increase as compared with both healthy controls and individuals with NAFL (P < 0.05). However, up-regulation of CD95/Fas was not accompanied by enhancement of the Fas ligand (Fig. 2B). Subsequently, histopathological examination was performed along with determination of serum alanine aminotransferase and aspartate aminotransferase values. The NAS is a well-established scoring system for patients with NASH as a progressive form of NAFLD.17 As expected, histopathological examinations from patients with NASH displayed an increase in NAS as compared with controls. In addition, a marked elevation in serum alanine aminotransferase and aspartate aminotransferase values was also observed in patients with NASH versus controls (Fig. 2C). Liver enzymes from individuals with NAFL were within the reference ranges.
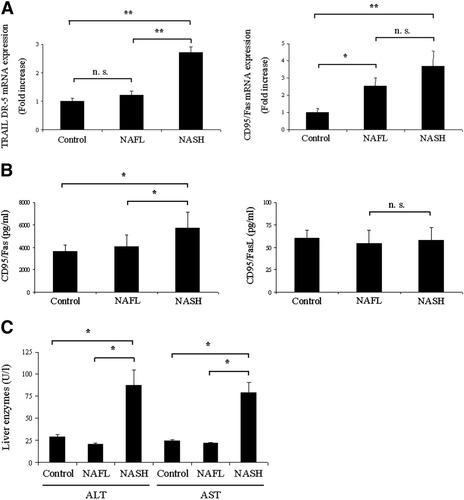
Hepatic expression of death receptors is increased during MIC A/B–mediated liver injury in patients with NASH. (A) qrt-PCR demonstrated increased mRNA expression levels of TRAIL–DR5 and CD95/Fas mRNAs in NASH patients. (B) Performance of ELISA demonstrated a significant up-regulation of CD95/Fas in NASH, whereas no alterations were observed in the Fas ligand. (C) A marked elevation in liver enzymes was also observed in patients with NASH.
Hepatocyte Apoptosis Is Up-regulated in NASH.
In order to examine potential effects of MIC A/B on hepatocyte death rates in NASH, we quantified the intrahepatic rates of apoptotic (by M30 and confirmatory TUNEL assays) and overall cell death (by M65 assay). As expected, M30 expression was significantly elevated in patients with NASH (440.5 ± 71.1 U/L) as compared with controls (94.4 ± 30.9 U/L; P < 0.05) (Fig. 3A), which went along with a likewise significantly increased M65 expression in patients with NASH versus controls (784.9 ± 129.9 U/L versus 230.3 ± 43.5 U/L; P < 0.05). In contrast, M30 levels were significantly reduced in individuals with NAFL (139.5 ± 20.9 U/L), which was again reflected in the significantly lower M65 expression in NAFL (298.2 ± 35.4 U/L). To further investigate whether an increase in MIC proteins is associated with enhanced liver injury and fibrosis, we determined correlation coefficients both for patients with NAFL and NASH. As shown in Fig. 3B, increased MIC A mRNA expression in patients with NAFL revealed a relevant correlation with NAS (r2 = 0.87), whereas the correlation coefficient for the stage of fibrosis was less evident (r2 = 0.68) (Fig. 3B). Higher correlation coefficients were found for MIC B transcripts in relation to NAS (r2 = 0.93) and fibrosis stage (r2 = 0.79). In patients with NASH, significant positive correlations were found for MIC A mRNA and NAS (r2 = 0.89) as well as the stage of fibrosis (r2 = 0.83) (Fig. 3C). Moreover, the correlation coefficients of MIC B transcripts to the severity of disease in NASH were also significant for both NAS (r2 = 0.72) and fibrosis stage (r2 = 0.85). In the final common step of the apoptotic machinery, activated effector caspases—in particular caspase-3 and caspase-7—cleave cytokeratin-18 as the major hepatic intermediate filament protein. Cytokeratin-18 fragment levels independently predict NASH. A cutoff value of 395 U/L holds 99.9% specifity and 85.7% sensitivity for the diagnosis of NASH.30 Regarding the role of MIC A/B in NASH severity, we further stratified the levels of these stress-induced proteins versus apoptosis-indicating M30 levels. Figure 3D shows a significant correlation within the NASH cohort. Confirmatory TUNEL assay demonstrated numerous clusters of apoptotic cells on an altered hepatic micro-architecture in obese patients with NASH (Fig. 3E). In contrast, only few apoptotic cells were observed in control livers or livers affected by fatty infiltration in patients with NAFL. Consistent with the previous results, the number of TUNEL-positive hepatocytes was also significantly higher in NASH patients than in controls and in individuals with NAFL. Quantitation of TUNEL-positive hepatocytes demonstrated a four-fold increase in patients with NASH compared with healthy controls (8.8 ± 0.8 versus 2.2 ± 0.2 TUNEL-positive cells per 10 high-power fields; P = 0.005) (Fig. 3E). Because hepatocyte apoptosis can induce liver inflammation and lead to fibrosis,31 we next investigated markers of liver fibrosis during MIC A/B–mediated liver injury.
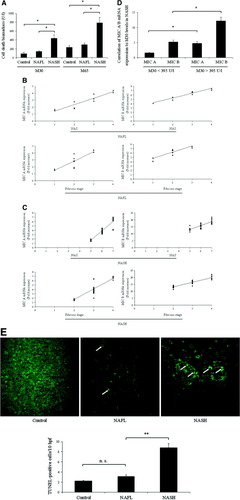
Hepatocyte apoptosis is enhanced in patients with NASH. (A) Cell death was first determined on the systemic level by the caspase-cleaved biomarkers M30 (for apoptosis) and M65 (for overall cell death) and revealed a significant increase in patients with NASH. (B) Correlation coefficients for MIC A/B to NAS and the stage of fibrosis in patients with NAFL. (C) Levels of MIC A/B mRNA were significantly associated with markers of disease severity in patients with NASH. (D) Further subanalysis of MIC A/B levels in relation to M30 levels revealed a significant correlation within the NASH cohort. (E) Next, apoptosis was measured using the TUNEL assay to quantify intrahepatic cell death. Patients with NASH demonstrated numerous clusters of apoptotic cells (arrows) in a background of altered hepatic micro-architecture. Quantitation of these TUNEL-positive hepatocytes demonstrated a significant increase in patients with NASH as compared with control and NAFL. Some of the fluorescent cells in control, NAFL, and NASH specimens represent red blood cells and were thus not regarded as TUNEL-positive. Representative photomicrographs of liver sections are shown.
Markers of Hepatic Fibrogenesis Are Enhanced in NASH.
Hepatic stellate cells are the principal cell type responsible for collagen deposition in the liver.32 We thus quantified transcripts indicating hepatic stellate cell activation by qrt-PCR. As expected, mRNA for α-smooth muscle actin, a cardinal marker for hepatic stellate cell activation, was increased 2.6-fold in patients with NASH as compared with controls (Fig. 4A). Conversely, α-small muscle actin transcripts were not up-regulated in individuals with NAFL. To ascertain whether HCS activation was associated with enhanced hepatic fibrogenesis, mRNA for hepatic collagen 1α (I) was quantified next. Collagen 1α (I) mRNA expression was increased 4.4-fold in patients with NASH versus controls (Fig. 4B). However, collagen 1α (I) mRNA was marginally up-regulated in NAFL. Hepatic collagen protein deposition was identified in liver specimens by Sirius red staining (Fig. 4C) and subjected to quantitative morphometry.26 Collagen staining by Sirius red was increased 2.7-fold in patients with NASH versus controls (Fig. 4C). Moreover, collagen deposition in livers of individuals with NAFL was significantly lower (5.7 ± 1.1%; P < 0.05) (Fig. 4C).

Hepatic fibrogenesis is enhanced in patients with NASH. (A) Transcript for α-smooth muscle actin, a cardinal marker for stellate cell activation, was significantly increased as compared with the control and NAFL groups. (B) Collagen 1α (I) mRNA expression was measured next and was also increased in patients with NASH. (C) Sirius red staining, a chemical stain of collagen deposition in the liver, was performed and quantitated using digital image analysis. Representative photomicrographs of liver sections are depicted. As demonstrated, Sirius red staining was quantitatively more intense in morbidly obese patients with NASH as compared with the control and NAFL groups.
Discussion
It is well known that the clinical presentation of NASH is highly variable, which can be attributed to host, genetic, environmental, and other factors.33 Some patients develop only minimal hepatic damage that rarely progresses to a truly chronic hepatopathy.34 Because these patients normally maintain this status, medical treatment is not required. However, some of these patients develop acute liver failure, liver cirrhosis, and even hepatocellular malignancy with the necessity of liver transplantation.35-38 Therefore, investigation of the underlying mechanisms leading to the development of NASH and its progression to fibrosis and liver cirrhosis is crucial to understand this entity—even more so on the background that is globally on the rise.39
The distant major histocompatibility complex class I homologs, MIC A/B, are recently identified human ligands for the NK cell receptor NKG2D.40 These stress-induced ligands can act as danger signals to alert NK cells by way of NKG2D engagement, and are increased in various chronic liver diseases. For example, patients with posttransplant HLA antibodies and expression of MIC A/B have a higher rate of chronic graft failure.41 The trend of rejection was more prevalent in patients with MIC A/B antibodies compared with those without antibodies. In another study, Jinushi and colleagues5, 27 investigated the role of MIC A in patients with hepatocellular carcinoma and detected elevated MIC A transcripts in hepatocellular carcinoma tissue but not in the surrounding noncancerous tissue. This elevation of MIC A was associated with down-regulated NKG2D expression and impaired activation of hepatic NK cells as a typical feature of malignant cells for escaping innate and adaptive antitumor immune responses. Changes in serum levels of MIC A/B were also observed by Kohga et al.6 in patients with HCC during arterial embolization. They showed that transcatheter arterial embolization therapy significantly decreased the levels of soluble MIC A/B and increased NKG2D expression by NK cells. Holdenrieder et al.42 analyzed the expression of MIC A/B in the sera of patients with autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, and healthy individuals. Similar to healthy controls, low levels of these stress-induced ligands were found in the sera of patients with hepatic autoimmune diseases. In contrast, significantly elevated concentrations of MIC A/B were observed in patients with cholestasis leading to increased serum levels of NKG2D.42 Zhang et al.43 explored MIC A/B signaling during host–parasite interactions in patients with alveolar echinococcosis and found that the expression of MIC A/B proteins was strongly up-regulated in hepatocytes and bile ducts in the areas of periparasitic infiltrates. In summary, the stress-induced expression of MIC A/B and their recognition by diverse NK cells may serve as a mechanism for the detection of damaged (for example, by fatty infiltration during NASH), infected, or malignantly transformed cells.44
In our present study, MIC A/B transcripts were detected in significant levels in patients with biopsy-proven NASH following bariatric surgery for obesity. Consistent with the PCR data, immunohistochemical analysis revealed that immunoreactivity of hepatic NK cells and MIC A/B was also enhanced in the livers of these patients. Immunohistochemically, MIC A/B revealed a diffuse tissue staining pattern. Due to loss of cell membrane integrity upon tissue injury, these stress-induced ligands may be internalized upon cellular stress as observed during fatty infiltration of the liver in NASH patients. The exact mechanisms remain to be clarified by further studies. In order to exclude the possibility that MIC A/B are up-regulated by obesity, we furthermore investigated a cohort of patients with NAFL and could demonstrate that the number of hepatic NK cells and transcripts for MIC B were significantly decreased in this study population, which was accompanied by lower degrees of liver injury, hepatocyte apoptosis, and fibrosis. Moreover, we found a significant positive correlation between MIC proteins and markers of disease severity in patients with NASH. Therefore, assessment of MIC A/B serum levels could be identified as a novel index to assess liver injury in NASH in a noninvasive fashion. Taken together, these results indicate that MIC A/B are expressed on hepatocytes from patients with NASH and that their expression plays an important role in their susceptibility to NK cells during hepatic inflammation as mediated by fatty infiltration of the liver.
Indeed, based on the crystal structure of the NKG2D-MIC A-complex, Zhang and colleagues synthesized 3 short peptides, mimicking functional α1 and 2 domain of MIC A, and demonstrated that MIC A–mimicking peptides might be useful for targeting the function of NK cells.45 In addition, Jinushi et al.46 demonstrated that NK-induced cytolysis was completely abolished by antibody-mediated masking of MIC A/B. MIC molecules can also be released from the cell surface by the activity of metalloproteinases, which can be inhibited by addition of a broad-spectrum metalloproteinase inhibitor.47 Metalloproteinases are known to be involved in tissue inflammation and repair.48 We recently demonstrated in a mouse model of murine obstructive cholestasis that administration of CTS-1027—a broad-spectrum matrix metalloproteinase inhibitor—significantly decreased liver injury, hepatocyte apoptosis, and fibrosis.49 This drug has already entered clinical trials evaluating the anti-inflammatory and antifibrogenic properties in hepatitis C. Modulation of MIC A/B expression may therefore be an attractive strategy to therapeutically control the development and progression of NASH.
To the best of our knowledge, this is the first study demonstrating that MIC A/B may contribute to disease severity in patients with NASH. Our results provide a basis to further investigate the biology underlying the NASH-associated expression of MIC A/B, as well as their potential significance as antigen-presenting molecules for activating hepatic NK cells. Further studies are needed to determine the precise mechanisms by which fatty infiltration of the liver activates MIC A/B expression.
Acknowledgements
We thank the anonymous reviewers for their valuable and constructive suggestions.




